The Beginning of the Genus Homo: The Concept of Species as an Influential Factor for Result
Abstract
The beginning of the genus Homo is not easy to be characterized, because its fossil remains are few and often quite fragmented. In addition, the recent discoveries relating to this topic, provided from Eastern and South Africa, and Dmanisi in Georgia has brought more debate because are founded on different explanatory views. Succinctly, the two main models of explanations for the birth of the Homo conceive, on one hand, a genus comprised by many distinct species, and on the other hand, a single lineage through which such a genus evolved gradually. In front of these two approaches we ask if there is an existence of a relation through which determines the identification of the number of species supposedly present in the group of fossils at the beginning of the genus Homo to the concept of species used to characterize it. In this discussion we intend to present such a relation considering two concepts of species: Biological Species Concept and Evolutionary Species Concept.
Introduction
The beginning of the genus Homo is not easy to be characterized. In addition to the amount of hominid fossils dating back to the Pliocene are few, and often quite fragmented.
Different from the fossils of the Upper Pleistocene, the biological material associated with hominid species approximately 1.8 million years old does not provide access to DNA information, which makes it difficult to draw conclusions about taxonomy. Furthermore, the recent discoveries relating to this topic, especially the fossils found in Dmanisi, Georgia [1], the findings of South Africa known as Homo naledi [2] the discoveries of African fossils in the Afar desert, Ethiopia, dated 2.8 million years [3] and the reconstruction of OH 7 [4] brought even more controversy to the debate, because these provide bases for different paths explanatory for the origin of our gender.
In synthesis, these discoveries can be understood through two different models used to explain the arising of genus Homo. While there are many arguments that support the hypothesis that the emergence of the such genus was strongly marked by distinct rate, the way it has been sustained by the researchers of the H. naledi [2], it has also been indicated that the high diversity of hominid types of this period, satisfies a single highly diversified species [1].
Due the fact in which each of these approaches is so hardly different, it arises to ask if their results are not influenced by some primordial conceptual orientation, or just the result of applying a particular methodology.
In general, in this kind of surveys it is not common to see any concern relevant to the concept of species used. The works are generally aimed at discussing of definitions, which indicate differences between groups capable of distinguishing them as species. However, disregard a plural reality concerning the concept of species in biology, which can influence or guide the results of such work.
The absence of reference to the concept of species in paleoanthropology research brings us to an understanding based solely on the Biological Species Concept, because this is the most traditionally used concept. However, the identification of key terms indicates specificities consistent with other concepts, although the very same are not disclosed within the premises of these works.
Before starting such a discussion, it is necessary to present two concepts used subliminally in some of the research that address this problem. Subsequently, we shall present the relation between the conceptual logic which is presented in two study perspectives associated with the beginning of the genus Homo and the two species concepts used in biology: Biological Species Concept and Evolutionary Species Concept.
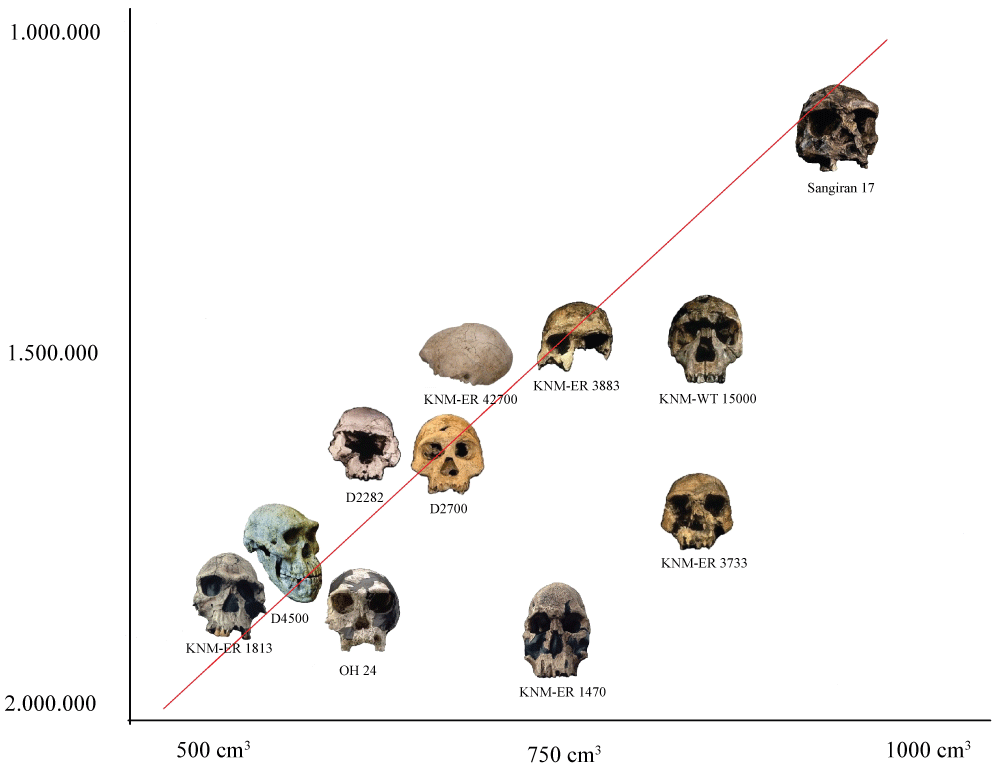
The next Table 1 shows the main fossils assigned as Homo discovered in African continent as well as Caucasus region, dated from 2.0 until 1.5 million of years. Concisely, the approach through which each species assigned as Homo is conceived as a biological species [5-18] may be correlated to the Biological Species Concept. Otherwise, the theoretical view thereby all earlier Homo specimen is thought to comprise one single evolutionary species is associated to the Evolutionary Species Concept [1,19-25].
Species Concept (S) used in Paleoanthropology
For more than two centuries of studies people have tried to define what biological species is.
According to Gonzalez-Forero [26], the species concept had originally been used by the naturalist Linnaeus in the 16th century, but he considered it as a means to designate typological objects, and may be of animal, vegetable and mineral origin, in other words, also included, particularities referring to the inorganic world [26,27].
Buffon considered the condition of interfertility in cospecific, i.e, reproduction between members of the same group. Whereas Dobzhansky [28] pointed out the importance of isolation.
Apart from these and other definition, only since 1942 that Mayr legitimately based which became known as the "Biological Species Concept" [29]. The aforementioned is a concept in which reproductive isolation presents itself with greater emphasis, because in this bias the species are considered as "groups of real populations, or potentially interbreeding, which are reproductively isolated from other groups" [30,31].
It should be noted that the Biological Species Concept makes no sense when directed to the inanimate world, because it only concerns of a biological concept. This systematization occurs for the first time in science, because prior to this, the species concepts could be applied to the inanimate world as well as to living beings [32].
Still within such a concept, it should be emphasized that the ability for groups or populations of organisms for interbreeding and leaving fertile offspring, or not, became the key criterion for defining boundaries between species [26,33]. Therefore, populations that are even geographically separated, although maintain the capacity to produce fertile offspring when in possible contact, are considered to be members of the same species, regardless of any other differences between them.
Although the Biological Species Concept can be considered a landmark to think of biological species, it is hardly used when compared to concepts developed by other researchers.
According to Mayden [33], there exist at least 22 species concepts being used, however, many of them are incompatible with the complexity related to biological diversity. Most of these concepts are functional constructs or definitions (classes) which the notion of species as a taxon (individual) is shown to be rejected. The search for a solution to this problem does not reflect the exclusion of such concepts, taking into account that they can be used according to a greater or lesser degree of operability and applicability, but rather to their conditioning to a primary concept of species-monistic, in a hierarchical manner. Nevertheless, the problem arises to the priority levels directed to information that one wishes to obtain relative to any group of organisms.
The study of species needs the definition of some concepts fundamental for distinguishing quantity inside a group and quantity between-groups. In this case, the number of species exiting within an ecosystem must be recognized as a diversity of species [34]. In addition, the variability is understood as a range of an existing feature within a single lineage.
In a general way, the species are the end products of speciation, and almost all settings available referring to this term taking for granted that the speciation process, whereby species originate must produce a basically uniform result [29,35]. Nonetheless, as they are visualized as speciation products, rather than uniquely defined and self-contained categories, it has been evolving species such groups, or dynamic entities, relatively cohesive [13]. In other words, it concerns as a distinct information derived from the Biological Species Concept, in which the capacity of intersecting between groups is essential, in order to define boundaries between species.
The definition of species used by Hennig [33,36,37] comprises any historically formed group, in which the ancestor and all their descendants are inserted into a phylogenetic nexus, monophilic, consisting as a non-operational theoretical definition, at least in the first moment. This definition includes the ability to reproduce as a characteristic of ancestral lineage that retains the anagenesis of some attribute closely related to reproductive success.
Mayden [33] indicates "Evolutionary Species Concept" as most suitable for this purpose, since this allows us to understand descent and speciation such as processes which occur in lineages, in other words, as a process corresponding to the formation of the rate.
The processes are designed in the Evolutionary Species Concept different from those in the Biological Species Concept. The lineage, key term to think about such a concept, evolves from a species and features its own trends [38]. This idea can be applied to asexual, unisexual and fossil species, which cannot be done within the Biological Species Concept, as the continuity of a lineage does not require the interbreeding of individuals [39]. Therefore, all the organisms of the past and of the present are grouped and are consistent with the same lineage or evolutionary species.
It is understood that the species, within the framework of the Evolutionary Species Concept, may be subdivided into other descendant ancestral species and that reproductive isolation must be effective enough to allow the maintenance of the "identity" of other contemporary lineages [39].
With those considerations about the two species concepts listed here, it is appropriate to get back to the paleontological problems chosen in this discussion-the beginning of the genus Homo-considering the examination of the processes and mechanisms related to speciation, as different mating, gene flow, adaptation, drift patterns, etc, in populations of living organisms in their natural habitats, together in the relation that is made around the cessation of genetic interchange between groups.
The difficulty to discern processes and evolutionary mechanisms arises insofar as there is not necessarily a predictive relationship between genetic and morphological divergence. In other words, we can use the morphology to create clusters which are essentially assumptions about species distinctions. Meanwhile, these should be tested against genetic and behavioral data, inaccessible in most of the paleontological cases. The impossibility of testing both data ends by directing an arbitrary choice to the concepts one is working on.
With regard to the studies related to the beginning of the genus Homo, one can note a recurrence in the presentation of results related to one of the two species concepts presented here. However, in these studies, the perspectives used to think on species diversity are presented at the end of the work, as a reading of the results, may compose the initial assumptions related to species concepts, placing epistemological against the background.
First Homo: An Evolutionary Species with Many Biological Species?
The first definitions for the beginning of the genus Homo present a species called Homo habilis, based primarily on the fact that stone artifacts have been potentially associated with findings [40]. But of course, such definitions also have distinct anatomical characteristics of those found in Australopithecus, as the larger brain, ranging from 600 to 700 cm³, total bipedalism [41], an increased roundness of the skull, mandible and reduced maxillofacial zygomatic area, a large supraorbital torus, a more rectilinear facial profile than designed forward, and a rounded alveolar arch with small canines and without sagittal crest [42].
Although there appears to be a single standard capable of characterizing the first Homo specimens, some researchers have argued that the variety of fossils within such a group could in fact correspond to at least two species-the H. habilis, in a strict sense, and H. rudolfensis [5-7,9,10,12,14-17]. However, other researchers seem to agree that the wide variation found in the group composed of the oldest fossils of the genus Homo relates only to an intraspecies variation, similar to paleo-deme found in Homo sapiens and genus Pan [1,21-25,43].
Part of the argument that sustains there is a single species at the beginning of the genus Homo, indicates the differences between fossils KNM-ER 1813 and ER 1470, mainly related to the size of 510 cm³ for the first, and 750 cm³ to the latter, as the result of sexual dimorphism [44-46]. However, the morphological and metric evidence shows that it is unlikely that both fossils belong to the same species, unless they represent a pattern of sexual dimorphism quite different from that found in other higher primates [9,12, 42,47]
The idea of high species diversity stipulated for the beginning of the genus Homo has also been supported by other archaeological and paleontological findings, such as the KNM-ER 62000, KNM-ER 60000 and KNM-ER 62003, which seem to emphasize the existence of the two species H. rudolfensis and H. habilis coexisting at the same timen [8]. The other example to reinforce such an idea is the skull OH 7, reconstructed through computer [4]. The cranial capacity of this fossil, dated 1.8 million years is estimated between 729 and 824 cm³, which is much greater than the variation of 500 to 700 cm³, estimated for the data set related to the first H. erectus. The characteristics of the mandible in OH 7, a fossil attributed to H. habilis, indicate that this anatomic part is more primitive than that of the AL 666-1. Indeed, a mixed set of derived and primitive features is naturally found in primate species [48]. However, considering the evolution of cranial form in Homo sapiens, it is expected to notice a trend through which skulls with bigger neurocranial elements should have smaller base-cranium apparatus, because of a modular integration occurred during the its development [49,50]. Therefore, the more primitive features in the OH 7's mandible, in addition to a larger brain, dated 500,000 years younger than the AL 666-1, is different from the expected, and may indicate multiple species.
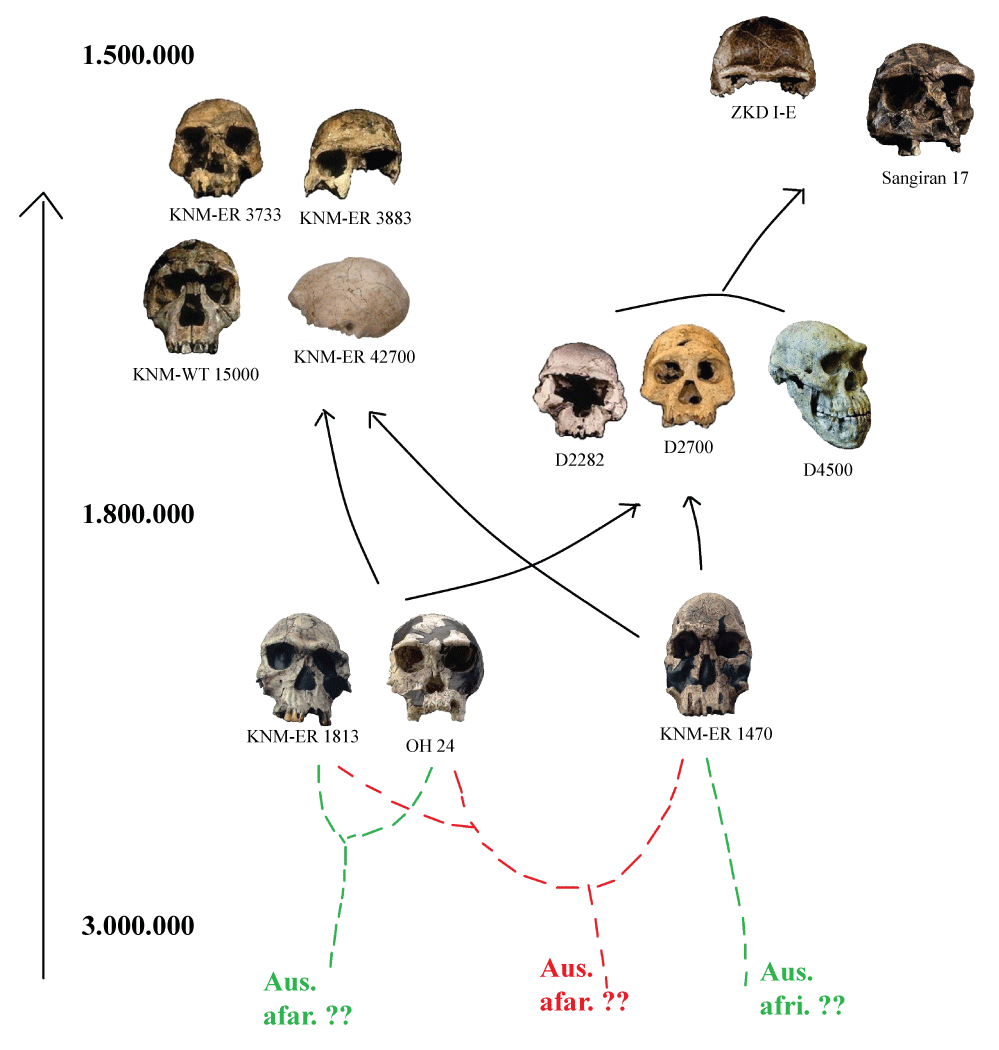
The argument that confirms the existence of several species was also established for the Homo erectus, a fossil group on which the data provide more recent evidence of 1.75 million years. In this sense, some researchers support the hypothesis of multiple taxa, derived from the older types found in the genus (Figure 1), distinguishing between the H. ergaster (African) and H. erectus sensu stricto (Asian) [18,19]. Other researchers suggest that the very H. ergaster may have been divided into several taxa [11,13].
Although not mentioned in the works, the arguments that underline a diversity of species at the beginning of the genus Homo are based on the Biological Species Concept, because they use the basic assumption, by which morphological differences may indicate speciation and consequently, species differentiation. On the other hand, although there may be many data and arguments capable to emphasize the taxa diversity at the beginning of the genus Homo, some researchers defend the idea of a single taxon in which both, H. erectus sensu stricto and H. ergaster should be grouped within the H. erectus [6,21,22,43]. In this way, these end up to be implicitly based on the Evolutionary Species Concept.
Perhaps the main study which indicates the as a signal relating to a single evolutionary species be the one led by Lordkipanidze and collaborators [1]. The studies are based on the findings of the archaeological and paleontological of the Dmanisi site, which is located in the Caucasus region and is dated to about 1.8 million years.
The bone remains of Dmanisi have been of great importance to the study of the first members of the genus Homo, mainly because these are oldest hominins found outside of Africa, for being well preserved, and for being confirmed as part of a single species: H. erectus [1,23-25]. Such a perspective, with basis on metric and non-metric character analysis, has indicated that the diversity within the five skulls of Dmanisi and their respective jaws, as well as other vestiges dated between 2.0 and 1.5 million years ago, could satisfy a single and gradual evolutionary lineage [1].
The main arguments serving this hypothesis sustain that the fossils of Dmanisi, have a paleodeme consisting of a combination of primitive craniodental traits and derivatives [1,20,23,51,52], so that the diversity within such a group is greater than that registered for African specimens of the same date, in addition to the set composed by the species of the genus Pan.
The skulls present a chronology between late Pliocene to 1.8 million years, up to 1.5 million years. Their cranial capacities range from 546 to 775 cm³ and the anatomical parts of the postcranial skeleton feature present plesiomorphic characteristics, as a greater medial orientation of the foot with regard to modern humans, the absence of torsion humeral, small body size and low encephalization quotient [53].
The assumption that considers the first Homo inserted into a single lineage gradually evolved, finds support when the evolution of the brain and the size of the body is considered together in space and time [54-57].
When considering the grouping of all the fossils located chronospatially, which are consistent with the line in question-the Homo genus-undergoing a gradual evolution, as shown in the Figure 2, and based on the explanatory base that defines these fossils for the Evolutionary Species Concept [43,58].
Discussion and Conclusion
The problem presented here refers to the fact of admitting the existence of a relation, which determines the identification of the number of species supposedly present in the group of fossils at the beginning of the genus Homo to the concept of species used to characterize it. It is therefore that the grouping of beings within the category 'species', depends otherwise, how variability and diversity are understood and used. The quantification, as well as the methods used in order to determine species diversity are based, in this way, how the concept will consider groups of organisms, and what will influence the decision, which will guide the way the species are visualized.
The lack of indication of the conceptual basis regarding the species, indirectly leads the reader to suppose that the works are oriented within the specificities of the Biological Species Concept, as this is the most traditionally known concept. However, it is worth mentioning that the results of such research, as well as the key terms used, indicate different assumptions that lead to either conclusion, although such an indication occurs indirectly, or subliminally.
The perspective that admits the fossils of the beginning of the genus Homo as distinct species, considers the morphological differences as useful attributes for distinguishing fossil species. In this way, it meets the premises of the Biological Species Concept. In contrast, when it is concluded that the fossils of the beginning of the genus Homo together meet a single evolutionary lineage, justified as own attributes of the Evolutionary Species Concept, admitted by Simpson [38], namely, an evolutionary trend in the lineage, which according to Henneberg [54,55] and Van Arsdale and Wolpoff [57] corresponds to the gradual increase of the brain over time, and a lower weight to the morphological variation as a means to distinguish species.
Obviously, each of the perspectives presents an importance to think about the evolution of genus, and in the speciation processes which involve the emergence of species.
Dmanisi´s research, for example, does not seem to consider morphological similarity in hominids as insufficient means, in order to define a group composed of a single species, as indicate in studies related to the genus Pan. In these studies we observed several groups that indicate great morphological similarities, despite the fact that they correspond to a genus definitely composed of at least two species, the Pan troglodytes and the Pan paniscus [59-63]. It should be highlighted that the speciation which carried out the division between chimpanzees and bonobos began at about 880,000 years ago [59,60,63], i.e. occurred in a shorter period than that used in the grouping of the fossils of the beginning of the genus Homo, of approximately 1.0 million years.
What is called into question in the present discussion is not the validity of the results in either work. Thus, thinking the group of the first Homo as a set consisting of several species does not imply saying that it is not also considered as a single evolutionary lineage. Apparently, such considerations must first and foremost, based on the conceptual basis that is working before being the result of the research results. On the other hand, older data seems to be more difficult to be used within a wider range of concepts, because they will be subordinate to the potential of the material to provide information. Due to complexities compelled in this puzzle, other explanatory modes may be justified in order to find a solution to our problem, as those provided by cultural and archaeological studies.
Culture is currently the largest vector for human diversity, able to directly influence the biological selection of various organisms [64]. Thus, it should serve to evaluate the diversity of species at the beginning of the genus Homo, if it weren't a so derisory force of influence over human biology, at the time of its emergence.
If we would evaluate the very little variety found in Oldowan technology we would conclude that we are dealing with only one species within that group. This finding goes back to the meaning of the Evolutionary Species Concept, since it relates a certain type (technology)/species (biology) to a gradual evolution toward more diverse forms over time. However, since the arising of culture is thought to have been very few in diversity, becoming gradually complex through time [65], it is reasonable to think that culture, in its emergence, was not influent for the development of biological differences able to create different species. In addition, the growing acceptance that other species of primates, as the case of the Sapajus libidinosus, are also capable to create instruments so similar to those associated with the initial Homo context [66], indicates that such Homogeneity is more related to a general question of cognition, natural of that biological order, than an reference to the existence of a single Homo species.
Perhaps, several species could make the same type of artifact at the beginning of their cognitive development. Thus, knowledge concerning the Homogeneity present in this technology may not be used for the study of diversity of species at the beginning of the genus Homo. The study should be reoriented to an eminently biological question.
The core of this problem, and its readdressed to a biological approach, indicates finally that placed limits on access to genetic material, will be difficult to fully work with the Biological Species Concept, still considered the most desirable to determine what become species. However, even methodology used in genetic studies could not determine a solution to this problem, because it is more oriented to other goals.
According to Ohl [67], the specificity of genetic studies in paleoanthropology is more related to taxonomy ordering, from which hypothesis of phylogeny might be elaborated. In such view genetic data and techniques collaborate largely to the understanding of divergence in human population as well as history and evolution of an ancient DNA. However, this kind of information is not enough to precise the boundaries able to determine a biological species clearly [68]. For instance, it reports the case study of interactions, or interbreeding occurred between H. neanderthal e H. sapiens. In this problematic, data has supported by 0.4% of European DNA corresponding to the Neanderthal [69-71] although morphological researches have indicated these two groups as different species [72-83].
Although some researches, for instance those leaded by Lordkipanidze and colleagues, have argued in favor of a single evolved species hypothesis for earlier Homo, we are looking toward a multiplicity of species to the group formed by such fossils as the better way of conceiving the diversity and variability of species. This idea is also based on the biological speciation stated before, in which the Homo sapiens and the Homo neanderthal are thought to being different species, in a process also occurred during about 0.5 million of years [69-71], i.e., within the same range of time used in our discussion, related to the group formed the earlier Homo.
Of course, the problematic related to the determination of the quantity of Homo species in the end of Pliocene and beginning of Pleistocene is unsolved and our positioning it is just a hypothesis amid others. The question discussed here was not exactly what is the best theory used to explain the birth of genus Homo, but how such ideas may be dependent on species concept used as background for think variability and diversity. Once there is one or other approach as the bases to drive a research, we may expect how result will be let at the end of a specific research.
References
- Lordkipanidze D, Ponce De Leon MS, Margvelashvili A, et al. (2013) A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo. Science 342: 326-331.
- Berger R, Hawks J, Ruiter D, et al. (2015) Homo naledi, A new species of the genus Homo from the Dinaledi Chamber, SouthAfrica. ELife 4: 1-35.
- Villmoare B, Kimbel W, Seyoum C, et al. (2015) Early Homo at 2.8 Ma. from Ledi-Geraru, Afar, Ethiopia. Science 347: 1352-1354.
- Spoor F, Gunz P, Neubauer S, et al. (2015) Reconstructed Homo habilis type OH 7 suggests deep-rooted species diversity in early Homo. Nature 519: 83-86.
- Chamberlain A (1987) A Taxonomic review and phylogenetic analysis of Homo habilis. Tese (Doutorado)-University of Liverpool.
- Chamberlain A (1989) Variations within Homo habilis. In: Giacobini (Org.), Hominidae: Proceedings of the 2nd International Congress of Human Paleontology. Milan: Editorial Jaca, 175-181.
- Donelly S (1996) How different are KNM-ER1470 and KNM-ER 1813? A multivariate comparison using randomization methods. American Journal of Physical Anthropology Supplement 22: 99.
- Leakey M, Spoor F, Dean C, et al. (2012) New fossils from koobi fora in northern kenyaconfirm taxonomic diversity in early Homo. Nature 488: 201-204.
- Lieberman D, Pilbeam D (1998) A probabilistic approach to the problem of sexual dimorphism in Homo habilis: A comparison of KNM-ER 1470 and KNM-ER 1813. J Hum Evol 17: 503-511.
- Lieberman D, Wood B, Pielbeam D (1996) Homoplasy and early Homo: An analysis of the evolutionary relationships of H. habilis sensu stricto and H. rudolfensis. J Hum Evol 30: 97-120.
- Schwartz J, Tattersall I (1999) Morphology and diversity in fossil hominids: Accepting Homo erectus and H. ergaster as separate taxa is just the beginning. Am J Phys Anthropol.
- Stringer C (1986) The credibility of Homo habilis. In: Wood B, Martin L, Andrews P, Major Topics in Primate and Human Evolution. Cambridge University Press, 266-294.
- Tattersal I (2007) Neanderthals, Homo sapiens, and the question of species in paleoanthropology. In: Journal of Anthropological Sciences 85: 139-146.
- Wood B (1992) Origin and evolution of the genus Homo. Nature 355: 783-790.
- Wood B (1993) Early Homo. How many species? In: Kimbel H, Martin B, Species, Species Concepts, and Primate Evolution. New York, 485-522.
- Wood B (1994) Koobi Fora research project, Volume 4: Hominid cranial remains. Journal of Anatomy 184: 178-179.
- Wood B, Collard M (1999) The Human Genus. Science 284: 65-71.
- Wood B, Richmond B (2000) Human evolution: taxonomy and paleobiology. Journal of Anatomy 197: 19-60.
- Clarke R (2000) Out of Africa and back again. International Journal of Anthropology 15: 185-189.
- Gabunia L, Vekua A, Lordkipanidze D, et al. (2000) Earliest pleistocene hominidcranial remains from dmanisi, republic of georgia: Taxonomy, geological setting, and age. Science 288: 1019-1025.
- Kramer A (1993) Human taxonomic diversity in the Pleistocene: Does Homo erectus represent multiple hominid species? Am J Phys Anthropol 91: 161-171.
- Rightmire G (2008) Homo in the middle pleistocene: Hypodigms, variation, and species recognition. Evolutionary Anthropology 17: 8-21.
- Rightmire G, Lordkipanidze D, Vekua A (2006) Anatomical descriptions, comparative studies and evolutionary significance of the hominin skulls from Dmanisi, Republic of Georgia. J Hum Evol 50: 115-141.
- Rightmire G, Lordkipanidze D (2010) Fossil skulls from Dmanisi: A paleodeme representing earliest Homo in Eurasia. In: Fleagle G, Shea J, Grine J, et al. The first hominin colonization of Eurasia Dordrecht. (1st edn), Springer, 225-243.
- Vekua A, Lordkipanidze D, Rightmire G, et al. (2002) A new skull of early Homo from Dmanisi, Georgia. Science 297: 85-89.
- GonzAlez-Forero M (2009) Removing ambiguity from the biological species concept. J Theor Biol 256: 76-80.
- Laurentii Salvii (1758) Systema Naturae, Per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. (10th edn), Stockholm, Sweden.
- Dobzhansky T (1937) Genetics and the origin of species. Columbia University Press, New York.
- De Queiroz K (2005) A unified concept of species and its consequences for the future of taxonomy. Proceedings of the California Academy of Sciences 56: 196-215.
- Mayr E (1943) Systematics and the origin of species. Columbia University Press, 289-291.
- Mayr E (1963) Animal species and evolution. Harvard University Press, MA, Cambridge.
- Mayr E (1982) The rise of the biological species concept. In: Mayr E, The growth of biological thought: diversity, evolution and inheritance. Harvard University Press, 270-285.
- Mayden R (1997) A hierarchy of species concepts: the denouement in the saga of the species problem. In: Claridge F, Dawah A, Wilson R (Org.), Species: The Units of Biodiversity. Chapman & Hall Press, 381-424.
- Hamilton AJ (2005) Species diversity or biodiversity? J Environ Manage 75: 89-92.
- De Queiroz K (1998) The general lineage concept of species, species criteria, and the process of speciation. In: Howard DJ, Berlocher SH, Endless Forms: Species and Speciation. Oxford University Press, 57-75.
- Hennig W (1950) Grundzuge einer theone der phylogentischen systematik, Aufbau Verlag, Berlin.
- Hennig W (1966) Phylogenetic systematics, University Illinois Press, Urbana, 280.
- Simpson G (1961) Principles of animal taxonomy. Columbia University Press, New York, USA.
- Wiley E (1978) The evolutionary species concept reconsidered. Systematic Biology 27: 17-26.
- Leakey R (1973) Evidence for an advanced Plio-Pleistocene hominid from east Rudolf, Kenya. Nature 242: 447-450.
- Cela-Conde C, Ayala F (2007) Human evolution-trails from the past. Oxford University Press, 437.
- Rightmire G (1993) Variation among early Homo crania from olduvai george and the koobi fora region. Am J Phys Anthropol 90: 1-33.
- Anton S (2003) Natural history of Homo erectus. Am J Phys Anthropol 122: 126-170.
- Lee S, Wolpoff M (2005) Habiline variation: A new approach using STET. Theory Biosci 124: 25-40.
- Miller J (1991) Does brain size variability provide evidence of multiple species in Homo habilis? Am J Phys Anthropol 84: 385-398.
- Tobias P (1991) Olduvai Gorge Volume IVa and IVb: Homo habilis, skulls, endocasts and teeth. Cambridge University Press.
- Guimaraes S, Lorenzo C (2015) Variability in first Homo: Analysis of the ratio between the skulls KNM-ER 1470 and KNM-ER 1813 based on sexual dimorphism of Homo sapiens. Homo 66: 387-398.
- Begun D (2010) Fossil record of miocene hominoids. In: Henke W, Tattersall, Handbook of Paleoanthropology. Springer, 921-977.
- Bastir M (2008) A system-model for the morphological analysis of integration and modularity in human craniofacial evolution. Journal of Anthropological Sciences 86: 37-58.
- Lieberman D, McBratney B, Krovitz G (2002) The Evolution and development of cranial form in Homo sapiens. Proc Natl Acad Sci USA 99: 1134-1139.
- Lordkipanidze D, Vekua A, Ferring R, et al. (2005) The earliest toothless hominin skull. Nature 434: 717-718.
- Lordkipanidze D, Vekua A, Ferring R, et al. (2006) A fourth hominin skull from Dmanisi, Georgia. Anat Rec A Discov Mol Cell Evol Biol 288: 1146-1157.
- Lordkipanidze D, Jashashvili T, Vekua A, et al. (2007) Postcranial evidence from early Homo from Dmanisi, Georgia. Nature 449: 305-310.
- Henneberg M (1995) A single-lineage hypothesis of hominid evolution. Evolutionary Theory 11: 31-38.
- Henneberg M (1997) The problem of species in hominid evolution. Perspectives in Human Biology 3: 21-31.
- Henneberg M, De Miguel C (2004) Hominins are a single lineage: brain and body size variability does not reflect postulated taxonomic diversity of hominins. Homo 55: 21-37.
- Van Arsdale A, Wolpoff M (2012) A single lineage in early pleistocene Homo: Size variation continuity in early Pleistocene Homo crania from east Africa and Georgia. Evolution 67: 841-850.
- Anton S (2012) Early Homo: Who, when, and where. Current Anthropology 53: S275-S298.
- Fischer A, Pollack J, Thalmann O, et al. (2006) Demographic history and genetic differentiation in apes. Current Biology 16: 1133-1138.
- Gonder M, Locatellia S, Ghobriala L, et al. (2010) Evidence from cameroon reveals differences in the genetic structure and histories of chimpanzee populations. PNAS 108: 4766-4771.
- Overmann K, Coolidge F (2013) Human species and mating systems: Neanderthal - Homo sapiens reproductive isolation and the archaeological and fossil records. J Anthropol Sci 91: 91-110.
- Shea B, Groves (1993) Multivariate craniometric variation in Chimpanzees: Implications for identification in paleoanthropology. In: Kimbel W, Species, species concepts and primate evolution. Springer Science, New York, 265-296.
- Won Y, Hey J (2002) Divergence population genetics of chimpanzees. Mol Biol Evol 22: 297-307.
- PJ Richerson, R Boyd (2005) Not by genes alone - how culture transformed human evolution. Chicago University Press, 342.
- Geertz C (1977) The interpretation of Cultures. Publisher Basic Books, New york, 480.
- Proffitt T, Luncz T, Falotico T, et al. (2016) Wild monkeys flake Stone tools. Nature 1-3.
- Ohl M (2010) Principles of taxonomy and classification: Current procedures for naming and classifying organisms In: Henke W, Tattersall I, Handbook of Paleoanthropology. Springer, 141-166.
- Hublin J (2010) Prospects and pitfalls. In: Henke W, Tattersall I, Handbook of Paleoanthropology. Springer, 815-829.
- Kuhlwilm M, Gronau I, Hubisz J, et al. (2016) Ancient gene flow from early modern humans into Eastern Neanderthals. Nature 530: 429-433.
- Meyer M, Fu Q, Aximu-Petri A, et al. (2014) A mitochondrial genome sequence of a hominin from Sima de los Huesos. Nature 505: 403-418.
- Prufer K, Racimo F, Patterson N, et al. (2014) The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505: 43-54.
- Benazzi S, Douka K, Fornai C, et al. (2011) Early dispersal of modern humans in Eurupe and implications for Neanderthal behavior. Nature 479: 525-528.
- Harvati K (2003) The Neanderthal taxonomic position models of intra- and inter-specific craniofacial variation. J Hum Evol 44: 107-132.
- Harvati K (2015) Neanderthals and their contemporaries. In: Henke W, Tattersall I, Handbook of Paleoanthropology. Springer, 1717-1748.
- Harvati K, Frost S, Mcnulty K (2004) Neanderthal taxonomy reconsidered: implications of 3D primate models of intra- and interspecific differences. Proceedings of the National Academy of Sciences (USA) 101: 1147-1152.
- Schwartz J, Tattersall I (2002) The human fossil record, vol. 1: Terminology and cranial morphology of genus Homo (Europe). Wiley/Liss, New York.
- Schwartz J, Tattersall I (2005) The human fossil record, vol. 4: Cranio dental Morphology of early Hominids (Genera Australopithecus, Paranthropus, Orrorin) and Overview. Wiley/Liss, New York.
- Stringer C, Gamble C (1993) In search of the Neanderthals: Solving the puzzle of human origins. Thames and Hudson, London, 247.
- Stringer C (1974) Population relationships of later Pleistocene hominids: A multivariate study of available crania. Journal of Archaeological Science 1: 317-342.
- Tattersall I (1986) Species recognition in human paleontology. J Hum Evol 15: 165-175.
- Tattersall I (1998) Neanderthal genes: What do they mean? Evolutionary Anthropology 6: 157-158.
- Tattersall I, Schwartz J (2000) Extict Humans. Boulder, Westview Press, USA.
- Tattersall I, Schwartz J (2006) The distinctiveness and systematic context of Homo neanderthalensis. In: Harvati K, Harrison T, Neanderthals Revisited. Springer, USA, 9-22.
- Alexeev, Valeri (1986) The origin of the human race. Progress, Moscow.
- Wood B (1991) Hominid cranial remains, vol. 4 of Koobi Fora research project. Clarendon, Oxford.
- Leakey R, Walker A, Ward C, et al. (1989) A partial skeleton of a gracile hominid from the Upper Burgi Member of the Koobi Fora Formation, east Lake Turkana, Kenya. In Hominidae: proceedings of the 2nd International Congress of Human Paleontology. Giacomo Giacobini, Milan, Jaca, 167-173.
- Spoor F, Leakey M, Patrick N Gathogo, et al. (2007) Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya. Nature 448: 688-691.
- Leakey M, Clarke R, Leakey L (1971) New hominid skull from Bed I, Olduvai Gorge, Tanzania. Nature 233: 317-323.
- Leakey L, Tobias P, Napier J (1964) A new species of the genus Homo from Olduvai Gorge. Nature 202: 7-9.
- Johanson D, Masao F, Eck G, et al. (1987) New partial skeleton of Homo habilis from Olduvai Gorge, Tanzania. Nature 327: 205-209.
- Leakey R, Walker A (1985) Further hominids from the Plio-Pleistocene of Koobi Fora, Kenya. Am J Phys Anthropol 67: 135-163.
- Walker A, Leakey R (1993) The Nariokotome Homo erectus skeleton. Springer.
- Rightmire G (1979) Cranial remains of Homo erectus from Beds II and IV, Olduvai Gorge, Tanzania. Am J Phys Anthropol 51: 99-116.
- Rightmire G (1990) The evolution of Homo erectus. Cambridge University Press, Cambridge.
- Walker A, Zimmerman M, Leakey R (1982) A possible case of hypervitaminosis A in Homo erectus. Nature 296: 248-250.
Corresponding Author
Santiago Guimaraes, Anthropology Graduate Program, Instituto de Filosofia e Ciências Humanas-IFCH, Federal University of Pará, Rua Augusto Corrêa, 01-Campus Universitário do Guamá, Belém-Pará, Brazil.
Copyright
© 2017 Guimaraes S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.