Intraoperative Analgesia Guided by the Nociception Level (NOL) Index in Laparoscopic Colorectal Surgery: A Preliminary Outcome Study
Abstract
Introduction: The majority of postoperative patients report moderate to severe pain, possibly related to opioid underdosing or overdosing during surgery. Nociception Level (NOL) Index has been proposed for the evaluation of the nociception–antinociception balance in the perioperative period and by that, may lead to a more appropriate analgesic regimen. The NOL-index is scaled from 0 to 100; with previous studies suggesting that values >25 nay guide analgesia dosing.
We designed a 2-cohort study (retrospective and prospective) trial to test the hypothesis that protocol-driven intraoperative analgesia guided by NOL during laparoscopic colorectal surgery may improve and reduce the intraoperative analgesic requirement.
Method: This single center, 2 cohort study aimed to compare perioperative data during laparoscopic colorectal surgery with or without the use of NOL monitoring (NOL-guided vs. control group).
Intraoperative analgesia was provided by fentanyl bolus injections, which was performed according to the clinician’s assessment in the control group or according to the NOL guided in the NOL-guided group. The primary outcome was a reduction in fentanyl consumption during surgery in the NOL-guided group.
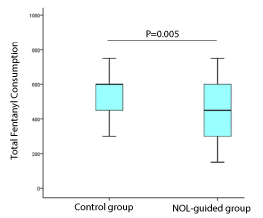
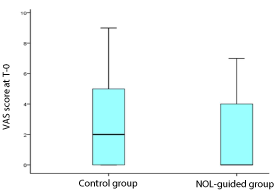
Results: Between 2017 and 2019, 70 patients (36 in the control group and 34 patients in the NOL guided group) were included. The median fentanyl consumption was significantly lower in the NOL guided group 450 mg versus 600 mg in the control group (P = 0.005). Median postoperative pain score at T-0, when the patient woke up in the operation room, was 2 (inter-quartile range 0-5) and 0 (0-4) in the control and NOL-guided groups, respectively (p = 0.132).
Conclusion: NOL guidance resulted in a statistically significant reduction of 25% in the doses of fentanyl administered in the NOL guided group compared with the standard of care group in laparoscopic colorectal surgery. This reduction in intraoperative analgesia requirement without negatively impacting pain scores suggests its interesting potential as a nociception monitor during general anaesthesia (GA). Future studies should employ a more robust design, be appropriately powered and seek to follow longer term outcomes.
Keywords
Nociception; Pain score; Fentanyl consumption; Laparoscopic colorectal surgery; NOL-perioperative pain management
Introduction
Balanced anaesthesia, the most common strategy used in the last decades, relies mostly upon the GABA-A receptor and mu-opioid receptor [1] .In colorectal surgery, the current practice is based on using a hypnotic for induction and maintenance of unconsciousness. Muscle relaxants are administered to produce immobility and opioids are the most commonly used drug to manage nociception intraoperatively and pain postoperatively [1,2]. Opioids are the ideal drug to block autonomic nervous system reactions whilst maintaining hemodynamic stability [2,3].
Nociception is intimately related to control of the autonomic nervous system and nociceptive disorders are a primary source of hemodynamic instability, as well as, postoperative chronic pain syndromes [4].
A sufficient level of antinociception has been reached when applied surgical stimuli, clinical responses as heart rate (HR) and blood pressure (BP) elevations no longer occur [1-3]. Nevertheless, according to a recent clinical trial, despite absent or mild clinical responses, nociceptive activation persists during deep general anaesthesia (GA). Therefore, the lack of clinical responses is not indicative of the absence of nociception specific activation [5].
Undoubtedly, opioids are effective antinociceptive agents and one of the three pillars of balanced anaesthesia is the administration of opioids in the perioperative period [1,6].
Although opioids are the most effective antinociceptive drug, they cannot be easily quantified or monitored during GA. Analgesics are administered somewhat theoretically, using population pharmacokinetic models, individual patient comorbidities, and generally adjusted to variations in HR and BP. Despite all this, inter-subject opioid pharmacological variability is very well established in the literature [7,8]. Furthermore, both HR and BP are influenced by other factors, including notably hypnotic anaesthetic agent blood concentration and haemodynamic status, and have been shown to be poor predictors of analgesic requirements [9].
Additionally, opioids that are the most effective antinociceptive drug, have undesirable side effects, such as respiratory depression, pharyngeal muscle weakness, postoperative nausea and vomiting, urinary retention, constipation, ileus, pruritus, tolerance and hyperalgesia that may progress into chronic pain syndrome [6,10,11]. Nausea and vomiting are particularly responsible for delayed patients' recovery, prolonged patient stay in the recovery area and, therefore, delayed hospital discharge. It is also known that opioids disorganize sleep patterns and may contribute to postoperative delirium [12].
Furthermore, patients receiving opioids as part of GA and leaving the hospital with opioid prescriptions, appear to have an increased risk of opioid dependence [13]. Therefore, it is debatable whether perioperative opioid administration is appropriate or necessary in current clinical practice [14].
Recently, nociception monitors have been introduced to track nociception during anesthesia and guide administration of analgesics, usually opioids. A reliable nociception monitors may help optimize analgesic management.
The Nociception Level (NOL) monitor (Medasense Biometrics Ltd, Ramat Gan, Israel) is able to reliably detect and quantify mild to intense noxious stimulation during anaesthesia and surgery and outperforms haemodynamic indices (BP, HR) in the ability to distinguish between noxious and non-noxious stimuli [15-17]. The NOL monitor uses an algorithm based on advanced statistical and machine learning technologies. The algorithm combines multiple autonomic signals (finger photo-plethysmograph amplitude, skin conductance, HR, HR variability, and their time derivatives) into a single index, the NOL index [18]. The NOL index ranges from 0 (absence of nociception) to 100 (extreme nociception) and the algorithm was validated in multiple studies [15-20].
Currently, there are only a few clinical outcome studies that assess whether the NOL index impacts GA care during surgery [19,20], and consequently; outcome studies using the NOL index have not yet been included in a meta-analysis study.
We therefore designed a 2-cohort trial to assess the ability of the NOL monitoring to modify anesthesia care during elective major abdominal surgery. Our study aimed to determine the effect of intraoperative NOL-guided fentanyl administration under GA. The primary hypothesis was that intraoperative NOL-guided fentanyl administration would reduce fentanyl requirement. The secondary hypotheses were that the NOL-guided group would show a reduction in the VAS pain score and in the total morphine consumption in the recovery room when compared to the standard practice.
Method
Ethics
This evaluation study was designed as an observational and interventional study. Two cohorts were analysed from 2017 to 2019:
Between April 2017 and October 2018, the retrospective cohort included 36 patients that provided consent to share their medical information including the physiological parameters recorded during surgery. In this cohort, intravenous analgesia with opioids was guided using standard of care hemodynamic monitoring.
In 2019, 34 patients were recruited after the prospective outcome protocol was approved by the local ethics committee.
All patients received written information about the protocol, had ample time to decide on their participation, and gave oral and written informed consent before enrolment into the study.
The study was performed at Complexo Hospitalário Universitário de Ourense, Spain.
Patients
American Society of Anesthesiologists (ASA) class I–IV patients (aged above 17) of either sex, scheduled for laparoscopic colorectal surgery under general anesthesia were recruited to participate in the study.
Exclusion criteria included inability to give informed consent, pregnancy/lactation, allergy to the opioids used, mean arterial pressure (MAP) at screen ingor on the day of surgery > 160 mmHg or < 55 mmHg, HR at screening or on the day of surgery > 9 0beats. min-1 or < 45 beats. min-1, any CNS-related disorder and body mass index (BMI) above 40.
Study Design
Standard Clinical Care at Complexo Hospitalário Universitário de Ourense, Spain: Before induction of anesthesia, dexamethasone 4mg and ondansetron 4mg were given. Patients received an IV line, BP cuff, Train-of-Four (TOF) for neuromuscular monitoring and were monitored with bispectral index (BIS) monitoring (Philips, Eindhoven, the Netherlands) for measurement of depth of anesthesia.
Anesthesia was induced with 150 mcg fentanyl followed by 2 mg/kg propofol. After loss of consciousness as detected by BIS values <60, absence of eyelash reflex andno response to verbal stimulation, a neuromuscular blocking agent, rocuronium 0.6 mg/kg, was administered. After full relaxation, the trachea was intubated. If HR was lower than 60 bpm, atropine 0.01 mg /kg was also administered.
After orotracheal intubation (IOT), patients received 0.5-3% sevoflurane to maintain a BIS between 40-60 and a rocuronium bolus of 10% of the initial dose to maintain a TOF of 1-2.
At the end of surgery, 1g of paracetamol, metamizole and dexketoprofen were given. Inspired sevoflurane concentration was tapereddown. All patients with a residual neuromuscular block (TOF ratios < 0.9) were reversed with sugammadex 2 mg/kg.
Maintenance in the Retrospective Cohort – The Control Group: In the control group (retrospective cohort), fentanyl dosing was dependent solely on hemodynamics (MAP, HR) as NOL-index values were not available. If systolic BP and/or HR increased by 20% from the baseline, 150 mcg fentanyl was administered. Vasodilation medication was given at the discretion of the anesthesiologist.
Maintenance in the Prospective Cohort Group – NOL-Guided Group: In the test group, fentanyl dosing was dependent on the NOL-index intervention. In cases where the NOL index was >25 for at least60sec, 150mcg fentanyl was administered. After fentanyl was given, 5 to 10 min were allowed before the next evaluation took place. If the NOL-index was below 25, no fentanyl was given.
If NOL-index was below 25 and the MAP was below 55 mmHg, vasoactive medication (ephedrine, phenylephrine, norepinephrine), crystalloids, or both could have been given.
Treatment in the Post-Anesthesia Care Unit (PACU): Patient monitoring and medical treatment followed standard hospital practice and were identical in both cohorts. After admission to the PACU, standard monitors were applied measuring heart rate, non-invasive blood pressure and oxygen saturation. Patients in the NOL-guided group remained connected to the NOL device for observational monitoring during their PACU stay.
Immediate postoperative pain intensity was assessed at T-0 i.e., as soon as the patient awoke from surgery, and at 1 hour intervals for the 3 hours of PACU stay using a 0–10 VAS score (0=no pain and 10=worst pain imaginable), with VAS < 3 corresponding to no or mild pain and VAS >3 corresponding to moderate-to-severe pain. In this study, patients reporting a VAS above 4, received 2 mg of morphine IV and the treatment was repeated every 10 min. until the VAS pain score was below 5.
The same discharge criteria were applied to both groups: controlled pain, hemodynamic stability and a modified Aldrete score. The Aldrete score is a composite index with 0-2 points for limb movement, respiration, circulation, consciousness, and oxygen saturation, and reflects the level of recovery from anesthesia. An Aldrete score of 9 or 10 indicates full recovery and PACU discharge readiness, although pain and other issues (nausea/vomiting, surgical complications, logistics) will delay discharge from the PACU.
Statistical analyses
Since at the time of writing the protocol, no studies had been published reporting treatment effect of NOL-guided analgesia, we could not estimate the standard deviation (SD)s. Based on our clinical experience in management of anesthesia, we know that in colorectal surgery, the patients receive about 750 mcg of fentanyl IV. For an expected decrease of at least 20% (600 mcg) in intraoperative fentanyl consumption in the NOL-guided group, with a type I error α= 0.05 (two-tailed), and 90% power, the total sample size needed was 65 patients (30 patients per cohort). To account for an estimated 5% rate of loss to follow-up or missing data due to technical problems, a total of 70 patients were recruited.
Numerical variables with normal distribution are presented as mean ± standard deviation (SD), and those without normal distribution are presented as medians (25th–75th percentiles). Categorical variables are presented in terms of frequency (percentages). Differences between the groups were analyzed using the independent samples t-test for numerical variables with normal distribution, and the Mann–Whitney U test was used for those without normal distribution. The relationships between the categorical variables were evaluated using the Chi square test. A probability value of p < 0.05 was considered statistically significant.
Results
We conducted this study with 70 patients (36 in the retrospective cohort and 34 in the prospective cohort), aged above 17 yrs. old between April 2017 and April 2019. Recruitment ended when the number of included patients reached the calculated required sample size. Data from all 70 patients were analyzed. No patients were excluded from the study. There were no significant differences between the patients in terms of demographic data and ASA risk classifications (Table 1).
When the patients were compared in terms of duration of anesthesia and the surgery performed, there was no difference between the two cohorts (data not shown). Surgical procedure and anesthesia times were similar between treatment groups (data not shown). All study patients completed the trial without any adverse events.
Intraoperative Fentanyl Consumption
The intraoperative fentanyl administration was less in the NOL-guided group compared with the control group by 25%: mean fentanyl in the NOL-guided group was 450 mcg versus 600 mcg in the control group (mean difference of 150mcg with 95% CI 150-300mcg, p=0.005). Table 2 summarizes the total fentanyl consumption in the 2-cohort study.
Pain in the PACU
The individual pain scores (VAS) at T-0, i.e., immediately after the patient awoke in the OR, were consistently higher in patients who received standard care compared with those who received fentanyl dosing based on the NOL-index but the difference in median and the mean scores was not significant. The median pain score at T-0 was 0 (inter-quartile range 0-4) and 2 (inter-quartile range 0-5) in NOL-guided and control groups, respectively although not statistically significant (P = 0.132, Table 3).
There were no differences in median and mean pain scores after one and two hours between the NOL-guided and the control group, 1 (inter-quartile range 0-5.2) and 2 (inter-quartile range 0-6), respectively (P=0.663, Table 3) (Figure 2).
Morphine Consumption in the PACU
Cumulative mean morphine consumption in the PACU did not differ between groups (NOL-guided group 5mg, control group 4mg, mean difference 1mg, p=0.953).In both groups the Aldrete score reached values > 8 in the majority of patients in the PACU. The mean time spent in the PACU was 254 min. in the control group and 247 min. in the NOL-guided group (p=0.737).
Discussion
Most of the patients in the PACU are characterized with a number of physiological disturbances caused by emergence from anesthesia and surgery, which affect multiple organs and systems. Postoperative pain and following agitation usually add risks and deterioration to complications with their interactions, aggravating outcomes. According to the guidelines by the American Society of Anesthesiologists, routine assessment and monitoring of pain detects complications and reduces adverse outcomes, and should be performed during emergence and recovery. However, unconsciousness and/or inabilities of clear verbal expression increase the difficulties in assessment and treatment for pain in the PACU.
Intraoperative high-dose opioid consumption is associated with postoperative hyperalgesia and can be associated with delays in recovery, postoperative nausea, and vomiting, which may extend time to discharge from the recovery unit.
Hence, given all of the above, it is important to prevent high pain levels during and after surgery. As both opioid under-dosing and overdosing during anesthesia are associated with high postoperative pain scores, we designed a study in which we examined whether opioid dosing based on the individual nociceptive state of the patient during surgery, as measured by the NOL index, would improve postoperative pain scores as a secondary goal of our study. Our study shows that NOL monitoring statistically significantly lowers opioid consumption in patients undergoing colorectal surgery, under general anesthesia.
Currently, several nociception monitors are available for detection of nociceptive events during surgery. The algorithms used to derive the level of nociception differ among monitors and most monitors measure single physiological variables such as HR variability or combine two variables such as BP and HR. In contrast to the other nociception monitors, the multiparameter NOL-index that we used has an algorithm based on machine learning technology. Earlier studies indicate that the NOL-index outperforms other indices of nociception including BP, HR, the pulse-plethysmograph amplitude and the surgical pleth-index (GE, Healthcare, Helsinki, Finland; an index that combines pulsebeat interval and pulse wave amplitude), in discriminating noxious from non-noxious stimuli [15-17]. Additionally, Meijer F et al. recently showed that NOL-guided remifentanil dosing results in improved haemodynamic stability compared with the control group [19].
In the NOL-guided group we used the NOL-index absolute cut-offs value of 25. We based this threshold on previous outcome and validation studies with the NOL index [15,19].
The management of intraoperative nociception/antinociception of the patients in the control group, and hence, opioid consumption, was based on hemodynamic monitoring and clinical judgement.
Although hemodynamic data provide us some ideas about nociception/antinociception, it is not possible to state that they can precisely reflect nociception/antinociception. It is well known that hemodynamic are associated with several changes such as fluid status, patient age, and depth and type of anesthesia. We aimed in our study to decrease the total consumption of fentanyl by 25% by adjusting dosing based on changes observed in the NOL-index measurements in the NOL-guided group. Another possible reason for the reduction in fentanyl requirement may be that the the anesthesiologist in the NOL-guided group, is more attentive and focused on keeping the NOL-index below the nociception - antinociception balance of 25 according to the protocol.
These results indicate that NOL monitoring during anesthesia has certain advantages in terms of opioid consumption. Further studies with larger sample sizes are needed to address whether nociception monitoring results in improved hemodynamic stability. During the patients stay in our clinic none of them developed any major complications. Still, we did not follow our patients beyond their hospital stay and hence remain uninformed on possible long-term complications.
Postoperative pain is the most common concern of patients undergoing surgery [21]. Up to 40% will experience severe pain in the recovery period, with a subsequent increase in the risk of developing chronic pain [22,23]. Our study found that in NOL-guided fentanyl dosing group rather than dosing based on hemodynamic indices (BP and HR) in the control group, results in a 2-points reduction in VAS pain score in the OR, when immediately after patient woke up after the surgery. This initial observation of clinically significant reduced pain scores in patients receiving NOL-guided fentanyl dosing, indicates the added value of a multiparameter monitor driven by an artificial intelligence algorithm based on advanced statistical and machine learning technologies. This reduction was not statistically significant due to the limited number of patients in the study but according to many studies on the relevance of postoperative pain score, this change seems to be clinically meaningful and therefore is of interest. We do not believe that the lower pain scores reported in the OR may be attributed to remnants of opioids given during surgery because the cumulative amount of opioids in the NOL-guided group was significantly lower than in the control group.
We believe that the benefit of the monitor was in improved timing of the dosing providing more personalized analgesia management.
Limitations
The study has several limitations: first of all, in common with all studies on the influence of nociception monitor-guided anesthesia on various anesthesia endpoints, our study was single blinded. This may have influenced the outcome due to an implementation or performance bias.
Secondly, the sample size was relatively small, but our intention was to detect a relatively large difference in intraoperative fentanyl consumption that would be clinically meaningful. We observed that a reduction 25% in fentanyl requirement in the NOL guided group. This difference is clinically meaningful and statistically significant. Thirdly, we relied on intermittent non-invasive blood pressure measurements to guide anesthesia in the standard clinical care group. It may well be that a continuous blood pressure signal from an arterial line may have improved our ability to guide anesthesia with more rapid response to nociceptive input. Finally, this study was powered for a single primary objective and some of the secondary objectives evaluated in this study might have shown no difference between groups because of lack of power and too small number of patients. Studies with a larger number of patients are necessary to better evaluate these specific objectives.
Conclusions
We studied the influence of nociception-guided anesthesia using the NOL monitor and observed that, compared with standard clinical care, NOL guidance resulted in a statistically significant reduction in the doses of fentanyl administered of 25% in the NOL-guided group compared with the standard of care in laparoscopic colorectal surgery. Furthermore, the patients in this study experienced less pain upon emergence when opioid dosing was guided by the NOL monitor compared with standard of care. These changes resulted in clinically relevant rather than statistically significant results.
NOL monitoring had no effect on postoperative opioid requirements.
Future studies in larger, more diverse populations should address the long-term outcome benefit of intraoperative analgesia guidance by the NOL index.
References
- Talmage D Egan (2019) Are opioids indispensable for general anaesthesia? Br J Anaesth 122: e127-e135.
- Brown EN, Pavone KJ, Naranjo M (2018) Multimodal general anesthesia: Theory and practice. Anesth Analg 127: 1246-1258.
- Lavand'homme P (2019) Opioid-free anaesthesia: Pro: damned if you don't use opioids during surgery. Eur J Anaesthesiol 36: 247-249.
- Cividjian A, Petitjeans F, Liu N, et al. (2017) Do we feel pain during anesthesia? A critical review on surgery-evoked circulatory changes and pain perception. Best Pract Res Clin Anaesthesiol 31: 445-467.
- Lichtner G, Auksztulewicz R, Velten H, et al. (2018) Nociceptive activation in spinal cord and brain persists during deep general anaesthesia. Br J Anaesth 121: 291-302.
- Mulier J (2017) Opioid free general anesthesia: A paradigm shift? Rev Esp Anestesiol Reanim 64: 427-430.
- Kaye AD, Fox CJ, Padnos IW, et al. (2017) Pharmacologic considerations of anesthetic agents in pediatric patients: a comprehensive review. Anesthesiol Clin 35: 73-94.
- Silins V, Brasher C, Antus F, et al. (2017) Predicting postoperative morphine consumption in children. Anaesth Crit Pain 36: 179-184.
- Wessel DL (1993) Hemodynamic responses to perioperative pain and stress in infants. Critical Care Medicine 21: 361.
- Lavand'homme P, Steyaert A (2017) Opioid-free anesthesia opioid side effects: Tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol 31: 487-498.
- Sultana A, D Torres, R Schumann (2017) Special indications for opioid free anaesthesia and analgesia, patient and procedure related: Including obesity, sleep apnoea, chronic obstructive pulmonary disease, complex regional pain syndromes, opioid addiction and cancer surgery. Best Pract Res Clin Anaesthesiol 31: 547-560.
- Lavand'homme P, Estebe JP (2018) Opioid-free anesthesia: A different regard to anesthesia practice. Curr Opin Anaesthesiol 31: 556-561.
- Mauermann E,W Ruppen, O Bandschapp (2017) Different protocols used today to achieve total opioid-free general anesthesia without locoregional blocks. Best Pract Res Clin Anaesthesiol 31: 533-545.
- Hah JM, Bateman BT, Ratliff J, et al. (2017) Chronic opioid use after surgery: Implications for perioperative management in the face of the opioid epidemic. Anesth Analg 125: 1733-1740.
- Martini CH, Boon M, Broens SJ, et al. ( 2015) Ability of the Nociceptio Level, a multiparameter composite of autonomic signals, to detect noxious stimuli during propofol- remifentanil anesthesia. Anesthesiology 123: 524-534.
- Edry R, Recea V, Dikust Y, et al. (2016 ) Preliminary intraoperative validation of the Nociception Level index, a noninvasive nociception monitor. Anesthesiology 125: 193-203.
- Stockle PA, Julien M, Issa R, et al. (2018) Validation of the PMD100 and its NOL Index to detect nociception at different infusion regimen of remifentanil in patients under general anesthesia. Minerva Anestesiol 84: 1160-1168.
- Ben Israel N, Kliger M, Zuckerman G, et al. (2013) Monitoring the Nociception Level -A Multi-Parameter Approach. Journal of Clinical Monitoring and Computing 27: 659-668.
- Meijer F, Martini C, Broens S, et al. (2019) Nociception-guided versus standard care during remifentanil-propofol anesthesia: a randomized controlled trial. Anesthesiology 130: 745-755.
- Meijer F, Honing M, Roor T, et al. (2020) Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. British Journal of Anaesthesia 125:1070-1078.
- Apfelbaum JL, Chen C, Mehta SS, et al. (2003) Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 97: 534-540.
- Gerbershagen HJ, Aduckathil S, van Wijck AJ, et al. (2013) Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiol 118: 934-944.
- Perkins FM, Kehlet H (2000) Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 93:1123-1133.
Corresponding Author
Olalla Figueiredo González, Tel Aviv, scientific consultant for Medasense Ltd, Israel.
Copyright
© 2022 González OF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.