Critical Review of Alcohol, Alcoholism and the Withdrawal Symptoms. II. Treatment Strategies
Abstract
Alcoholic beverages, socially accepted drinks around the world, are consumed (legally by adults and illegally by minors) to socialize, celebrate, and relax. However, persistent drinking results in the development of tolerance that necessitates a perpetual increase in alcohol drinking to achieve desired effects. In genetically predisposed subjects and in the presence of certain environmental cue, chronic alcohol drinking induces addiction, characterized by excessive uncontrollable drinking associated with rapid onset of the withdrawal symptoms. Currently there are only three medications approved by the U.S. FDA to treat alcoholism: disulfiram (alcohol metabolizing enzyme inhibitor), naltrexone (opiate receptor antagonist), topiramat and acamprosate (NMDA receptor inhibitor). Because pharmacotherapy alone or in combination with behavioral approaches is only modestly effective in treating alcoholism symptoms, there is an urgent need to develop effective and safe therapies. In the present review, we describe upcoming Biopsychosocial (BPS), pharmacologic, pharmacogenetic, pharmacogenomic, genomic and phyto medicinal approaches for the treatment of alcoholism.
Keywords
Acetaldehyde, Alcoholism, Addiction, Ethanol, Genomics, Herbal therapy, Pharmacogenomics, Pharmacotherapy, Tolerance, Withdrawal
Introduction
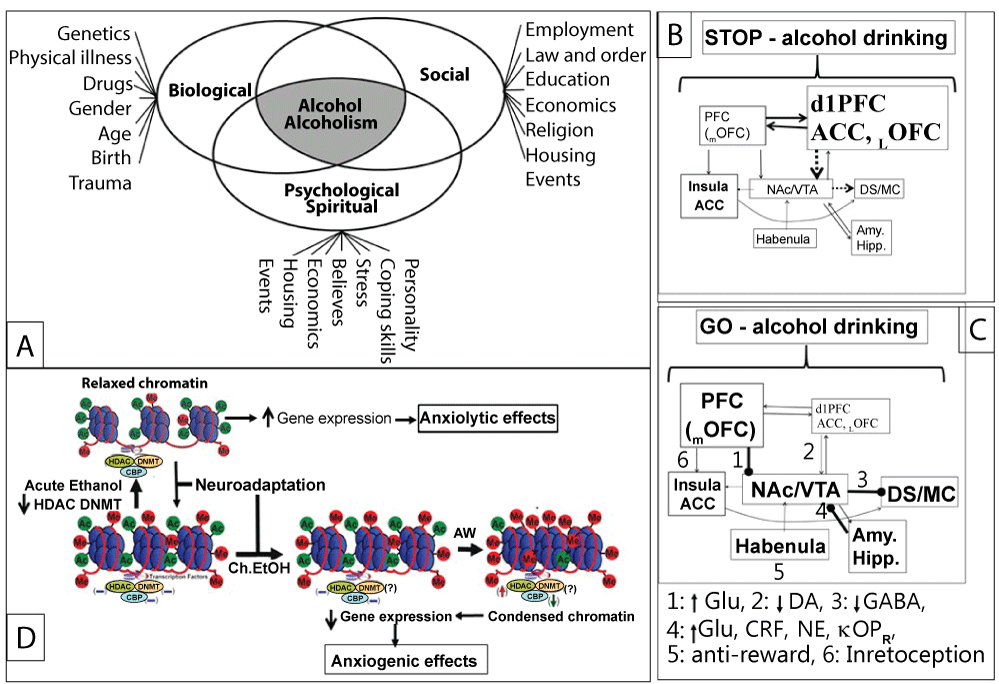
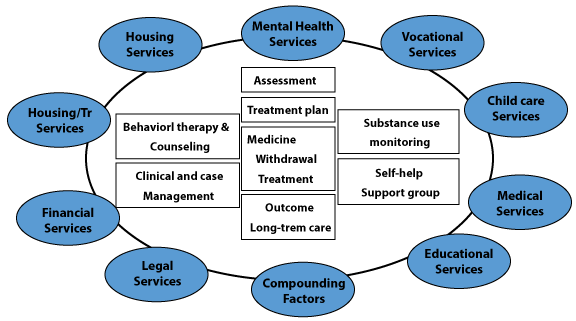
Alcoholism, a chronic disorder with often devastating health and economic consequences [1-3] has multiple causes with psychological, social and biological factors (Figure 1), also known as the Biopsychosocial (BPS) factor [4], determine the course of an individual's health-related outcomes [4]. Therefore, a single approach such as pharmacotherapy and/or behavioral counseling may not effectively treat the complex nature of the disorder [5,6]. In addition, every individual is not equally affected by each cause, necessitating a comprehensive individual assessment in order to adequately determine the individual's treatment needs. According to a 2016 National Institute of Drug Abuse (NIDA) report, a comprehensive alcoholism therapy must include a combination of behavioral therapy, psychosocial counseling, pharmacotherapy, exercise, relapse prevention and other services to meet the needs of the individual patient to accommodate issues as age, race, culture, sexual orientation, gender, pregnancy, parenting, housing, and employment, as well as physical and sexual abuse (Figure 2).
The comprehensive therapy proposed by NIDA, although effective, is expansive and require extensive resources and time commitment that may not be generally available in primary care settings. Therefore, novel strategies are needed that will effectively treat alcoholism and prevent relapse without some of the disadvantages listed above. Currently, many diverse approaches including the psychosocial therapies, non-traditional pharmacotherapy, Pharmacogenetics, pharmacogenomics, gene therapy and herbal therapy are being developed and tested for clinical use [7-11].
Despite considerable development in the quality of health care for people suffering from alcoholism, many deficiencies remain. Two major deficiencies are discussed below.
• According to a National Institute of Alcohol Abuse and Alcoholism report (2015) [12], only about 40% of the 7.9 million people suffering from alcohol dependence seek and/or receive treatment in the United States, possibly because people lack insurance or have limited financial resources, intimidated by the therapeutic agent's side effects and/or need for long-term commitment by the patients.
• Studies have shown that the chances of relapse (alcoholic reinstating alcohol consumption) are extremely high (approximately 54% relapse rate in the United States) in a large proportion of people who are in treatment program or have undergone available treatments [13]. Major precipitating factors for relapse include drug craving and stress, pre-attentive automatic reactions, and attention bias related to previous drug experiences [14-18].
For alcoholism therapy to be entirely successful, these deficiencies must also be addressed.
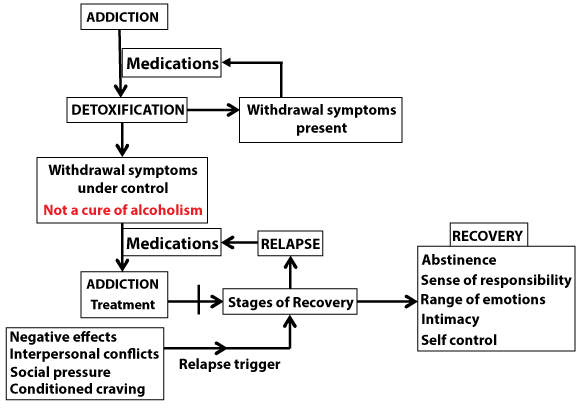
The overall aim of this review article is to elucidate current and upcoming approaches for treatment of alcoholism and other alcohol-related disorders. In general, alcoholism therapy is a three-step process: (1) Management of the withdrawal symptoms to facilitate abstinence and (2) Pharmacologic and non-pharmacologic treatments of the disease and (3) Prevention of relapse (Figure 3).Withdrawal management is not by itself an addiction treatment, but it is management of the withdrawal's aversive symptoms so that abstinence can be maintained. Relapse and even death from alcohol overdose may occur if the patients do not successfully transition to treatment and/or support program (Figure 3).
Management of the alcohol withdrawal symptoms
Cessation of alcohol drinking is the first step in treatment of alcoholism and other alcohol related disorders. Unfortunately, in alcoholics, self-detoxification is problematic because abrupt alcohol withdrawal results in rapid onset of symptoms such as agitation, anxiety, fever, tremors, seizures, and in some cases, hallucinations, delirium tremens (DT) and, if not treated, death [19] (Figure 4).
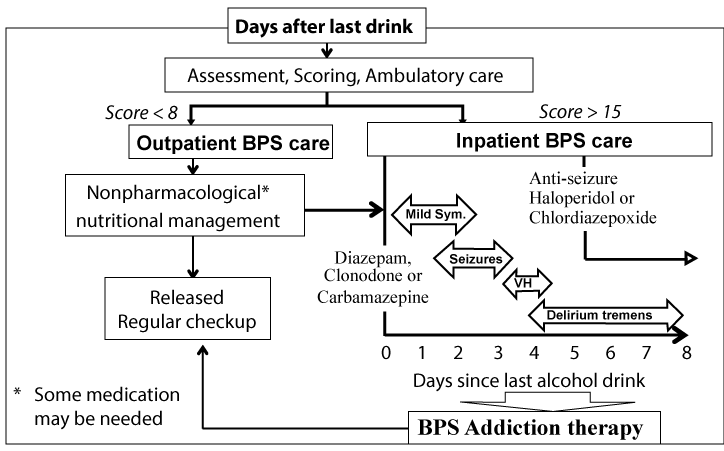
Alcoholics seek alcohol to get relief from the withdrawal symptoms. Thus, for detoxification, the withdrawal symptoms must be controlled as part of a comprehensive treatment program to increase the likelihood of successfully altering their drinking behavior [20]. Prior to deciding a treatment strategy, the severity of the withdrawal symptom is assessed using one of the assessment-protocols such as the Clinical Institute Withdrawal Assessment, CIWA-Ar [21]. Patients with CIWA-Ar score less than 8 (in some cases less than 15 in the absence of any co-morbidity such as psychiatric, cognitive or poly substance use problems) could successfully complete detoxification in an outpatient setting with managed supportive therapy and some pharmacological intervention [22]. Patents with CIWA-Ar score greater than 15 (or < 15 with co-morbidity such as recent surgery, psychiatric problems, pregnancy and lack of social support) may require hospitalization for more tests and treatments. In the following sub-sections, various pharmacological and non-pharmacological approaches of withdrawal management will be discussed.
Pharmacological management of alcohol withdrawal symptoms
Pharmacotherapy involves disease treatment with pharmaceuticals that act on a specific target, enzyme or receptor [23]. The interest in pharmacotherapy is growing because of identification of novel neurotransmitter systems that initiate and sustain alcohol drinking, synthesis of neurotransmitter analogues that may alter dependence, use of phyto-pharmacologic agents that reduce alcohol consumption, and medications that have improved the treatment of other addictive disorders, such as nicotine and opioid dependence [24]. The following drugs are approved for managing the withdrawal symptoms:
• Benzodiazepines: Benzodiazepines bind to the gamma-amino-butyric acid (GABA)A receptors at a site distant from the GABA-binding site and enhance the GABA-induced chlorideinflux [25,26]. Two commonly used benzodiazepines are chlordiazepoxide and diazepamthat, in addition to having anticonvulsant capabilities (diazepam >> chlordiazepoxide), also reduces everity of the withdrawal symptoms. However, its chronic use results in deterioration of cognitive functioning, physical dependence and tolerance [27-30]. Thus, in benzodiazepine treated patients, the drug-withdrawal may induce withdrawal symptoms that may be resistant to other drugs listed above.
• Carbamazepine: Carbamazepine is a GABA receptor agonist and has potency to suppress seizures, neuropathic pain and manic-depressive illness [31]. The drug has also been shown to be efficacious and can be chosen as an alternative to benzodiazepines in treatment of the withdrawal symptoms.
• Chlormethiazole: This drug is a positive allosteric modulator at the barbiturate/picrotoxin site at the GABAA receptor [32]. It effectively treats and/or prevents DTs in alcoholism patients, if given at an early stage.
• Buspirone, a non-benzodiazepine anxiolytic: Buspirone may reduce anxiety with less side effects and addiction potential than benzodiazepines [33-36].
• Adjuvant treatments: Adjuvants such as atenolol, propranolol and clonidine may be used in conjunction with benzodiazepines in patients with coexisting conditions such as coronary artery disease [19]. Bromocriptine (a dopamine agonist) and chlormethiaxole (a CNS depressant) have also been used in supportive treatment of alcohol withdrawal [36]. Acupuncture can also be used as an adjunctive treatment to the alcohol withdrawal symptoms in combination with other medication [37,38]. Further investigation of this treatment modality appears to be warranted.
Application of herbal remedies in withdrawal management
There have been promising results from different research groups studying the therapeutic effects of plant extracts on the withdrawal symptoms.
• Passiflora incarnata Linneaus and a tri-substituted benzoflavone moiety (BZF) isolated from the extract may counter the dependence produced by benzodiazepine or other addiction-prone substances like morphine, nicotine, and alcohol [39-42].
• Poyares, et al. [43] showed that valerian, a naturally occurring root, decreased WASO (wake time after sleep onset) with the mild anxiolytic effect in patients experiencing withdrawal symptoms in response benzodiazepine withdrawal after receiving 2-week of treatment.
• The aqueous extract of kudzu root or purified puerarin, in addition to suppressing alcohol intake, also suppressed the severity of alcohol withdrawal symptoms [44-46]. More clinical studies are warranted to identify the active ingredients and decipher the underlying mechanisms for the beneficial effects of herbal extracts in humans.
Management of addiction and relapse
As discussed earlier [47], a journey from responsible alcohol drinking to alcoholism involves the following stages: (i) Positive (pleasure seeking) and negative (pain avoidance) reinforcements, (ii) Tolerance, (iii) Physical addiction or alcoholism and (iv) Physical signs of withdrawal from alcohol abstinence [48,49]. Although substantial evidence support that the brain genomic, metabolic, cellular, and molecular processes play a critical role in the development of alcoholism and other alcohol-related disorders, an integrated and unified mechanism has not been established, thus hindering development of effective and safe therapeutic approaches. As discussed earlier, alcoholism is a multifaceted disease with complex biological, psychological and social interactions (Figure 1), thus requiring a multifaceted treatment approaches. In following sub-sections, current upcoming strategies for treatment of alcoholism and prevention of relapse have been discussed.
Non-pharmacological psychosocial management of alcoholism
Psychosocial approaches are based on an interrelation of social factors with individual thought and behavior including a patient's mental, emotional, social, and spiritual health. As shown in Figure 1, an interaction between the genetic and environment factors plays a critical role in the development of alcoholism in people abusing alcohol. Pharmacotherapy, Pharmacogenetics, pharmacogenomics and gene therapies (discussed in section 3.2) may address the only the biological abnormalities, not the psychological, social and economic issues. In the following sub-sections, psychosocial and behavioral approaches have been discussed.
• Involvement of a social worker: Abnormal alcohol consumption and alcoholism management can have adverse social and economic consequences on the individual, his family and society as a whole in terms of resources required for criminal justice, health care and other social institutions. A social worker, not the clinic or hospital, many assist the patients and the family to deal with social and/or economic issues.
• Biopsychosocial (BPS) Therapy/Interventions: The 'Bio' part of BSP therapy involves pharmacotherapy and gene therapy that are discussed later. The Psychosocial therapy deals with an individual's psychological development in and interaction with their social environment. The therapy includes structured counseling, motivational enhancement, care coordination, psychotherapy, and relapse prevention. To improve the patient's motivation to change, he/she is subjected to (i) Brief motivational intervention (BMI) and (ii) Motivational interviewing (MI) [50]. MI improves motivation to change, self-efficacy, and social support for abstinence. BMI, via 1 to 4 short sessions (10- to 60-minute), provides information and advice on the negative consequences of alcohol abuse with the goal to reduce alcohol intake rather than abstinence [51].
The cognitive behavioral therapy (CBT) improves the patients' cognitive and behavioral skills for changing their drinking behavior. In general, CBT is based on a model developed by [52] of relapse prevention, and often includes the following strategies: (1) Identifying triggers for relapse (intrapersonal and interpersonal), (2) Coping-skills training, (3) Refusal skills training, (4) Functional analysis of substance use, and (5) Fostering nonuse-related activities [53].
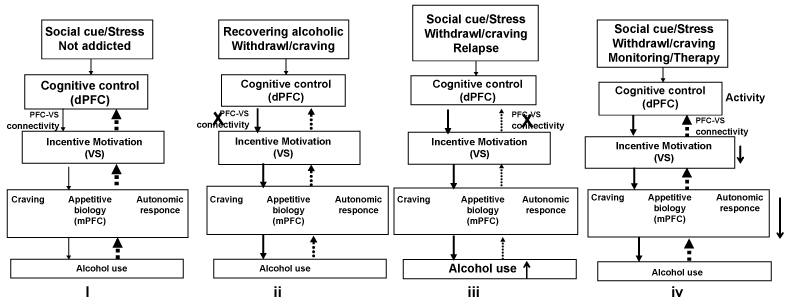
Although mechanisms for regulation of alcohol drinking is not fully understood, [54] have proposed key roles of (1) Dorsal prefrontal cortex (dlPFC) that sends a STOP alcohol drinking signal to VS, (2) Medial orbitofrontal cortex (mOFC) that sends GO drink alcohol signal to VS, and (iii) Ventral striatum (VS) that integrates the dlPFC and mOFC and regulates incentive motivation for alcohol seeking behavior. In healthy subjects, dlPFC is more active than mOFC, thus social alcohol drinking activates dlPFC, but not mOFC, and sends an inhibitory signal to the VS and ensuing incentive motivation to consume alcohol (Figure 5i). This reduces alcohol drinking. In patients during the early phase of alcoholism recovery, mOFC is more active then dlPFC, and sends a positive signal to VS, resulting in an increase in craving and incentive to consume alcohol (Figure 5ii). Alcohol consumption transiently activates dlPFC that suppress the withdrawal symptoms, beginning of the 'relapse' process (Figure 5iii). The behavioral/motivational exercises that activate dPFC and restores the dlPFC-VS cross-talk may suppress craving and incentive motivation to drink alcohol, thus preventing relapse (Figure 5iv).
Pharmacological management of alcohol addiction
Table 1 lists the currently approved and experimental therapeutic agents at various stages of development. At present, disulfiram, topiramate, naltrexone and acamprosate, alone or in combination, are commonly used to treat alcoholism [55,56], although studies have shown variability in their efficacy. [57,58] conducted a multi-state project sponsored by the NIAAA, to evaluate the efficacy of naltrexone, acamprosate, or both, with or without additional non-pharmacological treatment of alcoholism. They reported that patients receiving medical management with naltrexone, behavioral therapy, or both fared better on drinking outcomes, while acamprosate was ineffective. Therefore, more research is needed to evaluate the efficacy of drug combination in alcoholism pharmacotherapy. [59,60] provided clinical and experimental evidence that varenicline, a partial agonist of nicotinic acetylcholine receptor (nAChR), reduced self-reported alcohol reactivity, decreased alcohol self-administration and modulated DA mediated activities. This suggests that nAChRs play an important role in the behavioral effects of alcohol. [61] suggest that endocannabinoids acting at cannabinoid receptor 1 (CB1) contribute to ethanol preference and caused an age-dependent decline in the appetite for both ethanol and food by decreasing coupling of CB1 to G proteins in the limbic forebrain by mechanisms other than altered receptor or G protein levels. Voronin, et al. (2008) [62] showed that aripiprazole reduced the amount of drinking (reducing drinks per day and increasing days abstinent) during a natural observation period. However, some of these studies are experimental and require more studies.
Herbal therapy of alcoholism
Although pharmaceuticals are commonly used for treatment of alcoholism and other alcohol-related disorders, they have limited efficacy and, in some cases, severe side effects. This limitation has led to an exploration of complementary treatment approaches such as traditional herbal medicines that is relatively new to western medicine, but used extensively in traditional therapies in China, India, Egypt and other countries [63]. As summarized in Table 2, crude extracts and/or purified ingredients from St. John's wort, ibogaine, kudzu root and other plants suppressed alcohol intake and severity of the withdrawal symptoms in animal models of excessive drinking. Although the mechanisms of action of these compounds on alcohol intake are not fully understood, these compounds may exert their effects by modulating several neuronal systems implicated in drinking behavior. Their role in the future of pharmacotherapy for alcoholism will depend upon the outcome of carefully conducted clinical trials.
Pharmacogenetics, Pharmacogenomics and Genomic Therapy of Alcoholism
Investigating the role of genetics in predicting treatment outcomes is one of the promising areas of research, since a genetic basis for alcoholism (heritability rate, 50 to 60%) is well established in the literature [64-66]. With the completion of the Human Genome Project and rapidly falling costs of genetic analysis, genetics is providing a better understanding of diseases including alcoholism. With increased understanding of disease etiology comes greater potential to match patients with treatments. In general, three gene-based treatment strategies are developing: pharmacogenetics that involves development of pharmaceuticals that selectively target people with certain gene alleles, pharmacogenomics that considers the entire genome to select appropriate pharmaceuticals, and gene therapy that involves direct manipulation of selected genes to achieve desired therapeutic effects.
Pharmacogenetics
Although pharmacotherapy is commonly used to treat alcoholism, currently available drugs have modest efficacy, high individual variability in treatment outcomes, and severe side effects listed below [67,68] limit positive outcomes of the treatments.
• Benzodiazepines themselves are addictive and generate severe withdrawal symptoms similar to alcohol addiction.
• Carbamazepine has a rather narrow therapeutic window, warranting the need to monitor serum levels, and its hepatotoxic effects.
• Naloxone may potentiate the acute withdrawal syndrome and cause catecholamine release, ensuing pulmonary edema and cardiac arrhythmias.
• The side effects of topiramate include anxiety, ataxia, confusion, diarrhea, diplopia, dizziness, drowsiness, dysphasia, fatigue, etc.
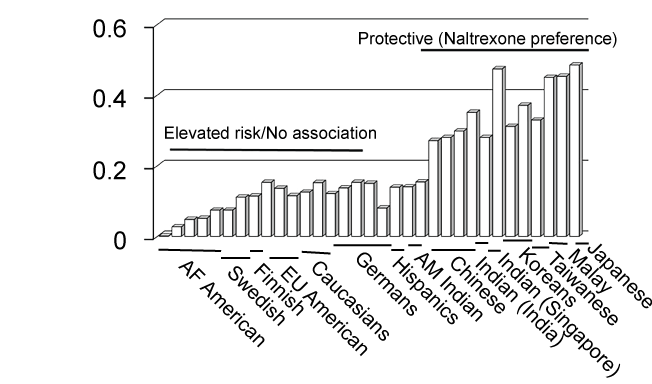
Thus, the pharmaceuticals currently being used may be poorly tolerated, especially when used for relatively longer term. The high variability may be due to a close association between the genetic makeup of the brain and the susceptibility to different pharmacotherapeutic agents. For example, the effectiveness of naltrexone treatment was determined by the patients having OPRM1-Asp 40 heterozygous OPRM1-Asn 40 homozygous proteins [58]. The frequency of the homozygous and SNP alleles differs considerably in different societies, so is their response to naltrexone (Figure 6) [69].
However, the experimental studies and clinical trials have yielded variable results regarding association between naltrexone and OPRM1 (A118G polymorph, Asp 40 protein substation) since a positive association [70-78] and lack of association [79-84] have both been reported between naltrexone and OPRM1-Asp 40. In addition to OPRM1, other pharmacogenetic targets that may differentially respond to therapeutic drugsare OPRK1 (G36T and C843T), OPRD1 (G8T (Cys27Phe)), GRIK1 (C > A) and SLC6A4 5), polymorphisms in alcohol metabolism, the 'reward pathway' (5HT, DA, GABA, Glu and β endorphin) and the behavioral stress response system (CRF and NPY).
A key advantage of pharmacogenetic approach is possible development of a personalized therapy that may provide better outcome [9,85]. Following are some of the genetic approaches currently being tested:
1. Naltrexone is more effective in patients possessing a polymorphic OPMR1 of the µ-opioid receptor gene than in patients lacking the polymorph [58,74,86].
2. Karpyak, et al. [87] have shown that patients containing single nucleotide polymorphism of GATA-binding protein 4 (GATA4) experiences very few relapses when treated with acamprosate [87].
3. 5-HTTLPR, a polymorph of the serotonin transporter (5-HTT) gene modulate the severity of alcohol consumption and predict a therapeutic response to the 5-HT(3) receptor antagonist, ondansetron. Individuals with this specific genotype had a higher percentage of abstinent days than all other genotype and treatment groups [88-90].
4. Enoch, et al. [91] have shown additive effects of a functional HTR3B rs1176744 SNP and the 5-HTTLPR polymorphism on alcohol and drug dependence. The genotype combinations of HTR3B variants and the 5-HTTLPR polymorphism may identify subgroups of alcoholics with varying response to ondansetron [92,93].
5. Franklin, et al. [94] proposed that the purinergic receptor P2X4R that binds adenine nucleotides [95] represents a novel target for drug development to prevent and/or treat alcoholism and other alcohol-related disorders. They hypothesized that there is an inverse relationship between P2X4R activity and ethanol consumption. Ivermectin, a positive modulator of P2X4Rs, antagonized ethanol-mediated inhibition of P2X4Rs in vitro and reduced ethanol intake in rodents.
Research is in progress to screen more pharmacotherapeutic alleles that can potentially be used to treat alcoholism.
Pharmacogenomics
Pharmacogenomics examines overall inherited variations in genes that dictate drug response and explores the ways these variations can be used to predict an individual's response to a drug [96]. Translation of the inherited variations in a gene gives rise to different proteins that may be functionally different. An alcoholic individual may possess different proportions of the gene alleles and exhibit different sensitivity to a particular pharmacotherapy. An example of this is the genes encoding alcohol dehydrogenase (ADH) for which variations in the gene sequences leads to the expression of different enzyme isoforms such as ADH1B, ADH1A, ADH1C and ADH4. People having different proportions of these isoenzymes may exhibit different degree of alcohol metabolism and alcoholism susceptibility [97,98].
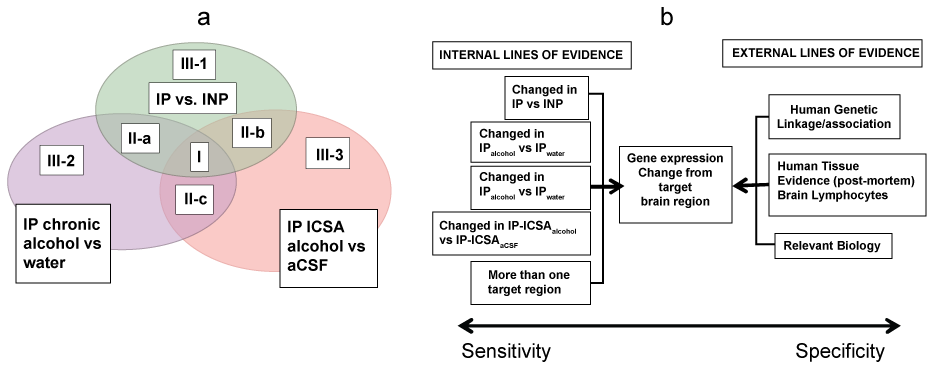
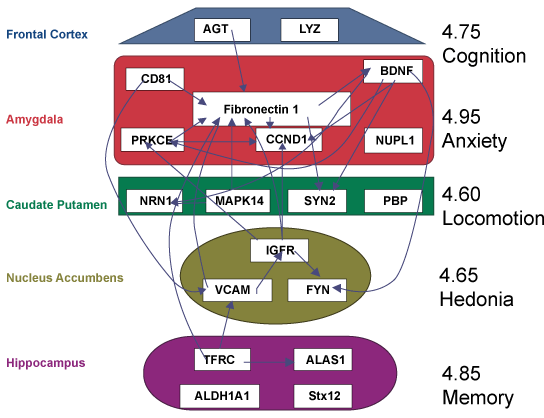
Earlier Rodd, et al. [99] applied a convergent functional genomics (CFG) approach to elucidate the genomics of alcoholism-related disorders. The three paradigms used were: (1) Innate neurological differences in basal gene expression in two rat-lines (IP and INP) selected for divergent propensity to consume alcohol, (2) The genetic alterations produced by chronic alcohol consumption in IP rats, and (3) Changes in gene expression following intracranial self-administration (ICSA) of alcohol into the posterior VTA that should primarily be the result of the reinforcing actions of alcohol in this region (Figure 7). Based on the patterns of change, genes were labeled in three categories shown in Figure 7. Rodd, et al. [99] showed that, out of top 20 differentially expressed genes, 14 interacted in a network with FN1 core that interacted with the following networks (Figure 8):
• cell adhesion and signaling - VCAM1 in nucleus accumbens [100] and CD81 in amygdale [101],
• iron-heme metabolism - TFRC [102] and ALAS1 [103] in hippocampus,
• cardiovascular regulation - AGT in frontal cortex [104] and PRKCE in amygdala [105],
• cell proliferation and differentiation-insulin-like growth factor 1 receptor - IGF1R [106] BDNF [107], CCND1 [108], FYN [109] and MAPK14 [110] in different brain regions, and
• synaptic transmission and neurite outgrowth - SYN2 [111] and NRN1 [112] in caudate putamen.
Some of the candidate genes in the data sets encode for proteins that are modulated by existing pharmacological agents (Table 3), which may potentially be applicable in alcoholism therapy.
Gene transfer therapy of alcoholism
As discussed above, pharmacogenomics therapy is based on how variations in the human genome affect the response to medications. However, the genomic therapy involves direct modulation (activation or suppression) of a gene to achieve desired therapeutic effects. Recombinant viral vectors (adenoviruses (AdV), adeno-associated viruses (AAV), and retroviruses including the leukemia virus and the human immunodeficiency virus) containing the gene of interest are administered to deliver genes for increasing protein expression. Conversely, antisense oligo nucleotides are used to block or destroy the mRNA, thus reducing the protein expression. The 3rd generation viral vectors allow the expression of the therapeutic gene for periods exceeding 1 year [113,114]. Earlier studies [115-119], using AAV or AdV vectors, have shown increased expression of the target genes in the brain reward centers such as the ventral segmental area (VTA), nucleus accumbens (NuA), and other corticolimbic structures, including the extended amygdala. [120,121] have described an AdV gene delivery vector that addresses the role of oxidants in alcohol-induced liver injury. [122-124] used antisense phosphorothioate oligonucleotides to inhibit TNF α synthesis in Kupffer cells and aldehyde dehydrogenase (ALDH2) and ethanol consumption in rodents [125] showed that an antisense oligonucleotide directed against a GGGA motif in rat mitochondrial ALDH2 markedly inhibited the synthesis of this enzyme, leading to a significant increase in acetaldehyde levels and decreases in alcohol consumption by rats.
Rivera-Meza, et al. [126] administered an adenoviral vector (AdV-ADH/asALDH2) encoding both a fast rat ADH and an antisense RNA against rat ALDH2 in rats and showed a 176% increase in liver ADH activity and 24% decrease in liver ALDH2 activity in control rats. Upon ethanol administration, their arterial acetaldehyde levels were 400% higher than those of animals administered control vector (AdV-C) with 60% reduction in their voluntary ethanol intake versus controls. Thus, the simultaneous increase of liver ADH and a reduction of ALDH activity by gene transfer could constitute a potential therapeutic strategy for the treatment of alcoholism.
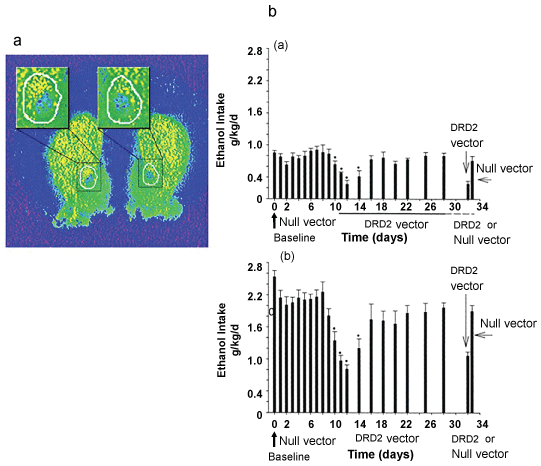
Thanos, et al. [127] administered an AdV-dopamine D2 receptor (DRD2) gene vector into the nucleus accumbens (NAc) of rats previously trained to self-administer alcohol, and assessed if DRD2 levels regulated alcohol intake in preferring (Figure 9a) and non-preferring (Figure 9b) rats.
They showed that a 52% increase in NAc DRD2 caused 43% reduction in alcohol preference (43%), and 64% reduction in alcohol intake of ethanol preferring/non-preferring rats, which recovered as the DRD2 returned to baseline levels. This was the first evidence that over expression of DRD2 reduces alcohol intake and suggests that high levels of DRD2 may be protective against alcohol abuse. Later [128], showed that animals receiving bilateral microinjections of a viral vector producing 5-HT1B over expression (HA1B/GFP) in nucleus accumbens consumed twice as much ethanol as the control groups, possibly because increased 5-HT1B expression led to either greater reward or reduced aversive effects. [129,130], by administering a null vector or a genetically modified adenoviral vector containing the rat D2R cDNA into the NAc of wild-type (Drd2+/+), heterozygous (Drd2 +/-), and receptor-deficient mice (Drd2-/-) mice showed significant decrease in ethanol intake by Drd2+/+ and Drd2+/- animals, but increase in ethanol intake in Drd2-/- animals. Ethanol intake and preference were then determined using the two-bottle choice paradigm. These observations provide proof of concept that gene manipulation can be a novel means to treat alcohol-related disorders, provided the functional genomics of the disorders are fully understood. With respect to alcohol and health outcomes, the strong association between alcohol drinking and smoking is often important because smoking has important relations to many medical conditions.
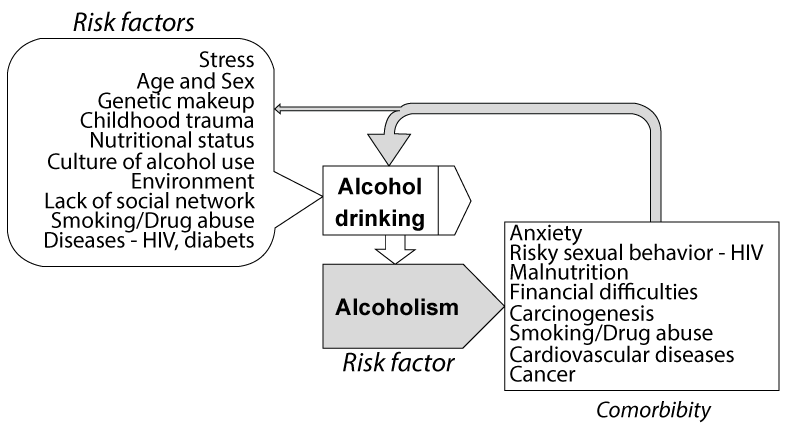
Risk Factors and Comorbidity
Risk factor is a factor that increases a person's chances of developing a disease, while comorbidity is presence of two or more illnesses, simultaneously or sequentially, in the same person. Figure 10 shows the risk factors for alcohol drinking and alcoholism and ensuing co morbid diseases.
Some of the key risk factors are dietary habits and nutritional deficiency [131], habitual smoking [132] cancer risk [133-135], cardiovascular diseases, liver diseases, etc. [136,137]. Some other risk factors are discussed below:
• Stress and Circadian Rhythms: Studies have shown that acute and chronic stress plays an important role in the motivation to abuse addictive substances including alcohol [15,138,139]. Conversely, alcohol drinking both reduces negative affect and increase positive effect, thereby reinforcing an effective, albeit maladaptive, coping strategy [138]. Stress management, therefore, is an important component of alcoholism therapy.
• Sex differences: Given the same amount of alcohol consumed, men and women can have differing morbidity and mortality from alcohol-related chronic disease and conditions, possibly because of pharmacokinetic and hormonal differences [140]. Women may attain higher blood alcohol concentrations than men after drinking an equivalent amount of alcohol [141-143].
• Age differences: Lazebnik, et al. [144] have suggested that the age of the drinker may determine possible pathology of alcohol-related ischemic heart disease and that differences may exist in the risk of ischemic heart disease in different age groups. However, additional research is needed to assess if age modifies the risk relationships between alcohol and other diseases.
• Genetic differences: Previous studies have shown that people carrying the ALDH Lys487 allele are at an elevated risk of cancer and digestive diseases from alcohol consumption [145]. This allele is more prevalent in Asian populations than in America, European or African populations [146].
Conclusions and Future Research
Currently there are only three medications approved by the U.S. Food and Drug Administration (FDA) for use in the treatment of alcohol abuse and alcoholism: disulfiram (alcohol aversion), naltrexone (opiate inhibitor), and acamprosate (NMDA receptor inhibitor). At present, pharmacotherapy alone or in combination with behavioral approaches is modestly effective in treating alcoholism symptoms in genetically diverse population. Thus, there is substantial room for improvement. Following are some of the important future research issues:
1. Potential use of new drugs such GABAB receptor agonists, cholinergic receptor partial agonists, corticotrophin-releasing factor and cannabinoid CB1 receptor antagonists [61], nociception receptor ligands, and the novel antipsychotic aripiprazole.
2. Further development of biopsychosocial (BPS) approaches to integrate biological, psychological, social and cultural issues will improve service to the patients. There is an urgent need to develop a comprehensive individual assessment to adequately determine the individual's treatment needs. Successful integration of non viral vectors containing specific gene's cDNA into one's genome will be an important improvement in gene therapy.
3. Although interest in herbal therapy is increasing, there is still a major problem of standardized herbal preparation and treatment protocols. Efforts are needed to improve the bioavailability of the herbs. A new approach is development of nano particle coupled herbs.
References
- Cami J, Farre M (2003) Drug addiction. N Engl J Med 349: 975-986.
- Room R, Babor T, Rehm J (2005) Alcohol and public health: A review. Lancet 365: 519-530.
- Weiss F (2005) Neurobiology of craving, conditioned reward and relapse. Curr Opinion Pharmacol 5: 9-19.
- Engel GL (1977) The need for a new medical model: A challenge for biomedicine. Science 196: 129-136.
- Longabaugh R, Morgenstern J (1999) Cognitive-Behavioral Coping-Skills Therapy for Alcohol Dependence. Current Status and Future Directions. Alc Res Health 23: 78-84.
- Willams SH (2005) Medications for Treating Alcohol Dependence. Am Fam Physician 72: 1775-1780.
- Hutchison KE (2010) Substance use disorders: realizing the promise of pharmacogenomics and personalized medicine. Annu Rev Clin Psychol 6: 577-589.
- Kranzler HR, McKay JR (2012) Personalized treatment of alcohol dependence. Curr Psychiatry Rep 14: 486-493.
- Oslin D (2011) Personalized addiction treatment: how close are we? Alcohol Alcohol 46: 231-232.
- Houben K, Wiers RW, Jansen A (2011) Getting a grip on drinking behavior: Training working memory to reduce alcohol abuse. Psychol Sci 22: 968-975.
- Feldstein Ewing SW, Filbey FM, Sabbineni A, et al. (2011) How psychosocial alcohol interventions work: A preliminary look at what FMRI can tell us. Alcohol Clin Exp Res 35: 643-651.
- National Institute of Alcohol Abuse and Alcoholism (NIAAA) (2015) Alcohol Facts and Statistics.
- Aseltine RH Jr, Schilling EA, James A, et al. (2008) An evaluation of national alcohol screening day. Alcohol Alcohol 43: 97-103.
- Tiffany ST (1999) Cognitive concepts of craving. Alcohol Res Health 23: 215-224.
- Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158: 343-359.
- Franken IH (2003) Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry 27: 563-579.
- Ingjaldsson JT, Thayer JF, Laberg JC (2003) Preattentive processing of alcohol stimuli. Scand J Psychol 44: 161-165.
- Garland E, Franken IA, Howard M (2012) Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology 222: 17-26.
- Kosten TR, O'Connor PG (2003) Management of drug and alcohol withdrawal. N Engl J Med 348: 1786-1795.
- Mann K, Lehert P, Morgan MY (2004) The efficacy of acamprosate in the maintenance of abstinence in alcohol‐dependent individuals: results of a meta‐analysis. Alcohol Clin Exp Res 28: 51-63.
- Sullivan JT, Sykora K, Schneiderman J, et al. (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84: 1353-1357.
- Soyka M, Horak M (2004) Outpatient alcohol detoxification: implementation efficacy and outcome effectiveness of a model project. Eur Addict Res 10: 180-187.
- Soyka M, Lieb M (2015) Recent developments in pharmacotherapy of alcoholism. Pharmacopsychiatry 48: 123-135.
- Finney JW, Hahn AC, Moos RH (1996) The effectiveness of inpatient and outpatient treatment for alcohol abuse: the need to focus on mediators and moderators of setting effects. Addiction 91: 1773-1796.
- Suzdak PD, Schwartz RD, Skolnick P, et al. (1986) Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci USA 83: 4071-4075.
- Sigel E, Steinmann ME (2012) Structure, function, and modulation of GABAA receptors. J Biol Chem 287: 40224-40231.
- Lader MH (1999) Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified? Eur Neuropsychopharmacol 9: S399-S404.
- Woods JH, Katz JL, Winger G (1987) Abuse liabilities of benzodiazepines. Pharm Rev 39: 251-413.
- Ashton H (1984) Benzodiazepine withdrawal: an unfinished story. Br Med J (Clin Res Ed) 288: 1135-1140.
- Busto U, Sellers EM, Naranjo CA, et al. (1986) Withdrawal reaction after long-term therapeutic use of benzodiazepines. N Engl J Med 315: 854-859.
- Granger P, Biton B, Faure C, et al. (1995) Modulation of the gamma-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol Pharmacol 47: 1189-1196.
- Malcolm R, Ballenger JC, Sturgis ET, et al. (1989) Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry 146: 617-621.
- Goa KL, Ward A (1986) Buspirone. Drugs 32: 114-129.
- Kranzler HR, Burleson JA, Korner P, et al. (1995) Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry 152: 391-397.
- Malcolm R, Anton RF, Randall CL, et al. (1992) Placebo‐Controlled Trial of Buspirone in Anxious Inpatient Alcoholics. Alcohol Clin Exp Res 16: 1007-1013.
- Williams D, McBride AJ (1998) The drug treatment of alcohol withdrawal symptoms: a systematic review. Alcohol Alcohol 33: 103-115.
- Weise R, Berglund M, Frank A, et al. (1999) Acupuncture in alcoholism treatment: a randomized out-patient study. Alcohol Alcohol 34: 629-635.
- Karst M, Passie T, Friedrich S, et al. (2002) Acupuncture in the treatment of alcohol withdrawal symptoms: a randomized, placebo-controlled inpatient study. Addict Biol 7: 415-419.
- Akhondzadeh S, Kashani L, Mobaseri M, et al. (2001) Passionflower in the treatment of opiates Passionflower in the treatment of opiates withdrawal: a double-blind randomized controlled trial. J Clin Pharm Ther 26: 369-373.
- Akhondzadeh S, Naghavi HR, Vazirian M (2001) Passionflower in the treatment of generalized Passionflower in the treatment of generalized anxiety: a pilot double-blind randomized controlled trial anxiety: a pilot double-blind randomized controlled trial with oxazepam. J Clin Pharm Ther 26: 363-367.
- Dhawan K, Kumar S, Sharma A (2002) Suppression of alcohol-cessation-oriented hyper-anxiety by the benzoflavone moiety of Passiflora incarnata Linneaus in mice. J Ethnopharmacol 81: 239-244.
- Dhawan K, Dhawan S, Chhabra S (2003) Attenuation of benzodiazepine dependence in mice by a tri-substituted benzoflavone moiety of Passiflora incarnata Linneaus: a non-habit forming anxiolytic. J Pharm Pharm Sci 6: 215-222.
- Poyares DR, Guilleminault C, Ohayon MM, et al. (2002) Can valerian improve the sleep of insomniacs after benzodiazepine withdrawal? Prog Neuro-Psychopharm Biol Psych 26: 539-545.
- Overstreet DH, Kralic JE, Morrow AL, et al. (2003) NPI-031G (puerarin) reduces anxiogenic effects of alcohol withdrawal or benzodiazepine inverse or 5-HT 2C agonists. Pharmacol Biochem Behav 75: 619-625.
- Benlhabib E, Baker JI, Keyler DE, et al. (2004) Kudzu root extract suppresses voluntary alcohol intake and alcohol withdrawal symptoms in P rats receiving free access to water and alcohol. J Med Food 7: 168-179.
- Benlhabib E, Baker JI, Keyler DE, et al. (2004) Effects of purified puerarin on voluntary alcohol intake and alcohol withdrawal symptoms in P rats receiving free access to water and alcohol. J Med Food 7: 180-186.
- Singh AK (2017) Critical Review of Alcohol, Alcoholism and the Withdrawal Symptoms I. Mechanisms of Addiction and Withdrawal Syndrome. Archives of Addiction and Rehabilitation, In Press.
- Gilpin NW, Koob GF (2008) Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health 31: 185-195.
- Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374: 363-371.
- Miller WR, Rose GS (2009) Toward a theory of motivational interviewing. Am Psychol 64: 527-537.
- Miller WR, Sanchez VC (1993) Motivating young adults for treatment and lifestyle change. In: Howard Gs, Nathan PE, Alcohol Use and Misuse by Young Adults. University of Notre Dame Press, Notre Dame, IN.
- Marlatt GA, Gordon JR (1985) Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford Press, New York.
- Magill M, Ray LA (2009) Cognitive-Behavioral Treatment With Adult Alcohol and Illicit Drug Users: A Meta-Analysis of Randomized Controlled Trials. J Stud Alcohol Drugs 70: 516-527.
- Goldstein RX, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Rev Neurosci 12: 652-669.
- Franck J, Jayaram-Lindström N (2013) Pharmacotherapy for alcohol dependence: status of current treatments. Curr Opin Neurobiol 23: 692-699.
- Miller PM, Book SW, Stewart SH (2011) Medical treatment of alcohol dependence: a systematic review. Int J Psych Med 42: 227-266.
- Anton RF, O'Malley SS, Ciraulo DA, et al. (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295: 2003-2017.
- Anton RF, Oroszi G, O'Malley S, et al. (2008) An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiat 65: 135-144.
- Chatterjee S, Bartlett SE (2010) Neuronal Nicotinic Acetylcholine Receptors as pharmacotherapeutic Targets for the Treatment of Alcohol Use Disorders. CNS Neurol Disord Drug Targets 9: 60-76.
- McKee SA, Harrison EL, O'Malley SS (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66: 185-190.
- Wang L, Liu J, Harvey-White J, et al. (2003) Endocannabinoid signaling via cannabinoid receptor 1 is invold in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A 100: 1393-1398.
- Voronin K, Randall P, Myrick H, et al. (2008) Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm--possible influence of self-control. Alcohol Clin Exp Res 32: 1954-1961.
- Manheimer E, Anderson BJ, Stein MD (2003) Use and assessment of complementary and alternative therapies by intravenous drug users. Am J Drug Alcohol Abuse 29: 401-413.
- Heath AC (1995) Genetic influences on alcoholism risk: a review on adoption and twin studies. Alcohol Health Res World 19: 166-171.
- Heath AC, Bucholz KK, Madden PA, et al. (1997) Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in men and women. Psychol Med 27: 1381-1396.
- Morozova TV, Mackay TF, Anholt RR (2014) Genetics and genomics of alcohol sensitivity. Mol Genet Genomics 289: 253-269.
- Wolanczyk T, Wojnar M, Cedro A (1997) Carbamazepine in the treatment of alcohol withdrawal. Psychiatr Pol 31: 189-196.
- King AC, Volpicelli JR, Gunduz M, et al. (1997) Naltrexone biotransformation and incidence of subjective side effects: a preliminary study. Alcohol Clin Exp Res 21: 906-909.
- Deb I, Chakraborty J, Gangopadhyay PK, et al. (2010) Single‐nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu‐opioid receptor and may contribute to the genetic risk for addiction. J Neurochem 11: 486-496.
- Ray LA, Hutchison KE, Ashenhurst JR, et al. (2010) Naltrexone selectively elevates GABAergic neuroactive steroid levels in heavy drinkers with the Asp40 allele of the OPRM1 gene: a pilot investigation. Alcohol Clin Exp Res 34: 1479-1487.
- Ray LA, Bujarski S, Chin PF, et al. (2012) Pharmacogenetics of naltrexone in Asian Americans: a randomized placebo-controlled laboratory study. Neuropsychopharmacology 37: 445-455.
- Setiawan E, Pihl RO, Cox SM, et al. (2011) The Effect of Naltrexone on Alcohol's Stimulant Properties and Self‐Administration Behavior in Social Drinkers: Influence of Gender and Genotype. Alcohol Cli Exp Res 35: 1134-1141.
- Setiawan E, Pihl RO, Benkelfat C, et al. (2012) Influence of the OPRM1 A118G polymorphism on alcohol-induced euphoria, risk for alcoholism and the clinical efficacy of naltrexone. Pharmacogenomics 13: 1161-1172.
- Kranzler HR, Armeli S, Covault J, et al. (2013) Variation in OPRM1 moderates the effect of desire to drink on subsequent drinking and its attenuation by naltrexone treatment. Addict Biol 18: 193-201.
- McGeary JE, Monti PM, Rohsenow DJ, et al. (2006) Genetic moderators of naltrexone's effects on alcohol cue reactivity. Alcohol Clin Exp Res 30: 1288-1296.
- Oslin DW, Berrettini W, Kranzler HR, et al. (2003) A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology 28: 1546-1552.
- Chamorro A, Marcos M, Mirón‐Canelo J, et al. (2012) Association of µ‐opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta‐analysis. Addict Biol 17: 505-512.
- Kim SG, Kim CM, Choi SW, et al. (2009) A mu opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 201: 611-618.
- Tidey JW, Monti PM, Rohsenow DJ, et al. (2008) Moderators of Naltrexone's Effects on Drinking, Urge, and Alcohol Effects in Non‐Treatment‐Seeking Heavy Drinkers in the Natural Environment. Alcohol Clin Exp Res 32: 58-66.
- Ooteman W, Naassila M, Koeter MW, et al. (2009) Predicting the effect of naltrexone and acamprosate in alcohol‐dependent patients using genetic indicators. Addiction Biology 14: 328-337.
- Boettiger CA, Kelley EA, Mitchell JM, et al. (2009) Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network. Pharmacol Biochem Behav 93: 291-299.
- Anton RF, Voronin KK, Randall PK, et al. (2012) Naltrexone Modification of Drinking Effects in a Subacute Treatment and Bar‐Lab Paradigm: Influence of OPRM1 and Dopamine Transporter (SLC6A3) Genes. Alcohol Clin Exp Res 36: 2000-2007.
- Gelernter J, Gueorguieva R, Kranzler HR, et al. (2007) Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res 31: 555-563.
- Coller JK, Cahill S, Edmonds C, et al. (2011) OPRM1 A118G genotype fails to predict the effectiveness of naltrexone treatment for alcohol dependence. Pharmacogenet Genomics 21: 902-905.
- Johnson BA, Ait-Daoud N (2010) Topiramate in the new generation of drugs: efficacy in the treatment of alcoholic patients. Curr Pharm Des 16: 2103-2112.
- Chen AC, Morgenstern J, Davis CM, et al. (2013) Variation in mu-opioid receptor gene (OPRM1) as a moderator of naltrexone treatment to reduce heavy drinking in a high functioning cohort. J Alcohol Drug Dependence 1: 101.
- Karpyak VM, Winham SJ, Biernacka JM, et al. (2014) Association of GATA4 sequence variation with alcohol dependence. Addict Biol 19: 312-315.
- Herman AI, Kaiss KM, Ma R, et al. (2005) Serotonin transporter promoter polymorphism and monoamine oxidase type A VNTR allelic variants together influence alcohol binge drinking risk in young women. Am J Med Genet Part B: Neuropsychiatr Genet 133: 74-78.
- Johnson BA (2000) Serotonergic agents and alcoholism treatment: rebirth of the subtype concept - an hypothesis. Alcohol Clin Exp Res 24: 1597-1601.
- Johnson BA, Roache JD, Javors MA, et al. (2000) Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA 284: 963-971.
- Enoch MA (2011) The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 214: 17-31.
- Johnson BA, Seneviratne C, Wang XQ, et al. (2013) Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT3 antagonist ondansetron. Am J Psychiatry 170: 1020-1031.
- Johnson BA, Seneviratne C, Wang X, et al. (2014) Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT3 antagonist ondansetron. Am J Psychiatry 170: 1020-1031.
- Franklin KM, Asatryan L, Jakowec MW, et al. (2014) P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders. Front Neurosci 8: 176.
- Burnstock G, Krugel U, Abbracchio MP, et al. (2011) Purinergic signaling: from normal behaviour to pathological brain function. Prog Neurobiol 95: 229-274.
- Hurley TD, Edenberg HJ, Li TK, et al. (2002) The pharmacogenomics of alcoholism. In: Licinio J, Wong ML, Pharmacogenomics: The Search for Individualized Therapeutics. (edn), Wiley, Weinheim, Germany, 417-441.
- Bosron WF, Ehrig T, Li TK, et al. (1993) Genetic factors in alcohol metabolism and alcoholism. Semin Liver Dis 13: 126-135.
- Maly IP, Toranelli M, Sasse D (1999) Distribution of alcohol dehydrogenase isoenzymes in the human liver acinus. Histochem Cell Biol 111: 391-397.
- Rodd ZA, Bertsch BA, Strother WN, et al. (2007) Candidate genes, pathways and mechanisms for alcoholism: an expanded convergent functional genomics approach. Pharmacogenomics J 7: 222-256.
- Lobb RR, Abraham WM, Burkly LC, et al. (1996) Pathophysiologic role of alpha 4 integrins in the lung. Ann NY Acad Sci 796: 113-123.
- Feigelson SW, Grabovsky V, Shamri R, et al. (2003) The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J Biol Chem 278: 51203-51212.
- Coppolino M, Migliorini M, Argraves WS, et al. (1995) Identification of a novel form of the alpha 3 integrin subunit: covalent association with transferrin receptor. Biochem J 306: 129-134.
- Ponka P (1999) Cell biology of heme. Am J Med Sci 318: 241-256.
- Tamura K, Chen YE, Chen Q, et al. (2000) Expression of renin-angiotensin system and extracellular matrix genes in cardiovascular cells and its regulation through AT1 receptor. Mol Cell Biochem 212: 203-209.
- Traub O, Monia BP, Dean NM, et al. (1997) PKC-epsilon is required for mechano-sensitive activation of ERK1/2 in endothelial cells. J Biol Chem 272: 31251-31257.
- Meyer A, van Golen CM, Kim B, et al. (2004) Integrin expression regulates neuroblastoma attachment and migration. Neoplasia 6: 332-342.
- Padovan CS, Jahn K, Birnbaum T, et al. (2003) Expression of neuronal markers in differentiated marrow stromal cells and CD133+ stem-like cells. Cell Transplant 12: 839-848.
- Han S, Sidell N, Roman J (2005) Fibronectin stimulates human lung carcinoma cell proliferation by suppressing p21 gene expression via signals involving Erk and Rho kinase. Cancer Lett 219: 71-81.
- Shima T, Nada S, Okada M (2003) Transmembrane phosphoprotein Cbp senses cell adhesion signaling mediated by Src family kinase in lipid rafts. Proc Natl Acad Sci U S A 100: 14897-14902.
- Wang L, Kwak JH, Kim SI, et al. (2004) Transforming growth factor-beta1 stimulates vascular endothelial growth factor 164 via mitogen-activated protein kinase kinase 3-p38alpha and p38delta mitogen-activated protein kinase-dependent pathway in murine mesangial cells. J Biol Chem 279: 33213-33219.
- Jovanovic JN, Czernik AJ, Fienberg AA, et al. (2000) Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 3: 323-329.
- Naeve GS, Ramakrishnan M, Kramer R, et al. (1997) Neuritin: a gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc Natl Acad Sci U S A 94: 2648-2653.
- Morral N, O'Neal W, Rice K, et al. (1999) Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci U S A 96: 12816-12821.
- Morsy MA, Gu M, Motzel S, et al. (1998) An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A 95: 7866-7871.
- McCown TJ, Xiao X, Li J, et al. (1996) Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res 713: 99-107.
- Betz AL, Shakui P, Davidson BL (1998) Gene transfer to rodent brain with recombinant adenoviral vectors: effects of infusion parameters, infectious titer, and virus concentration on transduction volume. Exp Neurol 150: 136-142.
- Bartlett JS, Samulski RJ, McCown TJ (1998) Selective and rapid uptake of adeno-associated virus type 2 in brain. Human Gene Therapy 9: 1181-1186.
- Carlezon WA, Thome J, Olson VG, et al. (1998) Regulation of cocaine reward by CREB. Science 282: 2272-2275.
- Myers AJ, Gibbs JR, Webster JA, et al. (2007) A survey of genetic human cortical gene expression. Nature Genetics 39: 1494-1499.
- Wheeler MD, Kono H, Rusyn I, et al. (2000) Chronic ethanol increases adeno‐associated viral transgene expression in rat liver via oxidant and NFκB‐dependent mechanisms. Hepatology 32: 1050-1059.
- Wheeler MD, Kono H, Yin M, et al. (2001) Delivery of the Cu/Zn–Superoxide dismutase gene with adenovirus reduces early alcohol-induced liver injury in rats. Gastroenterology 120: 1241-1250.
- Tu GC, Cao QN, Zhou F, et al. (1998) Tetranucleotide GGGA motif in primary RNA transcripts Novel target site for antisense design. J Biol Chem 273: 25125-25131.
- Ponnappa BC, Dey I, Tu GC, et al. (2001) In vivo delivery of antisense oligonucleotides in pH-sensitive liposomes inhibits lipopolysaccharide-induced production of tumor necrosis factor-α in rats. J Pharm Exp Ther 297: 1129-1136.
- Ponnappa BC, Zhou F, Aini M, et al. (2001) In vivo delivery of antisense oligonucleotide against TNF-a mRNA prevents lipopolysaccharide-induced liver damage in ethanol-fed rats. Hepatology 34: 463.
- Garver E, Tu GC, Cao QN, et al. (2001) Eliciting the low-activity aldehyde dehydrogenase Asian phenotype by an antisense mechanism results in an aversion to ethanol. J Exp Med 194: 571-580.
- Rivera-Meza M, Quintanilla ME, Tampier L (2012) Reduction of ethanol consumption in alcohol-preferring rats by dual expression gene transfer. Alcohol Alcohol 47: 102-108.
- Thanos PK, Volkow ND, Freimuth P, et al. (2001) Overexpression of dopamine D2 receptors reduces alcohol self‐administration. J Neurochem 78: 1094-1103.
- Hoplight BJ, Sandygren NA, Neumaier JF (2006) Increased expression of 5-HT 1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol 38: 73-79.
- Thanos PK, Rivera SN, Weaver K, et al. (2005) Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life sciences 77: 130-139.
- Thanos PK, Taintor NB, Rivera SN, et al. (2004) DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol. Clin Exp Res 28: 720-728.
- Kesse E, Clavel-Chapelon F, Slimani N, et al. (2001) Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC). Am J Clin Nutr 74: 322-327.
- Grucza RA, Bierut LJ (2006) Co-occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Res Health 29: 172-178.
- Rota M, Scotti L, Turati F, et al. (2012) Alcohol consumption and prostate cancer risk: a meta-analysis of the dose-risk relation. Eur J Cancer Prev 21: 350-359.
- Dennis LK (2000) Meta-analysis for combining relative risks of alcohol consumption and prostate cancer. Prostate 42: 56-66.
- Fillmore KM, Chikritzhs T, Stockwell T, et al. (2009) Alcohol use and prostate cancer: A meta-analysis. Mol Nutr Food Res 53: 240-255.
- Tuma DJ, Casey CA (2003) Dangerous Byproducts of Alcohol Breakdown--Focus on Adducts. Alcohol Res Health 27: 285-290.
- Rehm J, Gmel G, Sempos CT, et al. (2003) Alcohol-related morbidity and mortality. Alcohol Res Health 27: 39-51.
- Wills TA, Shiffman S (1985) Coping and substance abuse: a conceptual framework. In: Shiffman S, Wills TA, Coping and substance use. Academic Press, Orlando 3-24.
- Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278: 52-58.
- Wilsnack RW, Wilsnack SC, Kristjanson AF, et al. (2009) Gender and alcohol consumption: patterns from the multinational genacis project. Addiction 104: 1487-1500.
- Frezza M, di Padova D, Pozzato MD (1990) High Blood Alcohol Levels in Women - The Role of Decreased Gastric Alcohol Dehydrogenase Activity and First-Pass Metabolism. N Engl J Med 322: 95-99.
- Taylor JL, Dolhert N, Friedman L, et al. (1996) Alcohol elimination and simulator performance of male and female aviators: A preliminary report. Aviat Space Environ Med 67: 407-413.
- Eagon PK (2010) Alcoholic liver injury: Influence of gender and hormones. World J Gastroenterology 16: 1377-1384.
- Lazebnik LB, Komissarenko IA, Mikheeva OM (2011) Cardiovascular pathology associated with digestive system diseases. Exp Clin Gastroenterology 5: 69-74.
- Lewis SJ, Smith GD (2005) Alcohol, ALDH2, and esophageal cancer: A meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev 14: 1967-1971.
- Oze I, Matsuo K, Hosono S, et al. (2010) Comparison between self-reported facial flushing after alcohol consumption and ALDH2 Glu504Lys polymorphism for risk of upper aerodigestive tract cancer in a Japanese population. Cancer Sci 101: 1875-1880.
- Brunetti M, Di Tizio L, Dezi S, et al. (2012) Aripiprazole, alcohol and substance abuse: a review. Eur Rev Med Pharmacol Sci 16: 1346-1354.
- Kenna GA, Lomastro TL, Schiesl A, et al. (2009) Review of topiramate: an antiepileptic for the treatment of alcohol dependence. Curr Drug Abuse Rev 2: 135-142.
- Kenna GA, Zywiak WH, McGeary JE, et al. (2009) A within‐group design of nontreatment seeking 5‐HTTLPR genotyped alcohol‐dependent subjects receiving ondansetron and sertraline. Alcohol Clin Exp Res 33: 315-323.
- Williams D, Lewis J, McBride A (2001) A comparison of rating scales for the alcohol withdrawal syndrome. Alcohol Alcohol 36: 104-108.
- Te Beek ET, de Boer P, Moerland M, et al. (2012) In vivo quantification of striatal dopamine D2 receptor occupancy by JNJ-37822681 using [11C] raclopride and positron emission tomography. J Psychopharmacol 26: 1128-1135.
- Karam-Hage M, Brower KJ (2000) Gabapentin treatment for insomnia associated with alchohol dependence. Am J Psychiat 157: 151.
- Holbrook AM, Crowther R, Lotter A, et al. (1999) Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ 160: 649-655.
- Naranjo CA, Kadlec KE, Sanhueza P, et al. (1990) Fluoxetine differentially alters alcohol intake and other consummatory behaviors in problem drinkers. Clin Pharm Ther 47: 490-498.
- Naranjo C, Bremner K (1992) In Evaluation of the effects of serotonin uptake inhibitors in alcoholics: a review. Novel Pharmacological Interventions for Alcoholism 105-117.
- Naranjo CA, Bremner KE, Bazoon M, et al. (1997) Using fuzzy logic to predict response to citalopram in alcohol dependence. Clin Pharmacol Ther 62: 209-224.
- Gerra G, Caccavari R, Delsignore R, et al. (1986) Effects of fluoxetine and Ca-acetyl-homotaurinate on alcohol intake in familial and nonfamilial alcoholic patients. Curr Ther Res 52: 291-295.
- Babor TF, Caetano R (2006) Subtypes of substance dependence and abuse: implications for diagnostic classification and empirical research. Addiction 101: 104-110.
- Dundon W, Lynch KG, Pettinati HM, et al. (2004) Treatment outcomes in type A and B alcohol dependence 6 months after serotonergic pharmacotherapy. Alcohol Clin Exp Res 28: 1065-1073.
- Hwa LS, DeBold JF, Miczek KA (2013) Alcohol in excess: CRF1 receptors in the rat and mouse VTA and DRN. Psychopharmacology 225: 313-327.
- Zorrilla EP, Heilig M, de Wit H, et al. (2013) Behavioral, biological, and chemical perspectives on targeting CRF 1 receptor antagonists to treat alcoholism. Drug Alcohol Depend 128: 175-186.
- Rasmussen DD, Alexander LL, Raskind MA, et al. (2009) The α1‐Adrenergic Receptor Antagonist, Prazosin, Reduces Alcohol Drinking in Alcohol‐Preferring (P) Rats. Alcohol Clin Exp Res 33: 264-272.
- Volpicelli JR, Rhines KC, Rhines JS, et al. (1997) Naltrexone and alcohol dependence: role of subject compliance. Arch Gen Psychiatry 54: 737-742.
- Kranzler HR, Tennen H, Penta C, et al. (1997) Targeted naltrexone treatment of early problem drinkers. Addict Behav 22: 431-436.
- Kranzler HR, Armeli S, Tennen H, et al. (2003) Targeted naltrexone for early problem drinkers. J Clin Psychopharmacol 23: 294-304.
- Matsuzawa S, Suzuki T, Misawa M (2000) Involvement of μ‐Opioid Receptor in the Salsolinol‐Associated Place Preference in Rats Exposed to Conditioned Fear Stress. Alcohol Clin Exp Res 24: 366-372.
- Krishnan-Sarin S, Meandzija B, O'Malley S (2003) Naltrexone and nicotine patch smoking cessation: a preliminary study. Nicotine Tob Res 5: 851-857.
- Froehlich J, O'Malley S, Hyytiä P, et al. (2003) Preclinical and clinical studies on naltrexone: what have they taught each other? Alcohol Clin Exp Res 27: 533-539.
- Brothers SP, Wahlestedt C (2010) Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med 2: 429-439.
- Haddjeri N, Blier P (2008) Neurokinin-1 receptor antagonists modulate brain noradrenaline and serotonin interactions. Eur J Pharmacol 600: 64-70.
- Kranzler HR, Gage A (2008) Acamprosate efficacy in alcohol-dependent patients: summary of results from three pivotal trials. Am J Addict 17: 70-76.
- Johnson BA, Ait-Daoud N, Bowden CL, et al. (2003) Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 361: 1677-1685.
- Johnson BA, Rosenthal N, Capece JA, et al. (2007) Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA 298: 1641-1651.
- Johnson BA (2004) Topiramate-induced neuromodulation of cortico-mesolimbic dopamine function: A new vista for the treatment of comorbid alcohol and nicotine dependence? Addict Behav 29: 1465-1479.
- Weiss F, Porrino LJ (2002) Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci 22: 3332-3337.
- Blednov YA, Harris RA (2008) Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol 11: 775-793.
- Bin Zhao, Jun Wu (2006) Modeling particle deposition onto rough walls in ventilation duct. Atmos Environ 40: 6918-6927.
- De Vry J, Maurel S, Schreiber R, et al. (1999) Comparison of hypericum extracts with imipramine and fluoxetine in animal models of depression and alcoholism. Eur Neuropsychopharm 9: 461-468.
- Panocka I, Perfumi M, Angeletti S, et al. (2000) Effects of Hypericum perforatum extract on ethanol intake, and on behavioral despair: a search for the neurochemical systems involved. Pharm Biochem Behav 66: 105-111.
- Perfumi M, Panocka I, Ciccocioppo R, et al. (2001) Effects of a methanolic extract and a hyperforin-enriched CO2 extract of Hypericum perforatum on alcohol intake in rats. Alcohol Alcohol 36: 199-206.
- Perfumi M, Santoni M, Ciccocioppo R, et al. (2002) Blockade of gamma-aminobutyric acid receptors does not modify the inhibiton of ethanol intake induced by Hypericum perforatum in rats. Alcohol Alcohol 37: 540-546.
- Rezvani AH, Overstreet DH, Yang Y, et al. (1999) Attenuation of alcohol intake by extract of Hypericum perforatum (St. John's Wort) in two different strains of alcohol-preferring rats. Alcohol Alcohol 34: 699-705.
- Keung WM, Vallee BL (1993) Daidzin and daidzein suppress free-choice ethanol intake by Syrian golden hamsters. Proc Natl Acad Sci USA 90: 10008-10012.
- Heyman GM, Keung W, Vallee BL (1996) Daidzin decreases ethanol consumption in rats. Alcohol Clin Exp Res 20: 1083-1087.
- Rezvani AH, Overstreet D, Leef Y (1995) Attenuation of alcohol intake by ibogaine in three strains of alcohol-preferring rats. Pharm Biochem Behav 52: 615-620.
- Rezvani AH, Overstreet DH, Yang Y, et al. (1997) Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharm Biochem Behav 58: 615-619.
- Sweetnam P, Lancaster J, Snowman A, et al. (1995) Receptor binding profile suggests multiple mechanisms of action are responsible for ibogaine's putative anti-addictive activity. Psychopharmacology 118: 369-376.
- Sershen H, Hashim A, Lajtha A (1994) Ibogaine reduces preference for cocaine consumption in C57BL/6By mice. Pharm Biochem Behav 47: 13-19.
- Leal CG, de Gusmão Câmara I (2003) The Atlantic Forest of South America: biodiversity status, threats, and outlook. Island Press, Washinhton, USA.
- Nurul Hikmah Haruna, Abdi Wira Septamab, Ibrahim Jantanc (2015) Immunomodulatory effects of selected Malaysian plants on the CD18/11a expression and phagocytosis activities of leukocytes. Asian Pacific J Trop Biomed 5: 48-53.
- Steiner GG (2001) Kava as an anticraving agent: preliminary data. Pac Health Dialog 8: 335-339.
- Cairney S, Maruff P, Clough AR (2002) The neurobehavioural effects of kava. Aust NZJ Psychiatry 36: 657-662.
- Baum SS, Hill R, Rommelspacher H (1998) Effect of kava extract and individual kavapyrones on neurotransmitter levels in the nucleus accumbens of rats. Prog. Neuro-Psychopharmacol. Biol Psychiatry 22: 1105-1120.
- Kombian SB, Mouginot D, Pittman QJ (1997) Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron 19: 903-912.
- Kulkarni SK, Ninan I (1997) Inhibition of morphine tolerance and dependence by Withania somnifera in mice. J Ethnopharmacol 57: 213-217.
- Andrade MC, Menezes JS, Cassali GD, et al. (2006) Alcohol‐induced gastritis prevents oral tolerance induction in mice. Clin Exp Immunol 146: 312-322.
- Sudhir S, Budhiraja R, Miglani G, et al. (1986) Pharmacological studies on leaves of Withania somnifera. Planta Med 2: 61-63.
- Colombo G, Agabio R, Lobina C, et al. (1999) Salvia miltiorrhiza extract inhibits alcohol absorption, preference, and discrimination in sP rats. Alcohol 18: 65-70.
- Imanshahidi M, Hosseinzadeh H (2006) The pharmacological effects of Salvia species on the central nervous system. Phytotherapy Res 20: 427-437.
- Volkow ND, Baler RD (2014) Addiction science: Uncovering neurobiological complexity. Neuropharmacology 76: 235-249.
Corresponding Author
Singh Ashok K, Associate Professor, Department of Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota, Twin Cities, St Paul, MN 55108, USA, Tel: 763-234-9655.
Copyright
© 2017 Singh AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.