Critical Review of Alcohol, Alcoholism and the Withdrawal Symptoms I. Mechanisms of Addiction and Withdrawal Syndrome
Abstract
Alcoholic beverages are socially accepted around the world, consumed mostly to socialize, celebrate, and relax. The pleasant effects of alcohol are attributed to (i) an increase in GABAergic (inhibitory signals), OPergic and 5HTergic (euphoric effects) neuronal activities and (ii) a decrease in DAergic ('want' signal or craving), Gluergic (excitatory signals), NEergic (stress signals) neuronal, and the HPA axis (stress hormones) activities. If alcohol drinking continues, the receptors are sensitized, resulting in development of tolerance when alcohol drinking must be increased to achieve desired effects. In genetically/Environmentally predisposed subjects, chronic alcohol drinking results in the development of addiction, characterized by a condition when alcohol caseation results in rapid onset of withdrawal symptoms including, but not limited to, alcohol craving and moderate to severe discomfort. Because pharmacotherapy alone or in combination with behavioral approaches is only modestly effective in treating alcoholism symptoms, there is an urgent need to development effective and safe therapies. At present, a lack of clear understanding of the mechanisms underlying addiction hinders possible development of new treatment strategies. Therefore, the aim of this article is to discuss the mechanisms underlying (i) the euphoric, relaxing and adverse effects of alcohol drinking and (ii) addiction and the withdrawal symptoms.
Keywords
Acetaldehyde, Alcoholism, Addiction, Epigenetic, Ethanol, Genomics, Herbal therapy, Pharmacogenomics, Pharmacotherapy, Tolerance, Withdrawal
Introduction
Alcoholic beverages are socially accepted drinks, expected to bring pleasure, satisfaction, and relief from stress [1]. In general, most people drink alcohol responsibly, but, continued drinking may serves as a prelude to alcohol abuse and an escape route for social, personal or career pressures [2]. In the presence of a genetic predisposition and environmental cues, persistent drinking may result in the development of tolerance and addition or alcoholism (defined as a cluster of behavioral, cognitive, and physiological abnormalities developing after repeated alcohol use and resulting in a physical withdrawal state upon abstinence) that may interfere with a person's ability to function normally and impairing his/her daily life [3]. Abnormal alcohol drinking may also be an important risk factor for a number of diseases such as infection, cancer, atrial fibrillation, hypertension, etc [4-17]. In the United States alone, about 17 million people of different age suffer from alcoholism [18]. Thus, alcoholism may exert tremendous economic consequences not only for the drinking individuals, but also for the society at large [19-21]. The journey from responsible alcohol drinking to alcoholism involves four stages listed in Table 1.
The transition from the responsible use of alcohol to an excessive, uncontrolled alcohol consumption (alcohol addiction) results from a complex neuro adaptations involving various excitatory and inhibitory pathways in different brain regions. In alcohol-naïve subjects, the adaptation pathways such as aversive hangover response may keep the social intake of alcohol in check, while, in subject practicing abnormal alcohol drinking, the neuro adaptations in the brain may cause behavioral transitions, resulting in uncontrolled alcohol drinking [22]. Although, there are compelling evidence in support of causal relationship between the adaptive changes occurring in the brain (genomic changes, epigenetic modifications and changes and gene-environment interactions) and the development of alcohol's rewarding, aversive, addiction, and other alcohol-related effects, an integrated and unified mechanisms for these alcohol-related abnormalities are lacking. This gap may be hindering development of effective and safe medications to treat alcoholism and other alcohol-related disorders. Therefore, the aim of this review article is to critically evaluate the research papers published in the area of alcohol's euphoric effects and addiction, and then propose possible mechanisms underlying each stages of alcohol drinking disorders discussed above.
Literature Search Methodology
Overall literature search approach described by Meline [23] was used. Initial searches were performed on the following literature database: MEDLINE, Google Scholar and Web of Science using search words either alone or tagged with qualifying words. Some examples are listed below:
i. Alcohol drinking tagged with statistics, social, abnormal, gender, age, tolerance, addiction, blood alcohol concentrations (BAC), etc.
ii. Alcohol dehydrogenases or ADH tagged with genetic polymorphism, alcoholism, tolerance, demographic (Asians, Indians, Caucasians, Africans, etc.), acetaldehyde, acetaldehyde dehydrogenase-ALDH, blood alcohol concentration-BAC, etc.
iii. Alcoholism or alcohol addiction tagged with uncontrolled drinking, statistics, demographics, tolerance, brain pathways (dopamine, opiates, GABA, glutamate, NMDA, serotonin, adenosine), brain regions, abstinence, withdrawal syndrome, etc.
Other search words were tolerance, dependence, craving, genomics, epigenetics, hangover, etc. The initial results were subjected to certain inclusion and exclusion criterion listed in Table 2 and an example is shown in Table 3.
Acute Alcohol Intake, Blood Ethanol Concentrations and Alcohol's Positive/Negative Reinforcements
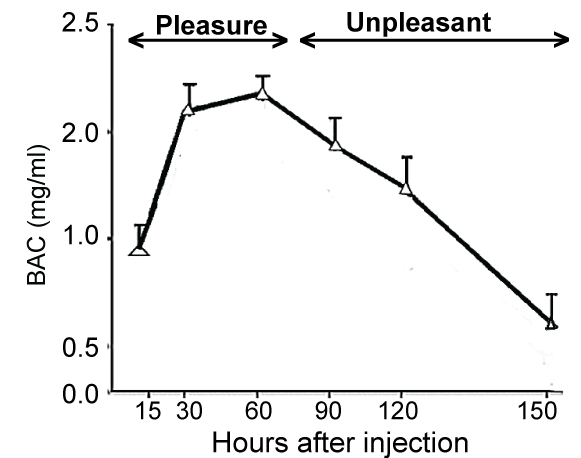
This section describes the possible mechanisms for the pleasant and aversive effects of acute alcohol drinking in people practicing responsible drinking. Acute alcohol drinking results in a rapid increase, followed by a gradual decrease (Figure 1) in the blood alcohol concentrations (BAC). The euphoric/pleasant effects correlated with the rising phase of BAC, while the falling phase of BAC correlated with a transition from rewarding effects to unpleasant and depressing effects [24,25]. During the ascending BAC phase, the brain's reward pathways are activated, while during the desending phase, the brain's aversive and pain patheays are activiated (Figure 2).
Mechanisms of alcohol's euphoric effects during rising BAC
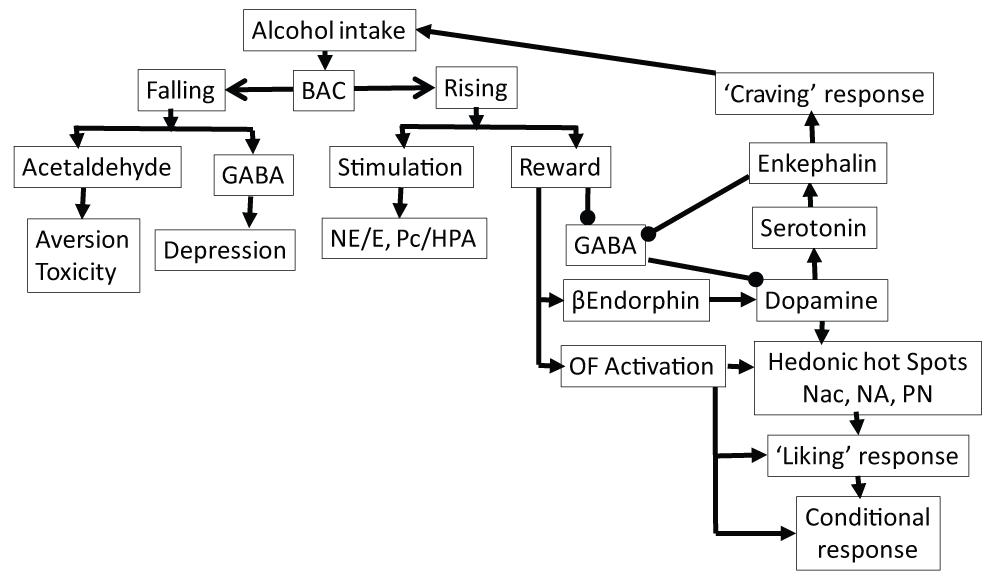
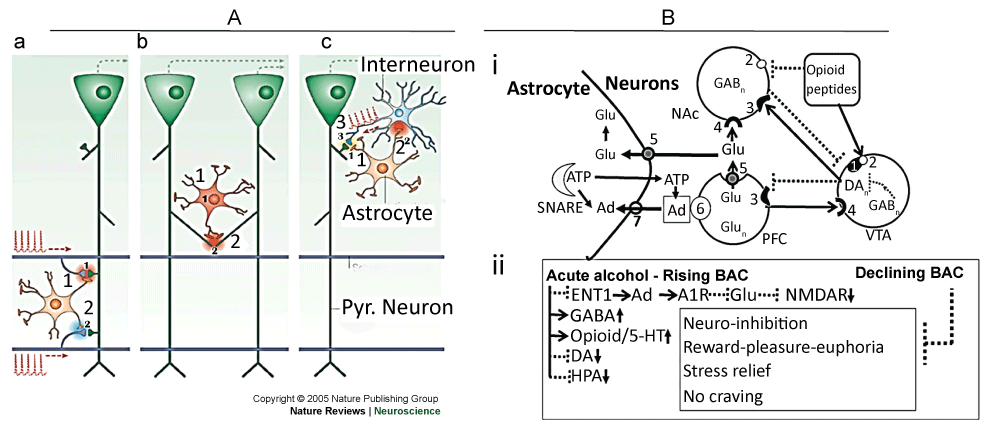
The rising BAC activates the rewarding pathways that further (i) activates the synthesis and release of of β-endorphin, (ii) inhibits GABAergic neurons and (iii) activates orbitofrontal (OFC) cortex [26,27]. A decrease in GABAergic signaling disinhibits DAergic neurons, while an increase in β-endorphine activated DAergic neurons, resulting in releases DA. β-Endorphin and DA activate the Hedonic hot spotes and ensuing sensory pleasures. DA also induces serotonin (5-HT) levels that induces enkephalin release. DA and enkephalin together may further inhibit GABAergic neurons and initiate alcohol 'craving'. Activagtion of the Hedonic hot spotes and OFC may develop a conditioned response. The 'stimulatent' effects of alcohol may be mediated via release of norepinephrine (NE) from adrenergic neurons and corticosteroids via the HPA axis [28-31]. As shown in Figure 3, GABAergic, AChergic, Gluergic, 5-HTergic and NAergic activities, directly or indirectly, are dynamically regulated via the NMDA receptors present on GABAergic, 5-HTergic and NAergic neurons [30,31]. Glu may regulate DA, opioid peptides, GABA, glutamate and 5-HT concentrations that mediates alcohol reinforcement by differentially modulating the GABAergic neurons. During the rising phase of BAC, the rewarding effects of alcohol drinking may also be associated with an inhibition of NMDA and DA receptors and HPA axis [30,31].
Mechanisms of alcohol's aversive effects during declining BAC
The descending phase of BAC, as shown in Figures 2, is characterized by an increase in acetaldehyde that (1) deactivates the NMDA receptors, resulting in an attenuation of GABAergic-mediated neuroinhibition and (2) react with DA and synthesized salsolinol that is shown to depolarize and disinhibit DAergic neurons, decrease GABAergic activity and enhance nitric oxide production [32,33]. Thus, acetaldehyde and salsolinol together may participate in development of alcohol-related negative reinforcement. In addition, the aversive response of the declining phase of BAC may block the rewarding effects as described below [34].
Hangover and Tolerance
Hangover (a feeling of general misery comprising of drowsiness, concentration problems, dry mouth, dizziness, gastro-intestinal complaints, sweating, nausea, hyper-excitability, and anxiety) is always associated to acute alcohol intoxication and/or heavy episodic drinking [35-37]. In addition to unpleasant feeling, hangover also has several social and clinical negative consequences such as work and academic absenteeism and neurocognitive impairments [38-40]. Tolerance is defined as a person's diminished euphoric response to alcohol when used repeatedly over time. As tolerance develops, person may need relatively higher quantity of alcohol to achieve the same level of response achieved initially. While hangover keeps a check on alcohol drinking, tolerance motivates people to drink more for desired effects. The following sections discuss the mechanisms underlying hangover and tolerance.
Hangover
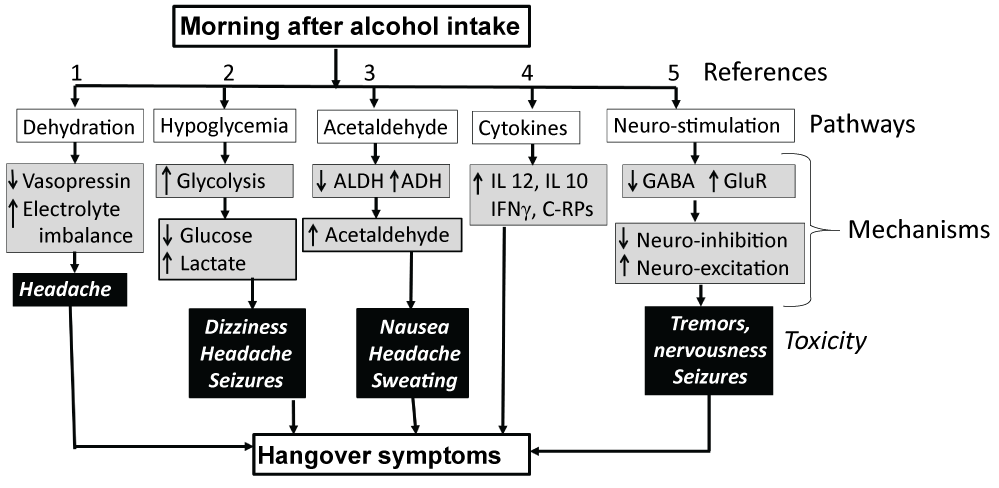
Although possible mechanisms underlying development of hangover are not fully understood, earlier studies have proposed possible roles of dehydration [41-44], hypoglycemia [45], acetaldehyde accumulation [46-52], pro-inflammatory cytokines [53] and neuro-stimulation [54] in the development of hangover (Figure 4).
Tolerance
Three basic types of tolerance have been described in literature [55].
• Cellular tolerance occurs at the level of a neuron or a network of many neuronal and supportive cells including astrocytes and glial cells,
• Molecular tolerance involves adaptation processes developed by individual molecules (e.g., ion channels) during exposure to ethanol and,
• Behavioral tolerance that involves the level of the activity of an entire animal.
Alcohol tolerance can also be classified as acute, rapid, and chronic, based on how long after exposure to alcohol tolerance develops. Possible molecular mechanisms proposed to explain the development of different types of tolerance are shown in Figure 5.
Development of Alcohol Addiction or Alcoholism
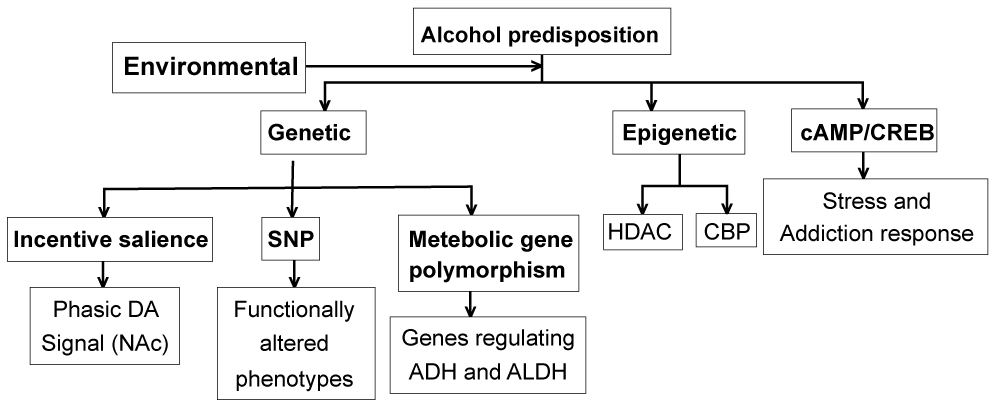
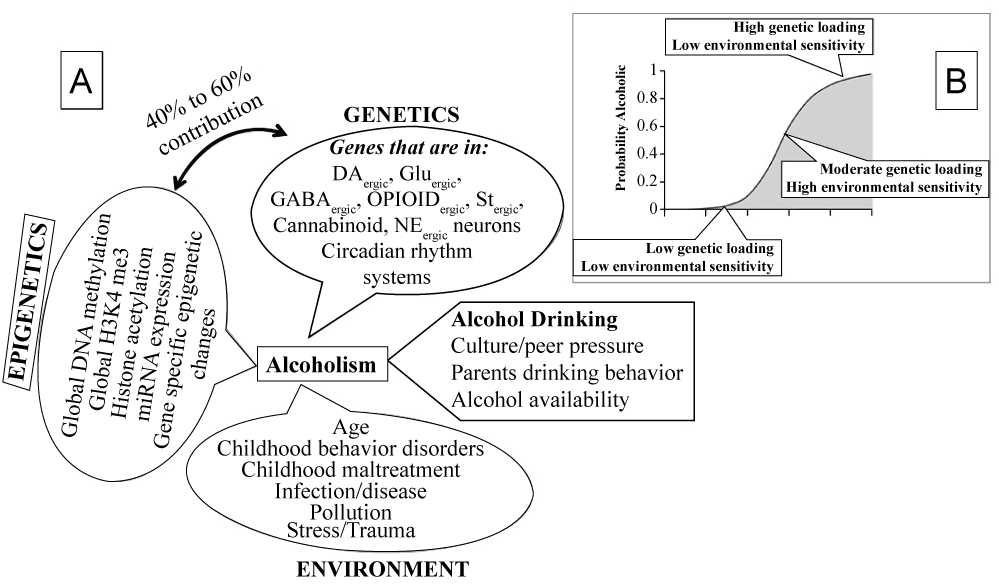
Many scientific studies have shown that children of alcoholics are about four times more likely than the general population to develop alcoholism, suggesting an important role of genetic factors in development of alcoholism [56-59]. However, studies have also shown that genetic factors are not the sole determinant of the development of alcoholism, the environmental (social, parental and peer) factors may also be involved (Figure 6) [60-62]. This is because the environmental factors they may either increase susceptibility for developing an alcohol use disorder or attenuate possible genetic risk by producing a level of protection for vulnerable individuals (Figure 6 inset) [63].
In this section, participation of genetic and environmental factors in development of alcoholism will be discussed in detail.
Genetic/Epigenetic predisposition to alcoholism
The evidence for genetic predisposition arises from studies involving the 'twins' of alcoholic parents that are more susceptible to develop alcoholism than children of normal parents, even when they were adopted and brought up in different environment (one adaptive parent abused alcohol while the other did not) [61-64]. Several important polymorphs associated with alcoholism predisposition have been identified (Table 4). The μ-opioid receptor A 118 G (OPRM1) having Asn40Asp SNP has been studied extensively, however, the results have been controversial. Some studies support an association between A 118 G (OPRM1) and addiction [65-68], while other studies do not [69-72]. Ray, et al. [73] showed that Asp40 carriers in adolescents may mediated the association between OPRM1/Asp40 genotype and alcoholism and that a significantly higher frequency (51.9%) of the Asp40 allele occurred among youth with alcohol-related disorder (ARD), as compared to non-ARD controls (16.3%). In addition to genetics, earlier studies have also provided strong evidence for epigenetic predisposition to addiction in people who have higher probably to develop addiction upon chronic alcohol drinking [74-79].
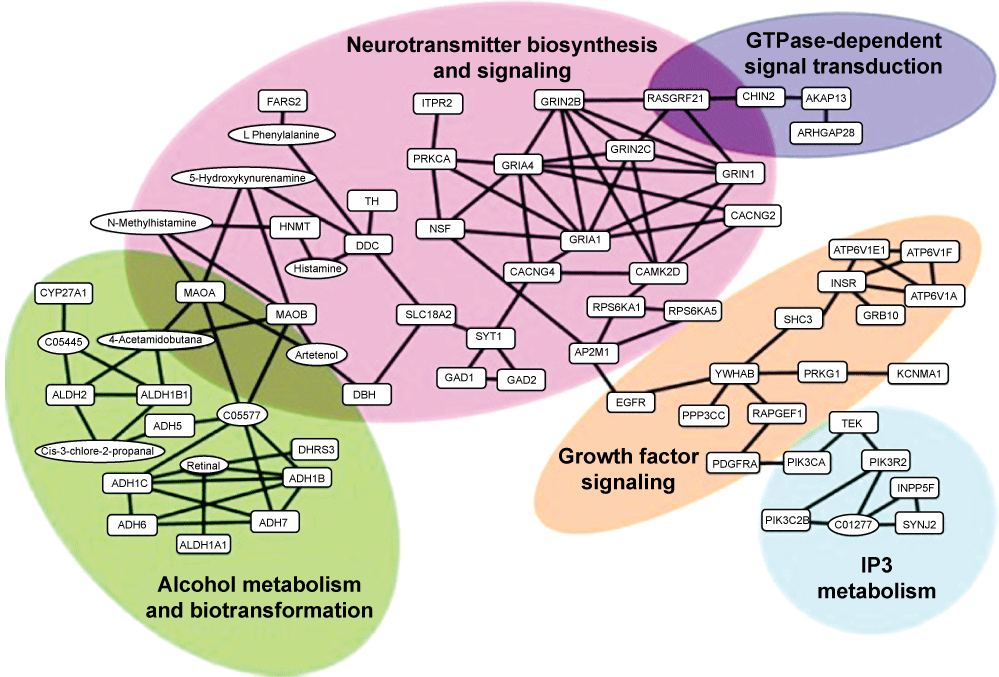
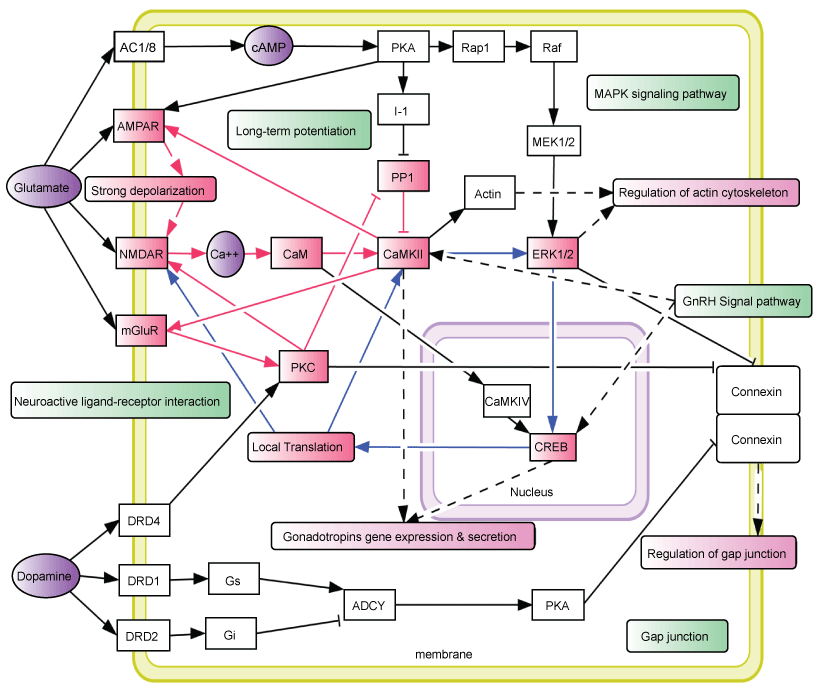
Li, et al. [80] analyzed data using a linkage association in chromosome regions of addiction and identified 1,500 addiction-related genes and five molecular pathways (neuro-active ligand-receptor interaction, long-term potentiation, GnRH signaling pathway, MAPK signaling pathway and gap-junction pathways) that were significantly enriched for alcoholism. They connected the common pathways into a hypothetical common molecular network for addiction. As shown in Figure 7, fast and slow positive feedback loops were interlinked through CAMKII, which may provide clues to explain some of the irreversible features of addiction. Activation of CAMKII may play a central role in the development and maintenance of addiction states. The fast and slow positive feedback loops interlinking through CAMKII may be essential for the development and consolidation of addiction.
Pandey and collogues [81-90] have provided compelling evidence that innate anxiety levels are important in initiating craving for ethanol consumption [91]. Predisposition to anxiety might alter the acquisition or expression of incentive salience for alcohol via the following pathways:
i. Dopamine receptor (DR) pathways for phasic (short term) and tonic (long term mediated by catechol-O-methyl transferase, Val 158 Met polymorph) DA release in NAc and PFC, respectively [91], and
ii. Cannabinoid receptor 1 (CNR1), C allele of rs2023239 in the PFC [92].
According to the incentive salience theory of addiction [92], repeated exposure to addictive drugs including alcohol can, in susceptible individuals and under particular circumstances, persistently change brain cells and circuits, a psychological process involved in motivated behavior (Figure 8). Individuals with the DR Val158Met polymorph and CNR1-C allele of rs2023239 may carry a genetic vulnerability that affects respective receptor-mediated signaling in the mesocorticolimbic structures, resulting in incentive salience to alcohol cues.
Gene-Environment (GxE) interaction
Although genetic predisposition (G) is associated with development of alcoholism, the GxE interaction potentiates (Figure 9) the effects of genetic factors. The joint effects of GxE are significantly greater than would be predicted from the sum of the separate effects [93]. The genetic influences on alcohol drinking behavior is commonly studied among sibling pairs reared in the same family and environmental influence, while the GxE is studied among sibling pair reared in different families and environmental influence [93].
Some of the studies reporting GxE interaction in development of alcoholism are described below.
ADH/ALDH x environment
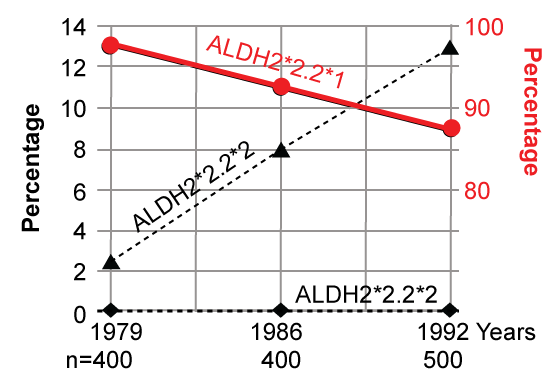
Earlier studies [94-96] have shown that liver may contain active and inactive alleles of liver mitochondrial aldehyde dehydrogenases (ALDH2s). The active forms are encoded by the gene ALDH (2*1/2*1), while the inactive form is encoded by the genes ALDH (2*1/2*2) or ALDH (2*2/2*2). The inactive ALDH form provides a genetic deterrent of heavy drinking and alcoholism among Asians. As shown in Figure 10, the normal active enzyme (ALDH2*1/2*1) decreased, while the partially inactive enzyme (ALDH2*1/2*2) increased between 1979 and 1992 in Japanese population. ALDH2 genetic polymorphisms have been shown to develop hypertension in a prospective cohort and that alcohol intake significantly modified the conferred risk [97]. These data strengthen GxE interaction in Asian populations.
5-HT transporter gene x environment
Nilsson, et al. [98] have shown that adolescents (aged 16 to 19) who had the heterozygous 5-HTTLPR l/s genotype and came from families with neutral or poor relationships had over 10-fold increased risk for high intoxication frequency, compared with heterozygous adolescents who had a good relationship with their families. Preadolescents or early adolescents having the heterozygous 5-HTTLPR l/s genotype and were maltreated exhibited a 40% greater risk for alcoholism then those who were not abused. Covault, et al. [99] reported that the 5-HTTLPR s-allele also may be linked with increased use of alcohol and other drugs among college students who have had multiple negative life events. Thus, an interaction between stress and 5-HTTLPR s/l or s/s alleles may predispose a person to abuse alcohol with increased risk of alcoholism.
GABRA x environment
A review of literature has provided substantial evidence for a key role the GABA receptors play in development of alcohol addiction [100-104]. An interaction between α1 (A > G SNP) and α6 (C > T SNP) subunits of GABRA1 and GABRA2 and the environment has been implicated in the development of alcoholism [105-107]. In family-based studies, GABRA2 has been associated with alcohol dependence exhibiting 13-28 Hz β electroencephalographic frequency in children of alcoholic parents, not in children of control parents [106,108-110]. People with the high-risk allele of GABRA2 and unhappy marriage had higher likelihood of developing alcoholism than people with the allele or marriage alone [106].
Dopamine transporter 1 gene × environment
There is some but not compelling evidence for involvement of dopamine transporter genes in development of alcoholism. Laucht, et al. [111] have shown that in adolescents who were homozygous for either of the DAT1 gene variants and grew up in psychosocially adverse familial conditions exhibited higher risk factors for alcoholism (impulsivity, hyperactivity, and inattention) than did adolescents with other genotypes or those with the same genotypes who grew up in less adverse family conditions. However, Vandenbergh, et al. [112] failed to show any correlation between DAT1 alleles and risk to alcoholism. More research is needed to clarify the role of DAT in alcohol predisposition. Taken together; these observations indicate that the gene-environmental interaction may play a key role in development of alcoholism phenotypes.
Anatomical Changes in Alcohol-Addicted Brain
Alcohol addiction is characterized by compulsion to seek and take alcohol, loss of control in limiting intake, and emergence of withdrawal syndrome when access to the drug is prevented [113]. As discussed earlier, the positive reinforcing effects (euphoria and reward) of acute alcohol ingestion is mediate through the cortico-mesolimbic DAergic pathways, extending from the ventral VTA to the NAc, AMY, HIP, PFC, SN, CP and related structures. The opposing effects (anxiety and stress), mediated through the HIP, muscarinic cholinergic neurons, NEergic neurons and HPA axis, remain inhibited during this phase [113,114]. The brain of alcoholic patients exhibited abnormalities in many of the brain regions associated with the rewarding and opposing effects of alcohol drinking [115,116]. In general, the addiction related pathology can be classified as 'complicated' and 'uncomplicated' disorders.
In complicated neuropathy, the addicted subjects, in addition to the cognitive deficits, also suffer from liver damage and vitamin B1 deficiency, a condition known as Wernicke-Korsakoff syndrome-WKS, [117-119] consisting of two separate syndromes, a short-lived and severe condition called Wernicke's encephalopathy and a long-lasting and debilitating condition known as Korsakoff's psychosis. Wernicke's encephalopathy [120] includes mental confusion, oculomotor disturbances, and difficulty with muscle coordination. Korsakoff's psychosis is a chronic and debilitating syndrome characterized by persistent learning and memory problems [121]. Patients with Korsakoff's psychosis are forgetful and quickly frustrated, have difficulty with walking and coordination and exhibit anterograde amnesia [122-124]. Abstinent WKS patients show significant impairment in neuropsychological tests compared to controls [125]. This suggests that the neurological effects of alcohol with thiamine deficiency may be more severe and permanent.
Uncomplicated (non-WKS) alcoholic patients display mostly neuropsychological and behavioral disorders that exhibit significant recovery of functions upon long alcohol abstentions, although some components of these functional domains recover faster or more fully than others [126-136]. Kopera, et al. [137] have shown only partial recovery in brain function of alcoholic patients abstinent for more than a year. Thus there may be some processes in the brain that do not easily recover over time.
The proceeding sections describe some of the permanent, reversible and dementia-like abnormalities associated with alcoholic brains.
Permanent brain damage
Chronic alcohol use may initiate neuronal loss in different brain regions (larger neurons (greater than 90 μm) are more sensitive than smaller neurons) and reduce the white matter volume in cortex and cerebellum. This may compromise the cerebellum-cerebral cortex loop (complicated >> uncomplicated) [138-141]. Neuronal loss in cortex, hypothalamus and cerebellum may result in lasting impairment in behavior such as decision-making activities [142,143]. Loss of GFAP in neurons (complicated >> uncomplicated) occurred without gross changes in brain pathology or brain weight and was not restricted to pathologically susceptible brain regions [144]. Alcoholic patients having vitamin B1 deficiency exhibits symptoms of alcoholism-related poly neuropathy (ALPN), a potentially debilitating disease associated with sensory, motor, and autonomic nerve dysfunctions [145,146]. Although B1 deficiency is believed to be the prime cause of ALPN, many studies have shown that ALPN was not significantly abated or reversed by thiamine repletion [147-149]. ALPN progresses slowly with burning pain and superficial loss of sensation due to irregular segmental [150], whereas thiamine deficiency result in acutely progressive deficits in superficial and deep sensation, due to degeneration of large fiber axons and sub-perineurial edema [151].
Transient brain damage
Substantial evidence associate alcoholism with global cerebral atrophy and ensuing cognitive dysfunction with age being a critical modulating factor [152-154]. Beck, et al. [155] have shown that alcohol addiction is associated with altered density of gray matter and white matter of specific brain regions, thus supporting the assumption that alcohol dependence is associated with both local gray matter dysfunction and altered brain connectivity (Table 5). The volumetric changes in the brain and certain cognitive impairments can be reversed with abstinence [156,157].
Earlier studies [158-160] have shown age related recovery of alcoholics, younger alcoholics improving to the level of the control groups in the area of visual-spatial functions (which are among those most sensitive to the effects of chronic alcohol abuse), whereas the older alcoholics continued to show deficits. Sclafani, et al. [161] have shown a positive relationship between age and ventricular volume (as % of total brain volume). Studies have shown relatively greater CSF filled spaces in frontal cortices and cerebellum of alcoholics, especially those with thiamine deficiency (Table 5). Shrinkage of Superior Cerebellar Vermis associated with Purkanje cells loss (complicated > uncomplicated) after chronic alcohol intake have been frequently reported [162]. They reported a 21%-40% reduction of Purkinje cell density in the cerebellar vermis with shrinkage of the molecular and granular layers.
Alcohol Withdrawal Syndrome
Earlier studies have shown that many of the alcohol-induced neurological abnormalities listed above (section 8.2.) were partially reversed with maintained abstinence [142,163]. However, in alcohol addicted subjects, abstinence (alcohol withdrawal) results in severe physical and emotional distress (paleness, excessive perspiration, nausea, stomach discomfort, heart palpitations, headaches, appetite loss, shakiness, seizures, nervousness, and, in extreme cases, death) that may increase alcohol craving [145]. Alcohol drinking rapidly reverses the withdrawal symptoms [164]. The aim of this section is to discuss the mechanisms underlying the development of the withdrawal symptoms in response to alcohol withdrawal.
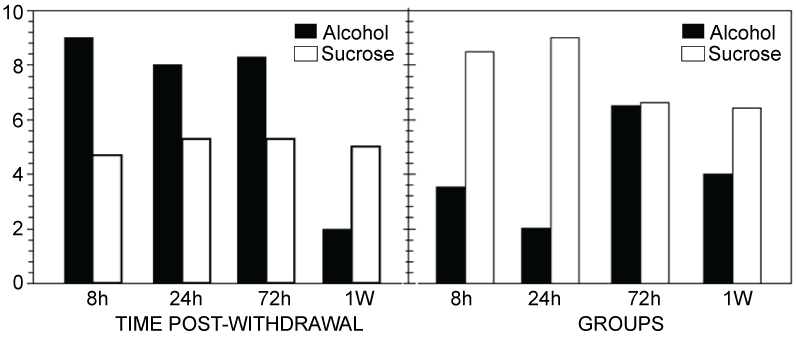
In general, a state of neural hyper-excitability, lasting for at least 24 h to 72 h following withdrawal may be central to the development of the withdrawal symptoms [165]. Geisler, et al. [148] have shown that neural hyper-excitability to primary electrical stimulation may exist for at least 72 h and then subsiding at 1 week after alcohol withdrawal in alcoholics. A secondary stimulation given a week after the first stimulation resulted in a state of hypo-excitability (Figure 11). This shows long-term effects of alcohol withdrawal in rats.
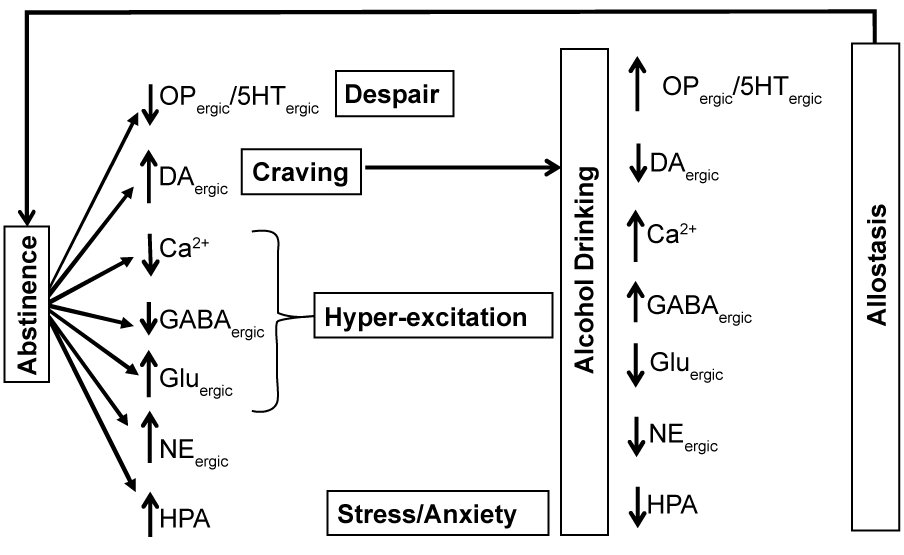
Earlier studies have suggested that chronic ethanol exposure induces a net excitation state in Dorsal Raphe (DR) neurons that contributes to enhanced anxiety and excitation during ethanol withdrawal [166-168]. The neurotransmitter changes occurring in the brain of alcoholics that may be associated with the withdrawal symptoms are shown in Figure-12. GABAergic neurons in NAc, inter neurons in VTA regions, and postsynaptic GABA receptors, notably GABAA receptors, may play a central role in development of addiction and withdrawal symptoms [113,169].
GABAA receptors are unique because they cause neuro-inhibition in alcohol-naïve subjects, but cause neuro-excitation in alcohol deprived alcoholic subjects by recruiting different pathways [170]. Chronic alcohol drinking may switch GABAA receptor function from inhibitory to excitatory, facilitating the withdrawal symptoms [171-173]. An increase in DA in the brain may augment craving for alcohol [174,175], while an increase in NA and activation of the HPA axis increases the stress response and anxiety, while a decrease in opioid and 5-HT receptors enhance the feeling of despair [175,176].
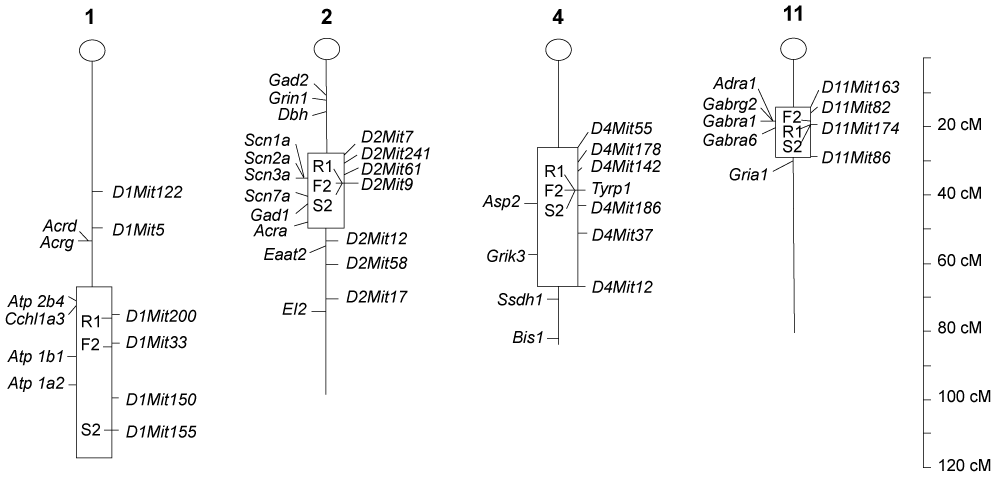
Earlier studies, using linkage-based genome scans of chromosomes, have identified markers of alleles that predispose to alcohol addiction and withdrawal symptoms [177-182]. Long, et al. [183] and Buck, et al. [177] have shown that the risk for alcohol withdrawal highly correlated with markers located at chromosomes 1, 2, 4 and 11. In chromosome 5, the marker was found at 38-42 cM from the centromere of chromosome 4 (Figure 13) in mouse that is syntonic with human 9p21-p23 and 1p32-p22.1. Buck, et al. [177] also detected a QTL near Gad 1, α-subunit of brain sodium channels (Scn1a, Scn2a, Scn3a) and a glial-specific sodium channel (Scn7a) of chromosome 2. Gad1 may be related to a candidate gene responsible for GABA synthesis. The differences in alcohol withdrawal severity among mice of different strains could be associated with differences in GAD enzyme activity and/or gene expression. Chromosome 11 contained a QTL in proximity to genes encoding the α1, α6, and γ2 subunits of GABAA receptors [184] that correlated with severity of the withdrawal syndrome.
Alcohol withdrawal, in addition to the rapid-onset withdrawal symptoms, also cause long-lasting post-abstinence changes including, but not limited to, sleep disturbances, cognitive deficits, anxiety, mood swings, depression mostly in females, panic disorder and suicide behavior [130,185-192]. Zorrilla, et al. and Koob [193,194] have shown that protracted withdrawal from ethanol or cocaine is associated with altered limbic CRF-LI and circulating CORT levels beyond the detoxification stage. These may play some role in the development of the delayed symptoms.
Conclusions
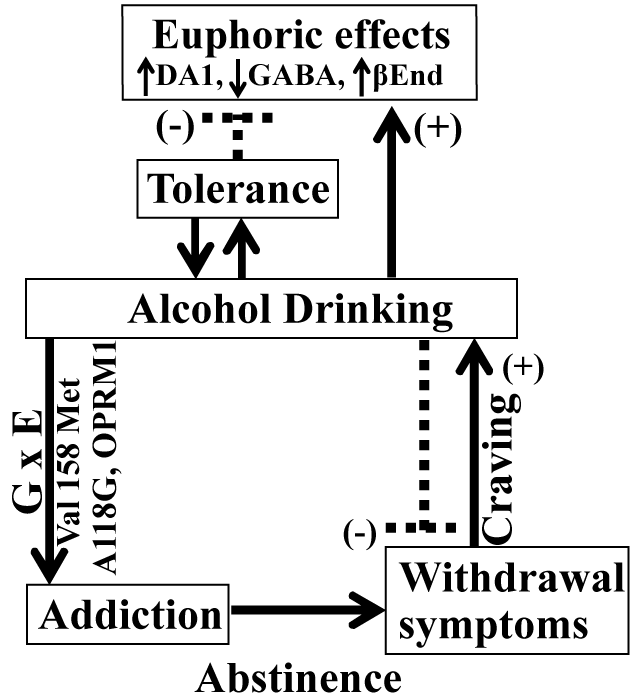
Alcohol exposure in alcohol-naive subjects elicits euphoric and relaxing responses that are attributed to (i) an increase in GABAergic (inhibitory signals), OPergic and 5HTergic (euphoric effects) neuronal activities and (ii) a decrease in DAergic ('want' signal or craving), Gluergic (excitatory signals), NEergic (stress signals) neuronal, and the HPA axis (stress hormones) activities. If alcohol drinking continues, the receptors are sensitized, resulting in development of tolerance when alcohol drinking must be increased to achieve desired effects (Figure 14). In genetically predisposed subjects, chronic alcohol drinking results in the development of addiction, characterized by a condition when alcohol caseation results in rapid onset of withdrawal symptoms including, but not limited to, alcohol craving and moderate to severe discomfort such as tremors, seizures, hallucinations, DT and, in extreme cases, death. Although mechanisms are not fully understood, in alcohol withdrawal, the neurochemical changes are opposite to those during the acute exposure phase: Gluergic, DAergic, NAergic and HPA activities are elevated, while GABAergic activity is reduced. Alcohol drinking rapidly reverses the withdrawal symptoms. Taken together, these observations suggest that alcoholism is a multifaceted disease, affecting almost every system in the body, including neurological, physiological/hormonal, and cardiovascular systems. Because of this, there is a great need to improve the success rates of all forms of treatment of alcoholism including prevention of relapse, curbing active alcohol consumption and craving and treatment of the disease.
References
- Peele S, Grant M (1999) Alcohol and pleasure: A health perspective. Brunner/Mazel, Philadelphia, 1-7.
- Artero A, Artero A ,Tarín JJ, et al. (2015) The impact of moderate wine consumption on health. Maturitas 80: 3-13.
- Samokhvalov AV, Irving H, Mohapatra S, et al. (2010) Alcohol consumption, unprovoked seizures and epilepsy: a systematic review and meta-analysis. Epilepsia 51: 1177-1184.
- Lonnroth K, Williams B, Stadlin S, et al. (2008) Alcohol use as a risk factor for tuberculosis − a systematic review. BMC Public Health 8: 289.
- Rehm J, Room R, Graham K (2003) The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease-An overview. Addiction 98: 1209-1228.
- Rehm J, Rehn N, Room R, et al. (2003) The global distribution of average volume of alcohol consumption and patterns of drinking. Eur Addict Res 9: 147-156.
- Rehm J, Mathers C, Popova S, et al. (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol use disorders. Lancet 373: 2223-2233.
- Rehm J, Samokhvalov AV, Neuman MG, et al. (2009) The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 9: 450.
- Rehm J, Baliunas D, Borges GL, et al. (2010) The relation between different dimensions of alcohol consumption and burden of disease-an overview. Addiction 105: 817-843.
- Rehm J, Taylor B, Mohapatra S, et al. (2010) Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev 29: 437-445.
- Rehm J, Shield KD, Joharchi N, et al. (2012) Alcohol consumption and the intention to engage in unprotected sex: systematic review and meta-analysis of experimental studies. Addiction 107: 51-59.
- Rehm J, Shield KD (2013) Alcohol and mortality: global alcohol-attributable deaths from cancer, liver cirrhosis and injury in 2010. Alcohol Res Curr Rev 35: 174-183.
- Baliunas D, Rehm J, Irving H, et al. (2010) Alcohol consumption and risk of incident human immunodeficiency virus infection: A meta-analysis. Int J Public Health 55: 159-166.
- Hendershot CS, Stoner SA, Pantalone DW, et al. (2009) Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr 52: 180-202.
- Azar MM, Springer SA, Meyer JP, et al. (2010) A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 112: 178-193.
- World Health Organization (2011) The global status report on alcohol and health. Geneva.
- Lim SS, Vos T, Flaxman AD, et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224-2260.
- Stahre M (2014) Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Preventing Chronic Disease 11: 130-293.
- Wickizer T, Maynard C, Atherly A, et al. (1994) Completion rates of clients discharged from drug and alcohol treatment programs in Washington State. Am J Pub Health 84: 215-221.
- Anderson P, Baumberg B (2006) Alcohol in Europe-A public health perspective. A report for the European Commission. Institute of Alcohol Studies, England.
- Sacks JJ, Roeber J, Bouchery EE (2013) State costs of excessive alcohol consumption, 2006. Am J Preventive Med 45: 474-485.
- Ron D, Barak S (2016) Molecular mechanisms underlying alcohol-drinking behaviors. Nature Rev Neurosci 17: 576-591.
- Nind M (2006) Conducting systematic review in education: a reflexive narrative. London Review of Education 4: 183-195.
- Martin CS, Earleywine M, Finn PR, et al. (1990) Some boundary conditions for effective use of alcohol placebos. J Stud Alcohol 51: 500-505.
- Giancola PR, Zeichner A (1997) The biphasic effects of alcohol on human physical aggression. J Abnorm Psychol 106: 598-607.
- Newlin SA, Mancillas-Trevino L, Bloom FE (1981) Ethanol causes increases in excitation and inhibition in area CA3 of the dorsal hippocampus. Brain research 209: 113-128.
- Crabbe JC, Harris RA, Koob GF (2011) Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci 1216: 24-40.
- Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4-26.
- Baez Mendoza, R Schultz W (2013) The role of the striatum in social behavior. Front Neurosci 7: 233.
- Parsian A, Cloninger CR (1997) Human GABA, Receptor αl and α3 Subunits Genes and Alcoholism. Alcoholism Clin Exper Res 21: 430-433.
- Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nature Rev Neurosci 6: 626-640.
- Hoffman PL, Rabe CS, MosesF, et al. (1989) N-Methyl-D-Aspartate Receptors and Ethanol: Inhibition of Calcium Flux and Cyclic GMP Production. J Neurochem 52: 1937-1940.
- Edenberg HJ (2007) The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 30: 5-13.
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445-1449.
- Versyer JC (2008) The alcohol hangover-a puzzling phenomenon. Alcohol Alcohol 43: 24-126.
- Jackson KM (2008) Heavy episodic drinking: Determining the predictive utility of five or more drinks. Psychol Addict Behaviors 22: 68-72.
- Piasecki TM, Robertson BM, Epler AJ (2010) Hangover and risk for alcohol use disorders: existing evidence and potential mechanisms. Curr Drug Abuse Rev 3: 92-102.
- Foster WH, Vaughan RD (2005) Absenteeism and business costs: does substance abuse matter? J Subst Abuse Treat 28: 27-33.
- Prat G, Adan A, PerezPamies M, et al. (2008) Neurocognitive effects of alcohol hangover. Addict Behav 33: 15¬-23.
- Stephens R, Ling J, Heffernan TM, et al. (2008) A review of the literature on the cognitive effects of alcohol hangover. Alcohol and Alcoholism 43: 163-170.
- Swift R, Davidson D (1998) Alcohol hangover: mechanisms and mediators. Alcohol Health Res World 22: 54-60.
- Linkola J, Ylikahri R, Fyhrquist F (1978) Plasma vasopressin in ethanol intoxication and hangover. Acta Physiologica Scand 104: 180-187.
- Linkola J, Fyhrquist F, Ylikahri R (1979) Adenosine 3', 5' monophosphate, calcium and magnesium excretion in ethanol intoxication and hangover. Acta Physiol Scand 107: 333-337.
- Ylikahri RH, Pösö AR, Huttunen MMO, et al. (1974) Alcohol intoxication and hangover: effects on plasma electrolyte concentrations and acid-base balance. Scan J Clin Lab Invest 34: 327-336.
- Siler SQ, Neese RA, Christiansen MP, et al. (1998) The inhibition of gluconeogenesis following alcohol in humans. Am J Physiol 275: 897-907.
- Tsukamoto S, Muto T, Nagoya T, et al. (1989) Determinations of ethanol, acetaldehyde and acetate in blood and urine during alcohol oxidation in man. Alcohol Alcohol 24: 101-108.
- Eriksson CJ, Fukunaga T, Sarkola T, et al. (2001) Functional relevance of human ADH polymorphism. Alcohol Clin Exp Res 25: 157S-163S.
- Yokoyama M, Yokoyama A, Yokoyama T, et al. (2005) Hangover susceptibility in relation to aldehyde dehydrogenase-2 genotype, alcohol flushing, and mean corpuscular volume in Japanese workers. Alcohol Clin Exp Res 29: 1165-1171.
- Quertemont E, Didone V (2006) Role of acetaldehyde in mediating the pharmacological and behavioral effects of alcohol. Alcohol Research Health 29: 258-265.
- Deelchand DK, Shestov AA, Koski DM, et al. (2009) Acetate transport and utilization in the rat brain. J Neurochem 109: 46-54.
- Maxwell CT, Spangenberg RJ, Hoek JB, et al. (2010) Acetate causes alcohol hangover headache in rats. Plos One 5: e15963.
- Penning R, van Nuland M, Fliervoet LA, et al. (2010) The pathology of alcohol hangover. Curr Drug Abuse Rev 3: 68-75
- Kim DJ, Kim W, Yoon SJ, et al. (2003) Effects of alcohol hangover on cytokine production in healthy subjects. Alcohol 31: 167-170.
- Liang J, Olsen RW (2014) Alcohol use disorders and current pharmacological therapies: the role of GABA(A) receptors. Acta Pharmacol Sin 35: 981-993.
- Pietrzykowski AZ, Treistman SN (2008) The molecular basis of tolerance. Alcohol Research Health 31: 298-309.
- Heath AC, Bucholz KK, Madden PA, et al. (1997) Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Med 27: 1381-1396.
- Chassin L, Pitts SC, DeLucia C, et al. (1999) A longitudinal study of children of alcoholics: Predicting young adult substance use disorders, anxiety, and depression. J Abnorm Psychol 108: 106-119.
- Kendler KS, Heath AC, Neale MC, et al. (1992) A population-based twin study of alcoholism in women. JAMA 268: 1877-1882.
- Kendler KS, Kessler RC, Walters EE, et al. (1995) Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatr 152: 833-842.
- Kendler KS (1995) Genetic epidemiology in psychiatry. Taking both gene and environment seriously. Arch Gen Psychiatry 52: 895-899.
- Slutske WS, Heath AC, Dinwiddie SH, et al. (1998) Common genetic risk factors for conduct disorder and alcohol dependence. J Abnormal Psych 107: 363-374.
- Cadoret RJ, Yates WR, Woodworth G, et al. (1995) Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry 52: 42-52.
- Hesselbrock MN (1991) Gender comparison of antisocial personality disorder and depression in alcoholism. J Substance Abuse 3: 205-219.
- Prescott CA, Kendler KS (1999) Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry 156: 34-40.
- Kranzler HR, Modesto Lowe V, Nuwayser ES (1998) Sustained-Release Naltrexone for Alcoholism Treatment: A Preliminary Study. Alcohol Clin Exp Res 22: 1074-1079.
- Bart G, Kreek MJ, Ott J, et al. (2005) Increased attributable risk related to a functional μ-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology 30: 417-422.
- Schinka JA, Town T, Abdullah L, et al. (2002) A functional polymorphism within the mu-opioid receptor gene and risk for abuse of alcohol and other substances. Mol Psychiatry 7: 224-228.
- Tan EC, Tan CH, Karupathivan U, et al. (2003) Mu opioid receptor gene polymorphisms and heroin dependence in Asian populations. Neuroreport 14: 569-572.
- Bergen AW, Kokoszka J, Peterson R, et al. (1997) Mu opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry 2: 490-494.
- Franke P, Wang T, Nöthen MM, et al. (2001) Nonreplication of association between mu-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. Am J Med Genet 105: 114-119.
- Shi J, Hui L, Xu Y, et al. (2002) Sequence variation in the mu-opioid receptor gene (OPRM1) associated with human addiction to heroin. Hum Mutat 4: 459-460.
- Luo X, Kranzler HR, Zhao H, et al. (2003) Haplotypes at the OPRM1 locus are associated with susceptibility to substance dependence in European-Americans. Am J Med Genet B Neuropsychiatr Genet 120B: 97-108.
- Ray LA, Hutchison KE, Ashenhurst JR, et al. (2010) Naltrexone selectively elevates GABAergic neuroactive steroid levels in heavy drinkers with the Asp40 allele of the OPRM1 gene: a pilot investigation. Alcohol Clin Exp Res 34: 1479-1487.
- Dick DM, Agrawal A (2008) The genetics of alcohol and other drug dependence. Alcohol Research Health 31: 111.
- Kohnke M, Kolb W, Kohnke A, et al. (2006) DBH* 444G/A polymorphism of the dopamine-β-hydroxylase gene is associated with alcoholism but not with severe alcohol withdrawal symptoms. J Neural Transm 113: 869-876.
- Starkman BG, Sakharkar AJ, Pandey SC (2011) Epigenetics-Beyond the genome in alcoholism. Alcohol Research Current Reviews 34: 293.
- Ponomarev ED, Veremeyko T, Weiner HL (2013) MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61: 91-103.
- Zhang TY, Labonté B, Wen XL, et al. (2013) Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology 38: 111-123.
- Pandey DP, Gerdes K (2005) Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 33: 966-976.
- Li H, Wetten S, Li L, et al. (2008) Candidate single-nucleotide polymorphisms from a genome wide association study of Alzheimer disease. Arch Neurol 65: 45-53.
- Pandey SC (2003) Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci 24: 456-460.
- Pandey SC, Roy A, Zhang H, et al. (2004) Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci 24: 5022-5030.
- Pandey SC, Ugale R, Zhang H, et al. (2004) Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci 28: 3729-3737.
- Pandey SC, Zhang H, Roy A, et al. (2006) Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci 26: 8320-8331.
- Misra K, Pandey SC (2003) Differences in basal levels of CREB and NPY in nucleus accumbens regions between C57BL/6 and DBA/2 mice differing in inborn alcohol drinking behavior. J Neurosci Res 74: 967-975.
- Zhang H, Sakharkar AJ, Shi G, et al. (2010) Signaling in the Central Nucleus of Amygdala Regulates Alcohol-Drinking and Anxiety-Like Behaviors of Alcohol-Preferring Rats. Alcohol Clin Exp Res 34: 451-461.
- Sakharkar AJ, Zhang H, Tang L, et al. (2014) Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol 17: 1207-1220.
- Moonat S, Sakharkar AJ, Zhang H, et al. (2011) The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol 16: 238-250.
- Arora DS, Nimitvilai S, Teppen TL, et al. (2013) Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: reversal by histone deacetylase inhibitors. Neuropsychopharmacology 38: 1674-1684.
- Arora P, Chanana A, Tejpal HR (2013) Estimation of blood alcohol concentration in deaths due to roadside accidents. J Forensic Legal Med 20: 300-304.
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247-291.
- Hutchison KE, Haughey H, Niculescu M, et al. (2008) The Incentive Salience of Alcohol Translating the Effects of Genetic Variant in CNR1. Arch Gen Psychiatry 65: 841-850.
- Kaufman J, Yang B, Douglas-Palumberi H, et al. (2007) Genetic and environmental predictors of early alcohol use. Biol Psychiatry 61: 1228-1234.
- Harada S, Zhang S (1993) New strategy for detection of ALDH2 mutant. Alcohol Alcohol Suppl 1A: 11-13.
- Takeshita T, Morimoto K, Mao X, et al. (1994) Characterization of the three genotypes of low Km aldehyde dehydrogenase in a Japanese population. Hum Genet 94: 217-223.
- Saito T, Lachman HM, Diaz L, et al. (2002) Analysis of monoamine oxidase A (MAOA) promoter polymorphism in Finnish male alcoholics. Psychiatry Res 109: 113-119.
- Moreb JS, Ucar D, Han S, et al. (2012) The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact 195: 52-60.
- Nilsson KW, Sjöberg RL, Damberg M, et al. (2005) Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcohol Clin Exp Res 29: 564-570.
- Covault J, Tennen H, Armeli S, et al. (2007) Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry 61: 609-616.
- Nowak K, McBride W, Lumeng L, et al. (1998) Blocking GABA(A) receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology (Berl) 139: 108-116.
- Chang L, Cloak CC, Ernst T (2003) Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J Clin Psychiatry 64: 7-14.
- Song J, Koller DL, Foroud T, et al. (2003) Association of GABA(A) receptors and alcohol dependence and the effects of genetic imprinting. Am J Med Genet B Neuropsychiatr Genet 117: 39-45.
- Dick DM, Agrawal A, Wang JC, et al. (2007) Alcohol dependence with comorbid drug dependence: genetic and phenotypic associations suggest a more severe form of the disorder with stronger genetic contribution to risk. Addiction 102: 1131-1139.
- Lappalainen J, Krupitsky E, Remizov M, et al. (2005) Association between Alcoholism and γ-Amino Butyric Acid α2 Receptor Subtype in a Russian Population. Alcohol Clin Exp Res 29: 493-498.
- Dick DM, Edenberg HJ, Xuei X, et al. (2004) Association of GABRG3 with alcohol dependence. Alcohol Clin Exp Res 28: 4-9.
- Edenberg HJ, Dick DM, Xiaoling X, et al. (2006) Variations in GABRA2, encoding the α2 subunit of the GABA A receptor, are associated with alcohol dependence and with brain oscillations. Am J Human Genetics 74: 705-714.
- Grucza RA, Bierut LJ (2006) Co-occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Res Health 29: 172-178.
- Bauer LO, Hesselbrock VM (1993) EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol 54: 577-589.
- Costa L, Bauer L (1997) Quantitative electroencephalographic differences associated with alcohol, cocaine, heroin and dual-substance dependence. Drug Alcohol Depend 46: 87-93.
- Rangaswamy M, Porjesz B, Chorlian DB, et al. (2002) Beta power in the EEG of alcoholics. Biol Psychiatry 52: 831-842.
- Laucht M, Becker K, Blomeyer D, et al. (2007) Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: results from a high-risk community sample. Biol Psychiatry 61: 87-92.
- Vandenbergh D, Thompson M, Cook E, et al. (2000) Human dopamine transporter gene: coding region conservation among normal, Tourette's disorder, alcohol dependence and attention-deficit hyperactivity disorder populations. Mol Psychiatry 5: 283-292.
- Koob GF, Le Moal M (2008) Addiction and the brain antireward system. Annu Rev Psychol 59: 29-53.
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35: 217-238.
- Heinz A, Lober S, Georgi A, et al. (2003) Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol Alcohol 38: 35-39.
- King AC, de Wit H, McNamara PJ, et al. (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68: 389-399.
- Butterworth RF, Kril JJ, Harper CG (1993) Thiamine-Dependent Enzyme Changes in the Brains of Alcoholics: Relationship to the Wernicke-Korsakoff Syndrome. Alcohol Clin Exp Res 17: 1084-1088.
- Zubaran C, Fernandes JG, Rodnight R (1997) Wernicke-Korsakoff syndrome. Postgrad Med J 73: 27-31.
- Reed LJ, Lasserson D, Marsden P, et al. (2003) FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex 39: 1027-1045.
- Sechi G, Serra A (2007) Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol 6: 442-455.
- Halliday G, Ellis J, Harper C (1992) The locus coeruleus and memory: a study of chronic alcoholics with and without the memory impairment of Korsakoff's psychosis. Brain Res 598: 33-37.
- Maurice V, Talland GA, Adams RD (1959) Psychological studies of Korsakoff's psychosis: I. General intellectual functions. J Nerv Ment Dis 128: 528-537.
- Maurice V, Angevine JB, Mancall EL (1961) Memory loss with lesions of hippocampal formation: Report of a case with some remarks on the anatomical basis of memory. Arch Neurol 5: 244-263.
- Frantzen E (1966) Wernicke's encephalopathy. Acta Neurol Scand 42: 426-441.
- Fujiwara E, Brand M, Borsutzky S (2008) Cognitive performance of detoxified alcoholic Korsakoff syndrome patient remains stable over two years. J Clin Exp Neuropsychol 30: 576-587.
- Roseribloom MJ, Pfefferbaum A, Sullivan EV (2004) Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology 18: 589-597
- Rosenbloom MJ, Sullivan EV, Sassoon SA, et al. (2007) Alcoholism, HIV infection, and their comorbidity: factors affecting self-rated health-related quality of life. J Stud Alcohol Drugs 68: 115-125.
- Sullivan EV, Deshmukh A, Desmond JE, et al. (2000) Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry 57: 894-902.
- Becker JT, Jaffe JH (1984) Impaired memory for treatment-relevant information in inpatient men alcoholics. J Stud Alcohol 45: 339-343.
- Brandt J, Butters N, Ryan C, et al. (1983) Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry 40: 435-442.
- Mann K, Gunther A, Stetter F (1999) Rapid recovery from cognitive deficits in abstinent alcoholics: a controlled test-retest study. Alcohol Alcohol 34: 567-574.
- Savage L (2015) Alcohol related brain damage. In: Savanberg J, Withall A, Draper B, et al. Alcohol and the Adult Brain. Psychology Press, New York, 108.
- Nixon SJ, Glenn SW (1999) Cognitive psychosocial performance and recovery in female alcoholics. Recent Developments in Alcoholism. Springer 12: 287-307.
- Parsons OA, Leber WR (1982) Alcohol, cognitive dysfunction and brain damage. NIAAA, Biomedical Processes and Consequences of Alcohol Abuse. Alcohol and Health Monograph 2: 213-225.
- Sullivan EV, Rosenbloom MJ, Lim KO, et al. (2000) Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology 14: 178-188.
- Fein G, Landman B, Tran H, et al. (2006) Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage 32: 1465-1471.
- Kopera M, Wojnar M, Brower K, et al. (2012) Cognitive functions in abstinent alcohol-dependent patients. Alcohol 46: 665-671.
- Harding A, Wong A, Svoboda M, et al. (1999) Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus 7: 78-87.
- Korbo L (1999) Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res 23: 164-168.
- Kril JJ, Halliday GM (1999) Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol 58: 381-387.
- Kubota M, Nakazaki S, Hirai S (2001) Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry 71: 104-106.
- Sullivan EV, Pfefferbaum A (2005) Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 180: 583-594.
- Parsons OA, Butters NE, Nathan PE (1987) Neuropsychology of alcoholism: Implications for diagnosis and treatment. Guilford Press, New York.
- Carlen PL, Wilkinson DA (1987) Reversibility of alcohol-related brain damage: Clinical and experimental observations. Acta Med Scand Suppl 717: 19-26.
- Bayard M, McintyreJ, Hill KR, et al. (2004) Alcohol withdrawal syndrome. American family physician 69: 1443-1450.
- Ripley TL, Whittington MA, Butterworth AR, et al. (1996) Ethanol withdrawal hyperexcitability in vivo and in isolated mouse hippocampal slices. Alcohol Alcohol 31: 347-358.
- MacDonnell MF, Brown SH, Davy B (1975) Hyperexcitability in the neural substrate of emotional behavior in cats after alcohol withdrawal. Evidence of a rapid development of alcohol dependence. J Studies Alcohol 36: 1480-1492.
- Geisler RF, Hunter BE, Walker DW (1978) Ethanol dependence in the rat: temporal changes in neuroexcitabilityfollowing withdrawal. Psychopharmacology 56: 287-292.
- Devaud LL, Fritschy JM, Sieghart W, et al. (1997) Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem 69: 126-130.
- Weiss F, Parsons LH, Schulteis G, et al. (1996) Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci 16: 3474-3485.
- Ravitz B, Nutt J (1991) Disturbances of Hypothalamic-Pituitary-Adrena1 Axis Functioning During Ethanol Withdrawal in Six Men. Am J Psychiatry 1: 1023-1025
- Hawley RJ, Major LF, Schulman EA, et al. (1981) CSF levels of norepinephrine during alcohol withdrawal. Arch Neurol 38: 289-292.
- Herz A (1997) Endogenous opioid systems and alcohol addiction. Psychopharmacology 129: 99-111.
- Van Eerdewegh P, Foroud T, Hesselbrock V, et al. (1998) Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet 81: 207-215.
- Beck A, Wüstenberg T, Genauck A, et al. (2012) Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry 69: 842-852.
- Sullivan E, Rosenbloom M, Pfefferbaum A (2000) Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res 24: 611-621.
- Shear PK, Jernigan TL, Butters N (1994) Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res 18: 172-176.
- Liepman MR, Nirenberg TD, Begin AM (1989) Evaluation of a program designed to help family and significant others to motivate resistant alcoholics into recovery. Am J Drug Alcohol Abuse 15: 209-221.
- Rourke SB, Grant I (1999) The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: a 2-year follow-up study. J Int Neuropsych Soc 5: 234-246.
- Dawson DA, Goldstein RB, Grant BF (2007) Rates and Correlates of Relapse Among Individuals in Remission From DSM-IV Alcohol Dependence: A 3-Year Follow-Up. Alcohol Clin Exp Res 31: 2036-2045.
- Sclafani V, Ezekiel F, Meyerhoff DJ, et al. (1995) Brain atrophy and cognitive function in older abstinent alcoholic men. Alcohol Clin Exp Res 19: 1121-1126.
- Torvik A, Torp S (1986) The prevalence of alcoholic cerebellar atrophy. A morphometric and histological study of an autopsy material. J Neurol Sci 75: 43-51.
- Harper C (2009) The neuropathology of alcohol-related brain damage. Alcohol Alcohol 44: 136-140.
- Becker HC, Hale RL (1993) Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol Clin Exp Res 17: 94-98.
- Hunter BE, Boast CA, Walker DW, et al. (1973) Alcohol withdrawal syndrome in rats: Neural and behavioral correlates. Pharm Biochem Behav 1: 719-725.
- Grahn RE, Watkins LR, Maier SF (2000) Impaired escape performance and enhanced conditioned fear in rats following exposure to an uncontrollable stressor are mediated by glutamate and nitric oxide in the dorsal raphe nucleus. Behav Brain Res 112: 33-41.
- Kirby LG, Pan YZ, FreemanDaniels E, et al. (2007) Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology 32: 712-723.
- LoweryGionta EG, Marcinkiewcz CA, Kash TL (2015) Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology 40: 590-600.
- Saitz R (1998) Introduction to alcohol withdrawal. Alcohol Health Res World 22: 5-12.
- Staley KJ, Soldo BL, Proctor WR (1995) Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 269: 977-981.
- Sanna E, Serra M, Cossu A, et al. (1993) Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcohol Clin Exp Res 17: 115-123.
- Grobin AC, Matthews DB, Devaud LL (1998) The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology 139: 2-19.
- Liang J, Zhang N, Cagetti E, et al. (2006) Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci 26: 1749-1758.
- Heinz A, Siessmeier T, Wrae J, et al. (2004) Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161: 1783-1789.
- Hillemacher T, Frieling H, Hartl T, et al. (2009) Promoter specific methylation of the dopamine transporter gene is altered in alcohol dependence and associated with craving. J Psychiatric Res 43: 388-392.
- Jackson JS, Knight KM, Rafferty JA (2010) Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health 100: 933-939.
- Buck KJ, Metten P, Belknap JK, et al. (1997) Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci 17: 3946-3955.
- Franke P, Schwab SG, Knapp M, et al. (1999) DAT1 gene polymorphism in alcoholism: a family-based association study. Biol Psychiatry 45: 652-654.
- Thompson MD, Gonzalez N, Nguyen T, et al. (2000) Serotonin transporter gene polymorphisms in alcohol dependence. Alcohol 22: 61-67.
- Dick DM, Foroud T (2003) Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin Exp Res 27: 868-879.
- Köhnke MD, Kolb W, Köhnke AM (2006) DBH* 444G/A polymorphism of the dopamine-β-hydroxylase gene is associated with alcoholism but not with severe alcohol withdrawal symptoms. J Neural Transmission 113: 869-876.
- Wetherill L, Schuckit MA, Hesselbrock V, et al. (2008) Neuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence. Alcohol Clin Exp Res 32: 2031-2040.
- Long JC, Knowler WC, Hanson RL, et al. (1998) Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 81: 216-221.
- Kumar S, Porcu P, Werner DF, et al. (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology 205: 529-564.
- George DT, Nutt DJ, Dwyer BA, et al. (1990) Alcoholism and panic disorder: is the comorbidity more than coincidence?. Acta Psychiatr Scand 81: 97-107.
- Hasin DS, Tsai WY, Endicott J, et al. (1996) Five-year course of major depression: effects of comorbid alcoholism. J Affect Disord 41: 63-70.
- Swendsen JD, Merikangas KR, Canino GJ, et al. (1998) The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Compr Psychiatry 39: 176-184.
- Frye MA, Altshuler LL, McElroy SL, et al. (2003) Gender differences in prevalence, risk, and clinical correlates of alcoholism comorbidity in bipolar disorder. Am J Psychiatry 160: 883-889.
- Le Bon O, Murphy JR, Staner L, et al. (2003) Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 23: 377-383.
- Sher L (2006) Alcoholism and suicidal behavior: a clinical overview. Acta Psychiatr Scand 113: 13-22.
- Stein MD, Friedmann PD (2006) Disturbed sleep and its relationship to alcohol use. Subst Abus 26: 1-13.
- Arnedt JT, Conroy DA, Brower KJ (2007) Treatment options for sleep disturbances during alcohol recovery. J Addict Dis 26: 41-54.
- Zorrilla EP, Valdez GR, Weiss F (2001) Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology 158: 374-381.
- Koob GF (2010) The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314: 3-14.
- Gilpin NW, Koob GF (2008) Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health 31: 185-195.
- Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374: 363-371.
- Sery O, Didden W, Mikes V, et al. (2006) The association between high-activity COMT allele and alcoholism. Neuro Endocrinol Lett 27: 231-235.
- Ham MI, Bettencourt LM, McDaniel FD, et al. (2008) Spontaneous coordinated activity in cultured networks: analysis of multiple ignition sites, primary circuits, and burst phase delay distributions. J Comput Neurosci 24: 346-357.
- do Prado‐Lima P, Chatkin J, Taufer M, et al. (2004) Polymorphism of 5HT2A serotonin receptor gene is implicated in smoking addiction. Am J Med Genet Part B: Neuropsychiatric Genet 128: 90-93.
- Blum K, Noble EP, Sheridan PJ, et al. (1990) Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 263: 2055-2060.
- Noble EP, Blum K, Ritchie T, et al. (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism or gene ism. Arch Gen Psychiatry 48: 648-654.
- Montag C, Markett S, Basten U, et al. (2010) Epistasis of the DRD2/ANKK1 TaqIa and the BDNF Val66Met polymorphism impacts novelty seeking and harm avoidance. Neuropsychopharmacology 35: 1860-1867.
- Kraschewski A, Reese J, Anghelescu I, et al. (2009) Association of the dopamine D2 receptor gene with alcohol dependence: haplotypes and subgroups of alcoholics as key factors for understanding receptor function. Pharmacogenetics Genomics 19: 513-527.
- Munafo MR, Brown SM, Hariri AR (2008) Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry 63: 852-857.
- Schoots O, Van Tol H (2003) The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics J 3: 343-348.
- Shih JC (1991) Molecular basis of human MAO A and B. Neuropsychopharmacology 4: 1-7.
- Vanyukov MM, Maher BS, Devlin B, et al. (2004) Haplotypes of the monoamine oxidase genes and the risk for substance use disorders. Am J Medi Genetics Part B: Neuropsychiatric Genetics 125: 120-125.
Corresponding Author
Singh Ashok K, Associate Professor, Department of Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota, Twin Cities, St Paul, MN 55108, USA, Tel: +763-234-9655.
Copyright
© 2017 Singh AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.