Extensive Medullary Thyroid Cancer: Aggressive Surgery with Mediastinal Dissection is Worthwhile?
Abstract
The purpose of this study is to analyze the treatment results of patients with medullary thyroid cancer, focusing on surgical complications, biochemical cures and survival. It is a retrospective analysis of 18 patients of is ferral oncological hospital in a developing country. Ten patients underwent initial surgery, 7 developed complications, and 2 developed major complications. Five patients require a second procedure after the first surgery elsewhere, 2 developed complications, 1 developed major complication. Three denied further treatment after diagnosis was made. After a median follow-up of 35.5 months (1-108), 7 (39%) patients are alive without disease, 4 (22%) are alive with disease and 7 (39%) died.
Fifteen patients after the first surgery with curative purposes, 4 reached normal calcitonin levels and still without evidence of recurrence after a mean follow-up of 47 months (3-86 months); five patients needed a second surgery and two of the reached normal calcitonin levels; one is still under remission after 36 months, and another one died 60 months later.
Nine patients did not reach normal calcitonin levels after first surgery or reoperation, but on the postoperative images was no evidence of residual disease; 6 patients are alive after a mean follow-up of 55 (12-108) months.
In conclusion, biochemical cure for advanced disease is rare after surgery. However, long-term survival is possible when judicious surgery produces no evidence of residual disease by imaging.
Keywords
Thyroid cancer, Medullary thyroid cancer, Surgical morbidity, Calcitonin, Reoperation, Head and neck cancer
Introduction
Thyroid cancer represents 2.5% of malignant neoplasms in Mexico [1,2]. Medullary Thyroid Cancer (MTC) (3-5%) is the second one after differentiated carcinomas (80-90%) and has a less favorable outcome. Sporadic disease represents 75% of the cases, whereas hereditary accounts for the remaining.
Age and stage are major prognostic factors. Stage III and IV patients have worse prognoses than those at early stages [3]. In addition, every year of age at diagnosis increases the risk of dying by 5.2%. Patients with regional disease have 2.69 times more risk of dying than those with local disease, but distant disease has a relative risk that reaches 4.47. MTC cells do not concentrate radioactive iodine and are not sensitive to hormonal manipulation; therefore surgery is the most effective option for curative therapy, reduction in tumor burden, or effective palliation. Prognosis of MTC is related to the stage of disease, as well as the extent of initial surgery. Extensive surgery is related to better survival [4]; however, its precise extension is controversial [5], especially in patients with initial extensive disease, or with persistent/recurrent disease since tyrosine kinase inhibitors targeting vascular endothelial growth factor receptors and RET emerged as alternative [6] and because reoperation is associated with morbidity and uncertain oncologic results. In our country unfortunately, most of the patients presents with advanced disease, when prognoses is dismal, but they request treatment. Our aim was to assess the results of surgical treatment in patients with advanced disease, with special focus on surgical complications, biochemical cures and survival.
Patients and Methods
Patients diagnosed with medullary thyroid cancer between July 1st, 2006 and December 31st, 2012 was selected. Patients were analyzed for clinical features, treatment and outcome. Survival was calculated from diagnoses to death. Major morbidity required reoperation, extended hospitalization or resulted in death. IRB dispensed approval of this retrospective analysis because no intervention was introduced and personal data cannot be tracked to specific patients.
Preoperative evaluation
Patients with thyroid nodules underwent a neck Ultrasonography (US) and Fine Needle Aspiration Biopsy (FNAB) of suspicious thyroid and neck nodes. Patients with palpable neck nodes underwent a CT scan of neck, thorax and upper abdomen. Serum CEA and calcitonin measurements were taken in all patients. Vocal cord mobility evaluation was mandatory. Patients with persistent high levels of calcitonin after surgery underwent a new complete evaluation; if complete resection seemed possible, reoperation was considered.
Statistics and definitions
We analyzed the demographic data and the outcome of patients. Pathologic material was revised in order to corroborate the diagnosis. Descriptive statistics were used in order to show results. Overall survival was defined as the time interval between medullary cancer diagnosis and the last follow-up visit to the hospital or death of the patient. Survival curves were estimated using the Kaplan-Meier Method. The Log-rank test was used to compare survival curves.
Results
Three hundred eighty one cases of thyroid cancer were identified, 24 corresponded to medullary carcinomas (6.3%). Six patients with incomplete files were excluded, 18 patients remained: 12 women and 6 men (ratio 2:1), with a mean age of 40 years (15-70). Mean age of women was 44.5 years (23-70) and 32 years for men (15-50), (p < 0.05). There were two cases of MEN 2B corroborated by molecular diagnoses, and 2 with MEN 2B and 2 with MEN 2A by clinical characteristics. All patients were index cases and no detected by molecular screening. Seventeen out of 18 had advanced disease (corresponding to T1-T4 with N1a or N1b, or M1 disease according AJCC, 2010). All patients were treated 2 months before diagnoses or referral (Table 1).
Treatment
Ten patients were submitted to initial surgery with therapeutic purposes. All had palpable disease in thyroid, neck or both. After imaging, 5 had a thyroid-confined tumor, 4 had a thyroid tumor with bilateral positive nodes. Of them, three obviously positive neck nodes, and 1 had a thyroid tumor, bilateral neck and mediastinal obviously patients underwent total thyroidectomy, bilateral and central neck dissection; 5 patients underwent total thyroidectomy, bilateral neck, mediastinal and central neck dissections, 1 had a total thyroidectomy with central neck dissection, and 1 patient underwent a total thyroidectomy only (Table 2).
Six additional patients were previously treated elsewhere resulting in persistent disease and subsequently they were referred. We reoperated four of them; one declined surgical treatment after total thyroidectomy, and another one after hemithyroidectomy and tracheotomy. In our institution, 1 additional patient was previously submitted to hemithyroidectomy due to a "Follicular neoplasia", but a medullary thyroid cancer resulted after definitive report. Thus five additional patients were reoperated.
Among reoperated patients, two had already undergone a total thyroidectomy, bilateral neck and central neck dissections (A GROUP) and 3 patients had undergone a form of thyroidectomy (B GROUP). Eight and nine months after initial treatment elsewhere, both patients of A GROUP underwent a mediastinal and a selective neck dissection due to high calcitonin levels, metastatic neck nodes and suspicious mediastinal nodes without distant disease. Three patients of B GROUP underwent bilateral neck and central neck dissections with or without a complementary thyroidectomy; two of them also underwent mediastinal dissection. Information about surgical morbidity was scant in previously treated patients (Table 2).
Finally, three patients were amenable only to palliative treatment. One patient was diagnosed by biopsy of a neck node, further study showed distant metastasis and the patient declined further treatment. Another patient underwent elsewhere a hemithyroidectomy and tracheotomy with unresectable residual macroscopic disease in the neck; he died 6 months later due to disease progression. The last patient underwent a total thyroidectomy with total laryngectomy with en-bloc resection and bilateral neck dissection for airway obstruction due to bilateral recurrent laryngeal nerve paralysis and invasion of cricoid with known distant metastases; he died due to progression, with a pharyngocutaneous fistula (Table 2).
All patients submitted to surgery in our institution showed metastatic nodes in dissected neck and at mediastinal levels. Six patients showed extranodal disease, extrathyroidal extension, or both. Five of them received postoperative radiotherapy.
Surgical morbidity
From 10 patients with primary surgery only 7 (70%) developed complications. Two of 3 patients with a central neck dissection without mediastinal dissection developed morbidity: 1 dysphonia, dysphagia and aspiration that improved with rehabilitation, and 1 developed a chylous fistula that required a muscle flap for closure.
From 5 patients with mediastinal dissection, 4 developed complications, 2 transient hypocalcemia; 1 permanent hypoparathyroidism, and 1 died in the postoperative period due to a neck hemorrhage. Complications were attributable to the cervical component of surgery. Morbidity was significant, 2 of 10 patients initially operated by us developed major complications: a chylous fistula, which required reoperation, and one died from a hemorrhage at the beginning of the period under review.
From 5 reoperated patients, 2 (40%) developed surgical complications; one a chylous fistula that healed spontaneously; this patient had previous dysphonia and dysphagia due to recurrent laryngeal nerve paralysis; the other patient developed dysphonia due to vocal cord paralysis; both underwent a bilateral neck and mediastinal dissection, one of them with additional central neck dissection (Table 2).
Additional treatment
Five patients received postoperative radiotherapy as a result of extrathyroidal, extranodal extension or macroscopic residual disease, 2 are alive with disease after 108 and 36 months. In addition, 1 received radiotherapy to spine after relapse; the patient is alive with elevated but stable calcitonin levels after 87 months. One case required tracheotomy 4 years after initial treatment because of bilateral vocal cord paralysis secondary to local recurrence. The patient additionally required bronchial dilatation due to compressive mediastinal nodes.
Survival
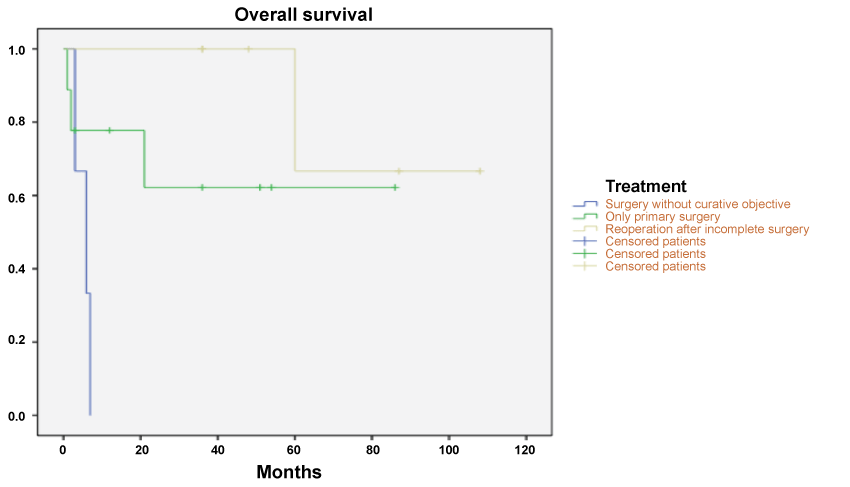
Overall, after median follow-up of 35.5 months (1-108), 7 (39%) patients are alive without disease, 4 (22%) are alive with disease and 7 (39%) died with disease. Survival was higher in previously treated and reoperated patients than those with only initial surgery (Log Rank Test: p = 0.011; Figure 1).
Calcitonin levels
Preoperatory calcitonin was recorded in 15 patients. Mean preoperative level was 5,996 pg/ml (26-50,000). Postoperatory calcitonin was measured two months after the surgery in 14 patients, with a mean of 685 pg/ml (0.3-4990). Only 4 patients reached normal values (< 10 pg/ml) after initial surgery and are alive without disease, with a mean follow-up time of 47 (3-86) months. All of them had lower preoperative calcitonin levels (315, 42, 30 and 200 pg/ml) when compared with the remaining patients. Two additional patients reached normal levels after reoperation, but 1 died after 60 months due to local and distant recurrence. However, 6 (42%) patients with elevated calcitonin after surgery but without measurable disease by imaging after definitive surgery are also alive after a mean follow-up of 55 (12-108) months, even with preoperatory calcitonin levels as high as 3550, 4990 and 10364 pg/ml (mean 4077, range 182-10, 364) (Table 3). Finally, 7 of 18 (39%) patients died after a mean follow up of 14.2 months (0-60). One of them was a surgical death, and the death of the remaining patients was due to disease progression.
Discussion
Surgery is keystone in the treatment of MTC, however surgery extent remains controversial in patients with primary local and regionally advanced disease and even more with recurrent disease [7]. Current ATA guidelines do not recommend routine bilateral lymph node dissection in primary disease, even with clinical evident disease in thyroid gland and ipsilateral neck, but states that a contralateral neck disection should be considered if basal calcitonin is greater than 200 pg/ml [8].
Whereas, Chen advocates aggressive surgery even with recurrent disease, when cure is less probable but adds: neck reoperations are associated with significant risks and reoperation should only be pursued if there is significant likelihood of benefiting the patients. Such benefits include achieving locoregional control or alleviating symptoms such as tracheal or esophageal compression and pain. Therefore, if patients develop symptomatic locoregional recurrence, even in the setting of metastatic disease, then they should be offered surgical resection when feasible [9].
Although with controversies, when primary tumor without metastatic neck nodes is present, a total thyroidectomy and central neck dissection are performed [10]. When thyroid tumor is larger than 2 cm, an ipsilateral selective neck dissection (II-V) is added due to risk of node metastasis. When nodal metastases in neck or mediastinum are demonstrated, the best local control is obtained with a bilateral neck dissection, but mediastinal dissection remains controversial [11].
Two months after surgery calcitonin is measured, if undetectable, patient is observed but with increased values reoperation is considered when previous surgery was incomplete. However, reoperation rarely produces normal values of calcitonin [12] and it is unknown its impact on survival. Clinical relapse is unusual when postoperative calcitonin values normalized, but patients who undergo surgical resection, even with distant metastasis, have better survival [13], and patients with high postoperative calcitonin values without noticeable disease by imaging may reach significant survival [14,15].
Thus, reoperation seems justifiable when calcitonin remains elevated after incomplete surgery, especially in absence of distant disease, but this must be tailored with potential morbidity. Central neck dissection is associated with recurrent laryngeal nerve injury and permanent hypoparathyroidism; meanwhile superior mediastinal dissection can produce hemorrhage, chylous leak, nerve injury and sternotomy dehiscence. Mediastinal dissection involves thymus resection along with adipose and lymphatic tissue up to the bifurcation of the trachea (metastatic low paratracheal nodes reaches 21%) [16] but it has been stated that less than 10% of patients with metastatic mediastinal nodes are cured.
In our experience, nine of 15 patients submitted to surgery with curative intent developed complications, 3 with major ones and one died. Two out of ten (20%) complications in the group of initial surgery only extended the hospitalization and one of them required reoperation, while one patient died from neck hemorrhage three days after surgery. From previously treated group one out 5 (20%) patients developed a chylous fistula that prolonged hospitalization. This suggests that reoperation is not significantly more morbid than the novo surgery.
In addition, in our series, 9 patients underwent a mediastinal dissection; all of them with proven disease and 6 are alive after a median of 53.5 (3-108) months. It is remarkable that mediastinal dissection was not associated with significant morbidity; thus, mediastinal dissection must be performed if the patient has obvious nodal disease and a complete resection with low morbidity can be done [17]. In addition, we propose that it should be strongly considered when there is obvious nodal disease in the neck. Elective mediastinal dissection is supported because: 1) The superior mediastinal metastasis rate is 2.6% in cN0 patients but 46.2% in CN+ patients [18], 2) In our series all patients had pathologic nodal disease at all dissected levels, including mediastinum, 3) Specific morbidity of mediastinal dissection was low, and 4) Of the outstanding survival of dissected patients.
It has been emphasized that patients with proven nodal disease at presentation or serum calcitonin levels higher than 1000 pg/ml before reoperation are rarely cured [11,19,20], but survival can be long with complete macroscopic resection. After a median follow-up of 35.5 months 7 (39%) patients are alive without disease, 4 (22%) are alive with disease and 7 (39%) died from the disease, including 3 with metastatic disease at diagnosis. In our experience, only 6 out of 15 patients reached biochemical cure, 4 out of 10 after initial surgery and 2 out of 5 after reoperation, but the mean survival after reoperation is 61 months (12-108), even better than the survival of patients with biochemical cure after initial surgery: 47 (3-86 months).
In our experience, all biochemical cures had initial low calcitonin levels (less than 400 pg/ml), but some with higher levels achieved prolonged survival after surgery; therefore, high isolated calcitonin levels must not preclude operation. Among patients with clinical and voluminous disease, only 33% reached biochemical cure, but we think surgery must be considered if it can be performed with low morbidity and can benefit the patient postponing progression or alleviating symptoms such as tracheal or esophageal compression.
Recently, Two Tyrosine Kinase Inhibitors (TKIs), Vandetanib and cabozantinib, were approved for use in patients with advanced, metastatic or progressive MTC, but its efficacy is questioned because toxicity and costs. Both drugs do not prolong survival or improve symptoms, despite a favourable effect on tumour imaging and certain laboratory parameters [21].
Preoperatory diagnosis of MTC is mandatory. Reoperations resulted in incomplete surgeries because of missed preoperative diagnoses. It is mandatory the preoperative determination of calcitonin in any patient with a suspicious thyroid nodule, since calcitonin is a sensitive and specific test [22], even more sensitive than standard cytology, and represents a cost-effective strategy [23] that promotes complete surgeries and better survival [24].
Our study has obvious limitations. It is retrospective with a limited number of patients and a relative short follow-up; however, surgical morbidity is assessed shortly after surgery and biochemical cure is a valid subrogate of final outcome. Finally, the mean follow-up is 36 months, but deaths occurred long time before our overall mean follow-up. We think our results and conclusions can be useful in countries or similar institutions where patients are diagnosed with advanced diseases.
Conclusion
Biochemical cure is infrequent in patients with obvious nodal disease. However, long survival is feasible after extensive surgery, if surgery results in no-measurable disease by imaging studies. Reoperation must be strongly considered, including mediastinal dissection, especially when surgery can be performed with low morbidity.
Conflict of Interest Statement
Nothing to declare.
References
- Ferly J, Soerjomataram I, Ervik M, et al. (2012) Cancer incidence and mortality worldwide: IARC cancer base lyon, france: International agency for research on cancer.
- Bray F, Ren JS, Masuyer E, et al. (2008) Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 132: 1133-1145.
- Pelizzo MR, Boschin IM, Bernante P, et al. (2007) Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol 33: 493-497.
- Roman S, Lin R, Sosa JA (2006) Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107: 2134-2142.
- Siironen P, Hagstrom J, Maenpaa HO, et al. (2016) Lymph node metastases and elevated postoperative calcitonin: Predictors of poor survival in medullary thyroid carcinoma. Acta Oncol 55: 357-364.
- Hadoux J, Pacini F, Tuttle RM, et al. (2016) Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol 4: 64-71.
- Maze H, Sippel RS (2012) Surgical management of medullary thyroid cancer. Minerva Endocrinol 37: 329-334.
- American Thyroid Association Guidelines Task Force, Kloos RT, Eng C, et al. (2009) Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19: 565-612.
- Chen H, Roberts JR, Ball DW, et al. (1998) Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg 227: 887-895.
- Lupone G, Antonino A, Rosato A, et al. (2012) Surgical strategy for the treatment of sporadic medullary thyroid carcinoma: our experience. G Chir 33: 395-399.
- de Groot JW, Links TP, Sluiter WJ, et al. (2007) Locoregional control in patients with palpable medullary thyroid cancer: results of standardized compartment-oriented surgery. Head Neck 29: 857-863.
- Malgorzata Wierzbicka, Edyta Gurgu, Elzbieta Wasniewska Okupniak, et al. (2014) The feasibility and efficacy of secondary neck dissections in thyroid cancer metastases. Eur Arch Otorhinolaryngol 271: 795-799.
- Nazanene H Esfandiari, David T Hughes, Huiying Yin, et al. (2014) The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metab 99: 448-454.
- van Heerden JA, Grant CS, Gharib H, et al. (1990) Long-term course of patients with persistent hypercalcitoninemia after apparent curative primary surgery for medullary thyroid carcinoma. Ann Surg 212: 395-400.
- K Pilaete, P Delaere, B Decallonne, et al. (2012) Medullary thyroid cancer: prognostic factors for survival and recurrence, recommendations for the extent of lymph node dissection and for surgical therapy in recurrent disease. B-ENT 8: 113-121.
- Liu J, Xu ZG, Wang XL, et al. (2007) Surgical treatment of thyroid carcinoma with the upper mediastinal metastasis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 42: 277-280.
- Machens A, Gimm O, Ukkat J, et al. (1999) Repeat mediastinal lymph-node dissection for palliation in advanced medullary thyroid carcinoma. Langenbecks Arch Surg 384: 271-276.
- Yan D, Zhang B, Li Z, et al. (2015) Cervical lymph node metastasis in medullary thyroid carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 50: 290-294.
- Larin OS, Cheren'ko SM, Nechai OP, et al. (2012) Efficacy of surgical treatment of medullary thyroid carcinoma in patients with regional and distant metastases. Klin Khir 5-8.
- Machens A, Dralle H (2013) Benefit-risk balance of reoperation for persistent medullary thyroid cancer. Ann Surg 257: 751-757.
- (2016) Cabozantinib (COMETRIQ0). In medullary thyroid cancer: more harmful than beneficial, as is vandetanib. Prescrire Int 25: 11-13.
- Hasselgren M, Hegedus L, Godballe C, et al. (2010) Benefit of measuring basal serum calcitonin to detect medullary thyroid carcinoma in a Danish population with a high prevalence of thyroid nodules. Head Neck 32: 612-618.
- Cheung K, Roman SA, Wang TS, et al. (2008) Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocrinol Metab 93: 2173-2180.
- Elisei R (2008) Routine serum calcitonin measurement in the evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab 22: 941-953.
Corresponding Author
Martín Granados García, MSc, MD, Head and Neck Cancer Department, Instituto Nacional de Cancerología, Mexico, Tel: +56280400-60530.
Copyright
© 2017 García MG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.