The Recultivation of the Soils, Contaminated with Oil, Petrol and Diesel Fuel, with the Help of Earthworms Dendrobena veneta and the Complex of Microorganisms
Abstract
The study was performed of the bioremediation efficiency of the soil contaminated with oil (20 to 100 g/kg), petroleum (20 to 60 g/kg) and diesel fuel (20 to 40 g/kg) with the help of earthworms Dendrobena veneta in the presence of bacteria Pseudomonas, nitrogen fixing bacteria Azotobacter and Clostridium, yeasts Saccharomyces, fungi Aspergillus and Penicillium, as well as Actinomycetales, all being components of biopreparation Baykal-EM. It was demonstrated that in oil-contaminated soil, the content of hydrocarbons decreased by 95% after 22 weeks in the presence of worms and bacteria. The microbiological preparation introduction increased the earthworms survivability in oil-contaminated substrate. The microbiological preparation introduction, therefore, allowed to use Dendrobena veneta for soil recultivation with higher oil concentrations that was impossible in the past. In petroleum-contaminated soil the content of hydrocarbons decreased by 97% after 22 weeks months. The presence of the diesel fuel in the amount of 40 g per 1 kg soil had an acute toxic effect and caused the death of 90% earthworm species in 14 days. Bacteria introduction enhanced the toxic effect of the diesel fuel and resulted in the death of 100% earthworms after 7 days. All studies demonstrated high survivability level for D. veneta in oil contaminated samples with added biopreparation Baykal EM-1, though in the field study the level was lower due to the substrate composition, and absence of additional nutrients and aeration. The oil content decreased more intensely in the soil samples containing earthworms as compared to the samples without earthworms. The decomposition efficiency and the rate of oil hydrocarbons removal depended on the oil content and the presence or absence of bio Baykal EM-1. The highest decomposition rate was registered in the sample with oil concentration of 40 g/kg. The obtained results are statistically important and demonstrate that microorganisms of biopreparation Baykal EM-1 have a positive effect on the earthworms' survival, their reproductive potential, and hydrocarbons degradation.
Keywords
Bioremediation, Earthworms, Oil
Introduction
The earthworms are known to enhance the process of contaminants removal from the soil. The earthworm changes physical and chemical properties of the soil by mixing it with organic substances through burrowing living habits thus improving the aeration and making contaminants accessible to the microorganisms [1]. The presence of the earthworms in soil contaminated with various organic compounds proves that the worms can survive in soils with pesticides, herbicides, polycyclic aromatic hydrocarbons (PAHs), polychlorinated Biphenyls (PCBs), and oil at low concentrations of contaminating substances [2].
Remediation of the soils contaminated with hydrocarbons is based on the chemical treatment or physical removal of the contaminants, but lately biostimulation, bioaugmentation and phytoremediation are used more and more often since they have less destructive effect on the environment [3,4]. The term 'vermiremediation' is applied to usage of the earthworms in contaminants removal from the soil [5] and enhancement of PAHs compounds dissipation. Some authors reported positive effect of the earthworms on the removal of the contaminants such as oil, PAHs, PCBs, pesticides, and heavy metals [1,6-9].
Thus, the earthworms facilitate and enhance the contact between the contaminants and soil microorganisms [10]. Lumbricus rubellus, Dendrobena octaedra and Aporrectodea caliginosa [11] are the most common cultivated species in soils, contaminated with heavy metals and waste sites (ash, sludge).
Oil contamination of the soil is the most spread in Russia and whole world. The earthworms are proven to be able to lower the concentration of the oil in the soil [1]. М Whitfield-Aslund, et al., observed that oil-contaminated soils are not toxic for the earthworms, 90% survival was registered at low oil concentrations up 25 g/kg, but the earthworm reproductive function disorder was also noted [12].
Edwards CA, Bater JE, noted that Dendrobena veneta has been used in agriculture for a long time. For the last 25 years the scientists of the Rothamsted Experimental Station investigated many aspects of the earthworm's application in soil recultivation and melioration. Dendrobena veneta is used in organic waste decomposition, and in recycling of animal and plant waste, sewage, and agricultural, household, urban and industrial sources [13]. There is no data on Dendrobena veneta usage in oil contaminated recultivation in scientific literature.
Viuoen SA, Reinecke AJ, Hartman L, described lifecycle of Dendrobena veneta proceeds at 15 ℃ and ends in 100-150 days, but at 25 ℃ the maturing progresses faster, the earthworms produce cocoons earlier and the number of cocoons per container is higher than at 15 ℃. Cocoons incubation period is shorter than at a higher temperature [14].
There are a number of studies on the use of earthworms to accumulate heavy metals from the soil. There are data on Dendrobena veneta use in lead removal from the soil with high concentration of lead and cadmium [15]. The information on heavy metals kinetics in the earthworms Dendrobaena veneta was accumulated. Marinussen MP, et al. made some tests on metals accumulation and removal in laboratory conditions using the soils contaminated with the heavy metals (Cu, Pb, Zn) [16]. The worms were incubated for 28-112 days in the soil contaminated with heavy metals (242 mg/kg of Cu, 109 mg/kg of Pb, 72 mg/kg of Zn). On day 112, an unexpected increase of Cu and Zn concentrations in the worm's tissue was noticed [16]. There are a small number of works on the use of worms Dendrobaena veneta for remediation of oil-contaminated soils.
Erlacher, et al., studied the influence of various oil concentrations on survivability of Dendrobena hortensis. At concentration 823 mg/kg the mortality of Dendrobena hortensis rose up to 100%. But after amendment with organic substrate, the mortality did not exceed 60% at higher oil concentrations from 1059 to 2241 mg/kg [17]. Hickman ZA and Reid noticed the decrease of 3-methylchlorantrene concentration in soil D. veneta cultivation [18].
Hickman ZA and Reid BJ studied the effect of compost and earthworms Dendrobena veneta on the level of hydrocarbon catabolism in PAH-contaminated soil. The survival of the earthworm D. veneta was studied in the soil contaminated with PAHs 10 g/kg for 84 days. It was noticed that the earthworm's survivability was the highest in the sample with compost (70%) and it was low in contaminated soil (13.2%). Compost addition resulted in a higher earthworm's survivability with the increased amount of compost (р < 0.05). The survival was 23% and 64% in soil samples with compost proportions 1:0.5 and 1:2, respectively. Hydrocarbons dissipation was observed for 84 days in the samples with compost addition in proportions 1:0.5 and 1:2 in the presence of D. veneta a without them. PAHs in concentration of 10 g per 1 kg of soil were introduced. The version without earthworm and compost had 70% of PAHs after 84 days, and the version with the earthworms had 65% of PAHs. The variant with compost (1:0.5) but without the earthworm had PAHs residual concentration of 40%, and the variant with the earthworm had 18%. The residual concentration of PAHs was 20% in the variant with the compost (1:2) and without the earthworms, and it was 8% in the variant with the earthworms. Compost and earthworms in PAHs-contaminated soil increases catabolic activity greatly [10,19].
Dendrobaena veneta can consume a wide range of organic waste. Rorat A and Kacprzak M, 2013 studied the earthworm survival in soil with Municipal Waste Sludge (MWS). There were versions with 0%, 25%, 50% and 100% of waste sludge. Three juvenile species and two cocoons were found in the variant with 0%, 2 cocoons were in the variant with 50%, 44 cocoons and 41 juvenile species were in the variant with 25%, and all earthworms died in the variant with 100%. Maximal earthworm biomass (1.5 g) was at MWS concentration of 25% [20].
Microorganisms are able to enhance the hydrocarbons biodegradation rate [21,22]. Moreover, Shuttleworth KL, Cerniglia CA, noted PAHs biodegradation rate varies greatly and depends not only on polycyclic aromatic hydrocarbons structure, but on soil structure determining bioaccessability and soil microbial community's composition and activity as well [23]. Singleton, et al., studied bacteria groups present in the earthworm intestines [24]. Some bacteria such as Pseudomonas, Alcaligenes and Acidobacterium ensure degradation of hydrocarbons and other organic compounds [25,26]. Besides that, fungi such as Penicillium, Mucor and Aspergillus, found in the earthworm intestines [27] and Penicillium fungi can dissipate PAHs [26]. Aerobiotic and anaerobiotic bacterial communities were found in the intestines of E. fetida [28]. Hong, et al., found the following types of bacteria in E. fetida: Aeromonas, Bacillus, Photobacterium, Pseudomonas, Clostridium, Cellulosimicrobium, and Streptomyces. Khalid A, Arshad M, Crowley D E, Tiquia S M, Wu X, et al., noted, that some of bacteria are able to degrade contaminants and PAHs, for instance, Bacillus, Clostridium, Pseudomonas, Streptomyces and Shewаnellа [29-31]. The earthworms encourage hydrocarbons concentration decrease in soil through soil microorganism's stimulation. The earthworms are known to regulate the microbial population consuming large amounts of soil [32].
Therefore, the undertaken analysis of the publications shows that the information on application in oil-contaminated soil recultivation is insufficient. The earthworms D. veneta were used in oil-contaminated soil bioremediation at hydrocarbons concentration of no more than 10 g per 1 kg of soil, and it was proved to me an efficient method. Taking into consideration the abovementioned, the present research is aimed to study oil-contaminated soil bioremediation with the help of D. veneta earthworms in the presence of bacteria such as Pseudomonas, nitrogen fixing bacteria Azotobacter and Clostridium, yeasts Saccharomyces, fungi Aspergillus and Penicillium as well as Actinomycetales and to evaluate the survivability of D. veneta earthworms in soil contaminated with various concentrations of oil (from 20 g/kg to 100 g/kg), petroleum (20-60 g/kg) and diesel fuel (20-40 g/kg).
Material and Methods
Earthworm species
Earthworms Dendrobena veneta. They are usually found near human habitats, in gardens, vineyards, forests, and in high mountains. Worm mean mass was 0.9 -1.42 g. Only the adult (clitellets) earthworms were used in the experiment. The adult earthworms were purchased from the Yermak farm (Saratov, Russia).
Biopreparation Baykal EM-1
Biopreparation Baykal EM-1 (NPO EM Center Ltd, Novosibirsk, Russia. License No. 226-19,156-1) served as a source of lactic acid and nitrogen fixing bacteria, and fungi. The microorganisms in biopreparation Baykal EM-1 were determined by MALDI-TOF (Matrix-assisted laser desorption ionization time-of-flight) mass spectrometry with the system VITEK MS. Microorganisms composition of biopreparation Baykal EM-1 was as follows: bacteria Paenibacillus pabuli, Azotobacter vinelandii, Lactobacillus casei, Clostridium limosum, Cronobacter sakazakii, Rhodotorula mucilaginosa, Cryptococcus albidus, yeast Saccharomyces, Candida lipolytica, Candida norvegensis, Candida guilliermondii, fungi Aspergillus and Penicillium, as well as Actinomycetales (КОЕ UFC = 2*1011 per mL).
The microorganisms identification with MALDI-TOF mass spectrometry
Biopreparation in the amount of 1.5-2 mL was taken from the vessel and centrifuged at 15,000 rpm for 20 min. After that the residual matter was resuspended in 1 mL of distilled water and centrifuged again at 5000 rpm for 10 min. Supernatant fluid was removed, and the residual matter was resuspended and centrifuged once more. Next, the chalky residue was collected, dissolved in 1mL of distilled water and centrifuged at maximum speed for 10 min, and then it was resuspended in 1 mL of 80% ethanol and centrifuged at 15,000 rpm for 10 min. The newly formed residue was dissolved in 15 μL of deionized water and 35 μL of formic acid. After adding 50 μL of acetonitrile, the sample was centrifuged at the maximum speed for 2 min. The resulting supernatant was applied to a chip and coated with 1 μL of matrix consisting of alpha-cyano-4-hydroxycinnamic acid in form of saturated solutions in the mixture of 50% acetonitrile and 2.5% trifluoroacetic acid (Bruker Daltonics, Germany). Mass spectrometric identification of the microorganisms was performed on mass spectrometer MicroFlex (Bruker, Germany). Each sample was tested 4 times. Spectra were registered automatically. The detection mode was standard MBT_FC. Spectra range started at 2-20 kDa. 240 spectra were obtained from each sample. Identification was performed by database Biotyper 3 (Bruker, Germany). Identification precision was almost 100% [33].
Soil substrate
All the experiments were carried out with the soil substrate represented by a sterilized meadow soil with the brand name "Living Earth (Terra Vita) Universal Nutrient Ground" that was manufactured by MNPP FART Ltd. The soil had the following characteristics: organic substance content 46%, pH 5.9-6.0, base exchange capacity 28-40 mg - eq per 100 g soil. Chemical composition of the soil: nitrogen (NH4 + NO3) content 150 mg/l, phosphorus (P2O5) content 270 mg/l, and potassium (K2O) content 300 mg/l.
Before the experiment, the soil was prepared in compliance with ISO (ISO 11268-1, 1993; ISO 11268-2, 1998). The soil was dried to a constant weight, sieved using 5 mm mesh filter, and homogenized by hand; this was followed by the addition of calcium carbonate to bring pH to 6.0 ± 0.5 and distilled water to obtain a soil moisture content of 60%. 1 kg of the prepared soil was placed in 2 L polypropylene containers. In the course of experiments, a water loss due to evaporation was monitored once a week, and the soil was humidified to bring the soil moisture content to 60%.
Three series of contaminated soil samples were prepared.
A series of oil contaminated soil samples was prepared. To obtain the samples, oil was added to the soil at concentration of 20,000-100,000 mg kg-1. The oil was obtained from the Samotlor (Russia) oil field, and had the following characteristics: relative density (ρ) 0.934, M = 367, V20 = 63.13, pour point 25 °C, the temperature of processing 22 °C, and flash point in closed crucible 120 °C. The composition of crude oil was as follows: 2.3% of paraffins, 0.96% of sulfur, 0.12% of nitrogen, 14% of sulfurous resin, 10% of silica resin, 1.36% of asphaltenes, 1.99% coking ability, 0.01% of ash, 0.01% of naphthenic acids, and 0.006% of phenols. The elemental composition was as follows: C - 85.9%, H - 12.93%, O - 0.15%, S - 0.92%, and N - 0.1% The petroleum with the label AI-92 produced by company Lucoil was introduced in the amount of 20-60 g/kg into the soil samples of the 2nd series. The petroleum characteristics were in compliance with GOST of Russia 51105-97 [34].
The diesel fuel with the label EURO GOST of Russia 52368-2005 (ЕН 590:2009) was introduced in the amount of 20-40 g/kg into the soil samples of the 3rd series [35].
Test protocols
1 kg of sterile soil was put into the 2 L plastic containers. 10 clitellets earthworms were added to each container. Over the entire experiment, the soil was humidified once a week by introducing 100 mL of distilled water into each container. Containers with the soil were covered by plastic cover with small-size air-holes and then sealed to prevent excessive moisture loss and earthworm escape. The earthworms were fed with 5 g of fresh grated potato once a week. Although D veneta exhibit the maximum activity at a temperature of 20 °C, all the earthworms were incubated at 15 ± 1 °C for 22 weeks because such temperatures are more typical for Siberia. The control soil samples and the experimental samples of contaminated soil with the introduced earthworms and biopreparation Baykal EM-1 were incubated for 22 weeks, from September 2013 to February 2014.
A model test species D. veneta. Adult earthworms (visible clitellum) ranging from 400 to 900 mg were used in all the experiments. Clitellets earthworms were weighed before the experiment. To measure the body weight, earthworms were sorted from the test soil by hand, washed with tap water, dried on absorbent paper, and then weighed in groups of ten on an electronic balance. The numbers of cocoons, adult and juvenile species were measured once in 2 weeks [36].
A series of oil contaminated soil samples was prepared. To obtain the samples, oil from the Samotlor (Russia) oil field was added to the soil at a concentration of 20-100 g/kg. (Table 1) shows the composition of soil samples used in the study. Oil was introduced as a contaminant into each experimental soil sample. Oil content varied from 20-100 g/kg. 10 adult earthworms were added into each container with contaminated soil, and some containers were supplemented with biopreparation Baykal EM-1 in the amount of 1 g/kg. Along with contaminated soil samples, control samples were prepared in each series of experiments; they were free of oil contaminant but had the earthworms and biopreparation Baykal EM-1 ((UFC) = 2*1011/mL).
To estimate the concentration of oil and organic substances in the soil, the sampling of oil contaminated soil was made according to GOST of Russia 28168, GOST of Russia 17.4.3.01, and GOST of Russia 17.4.4.02. The soil was ground in a mortar. 3-5 g sample of the ground soil was additionally milled to obtain the particle size smaller than 0.3 mm and then sieved using 0.25 mm screen [37-39].
Chemical analyses (total petroleum hydrocarbon (TPH) concentration)
To analyze the petrochemicals and organic substance content in the samples, the collection of the soil samples was performed according to GOST of Russia 54039-2010 Soil quality [40]. The measurements were made at room temperature (20 ℃). In order to wet Al2O3, 3 mL of carbon tetrachloride was poured into a glass column (1 cm in diameter) filled with alumina (a 5 cm layer). As soon as ССl4 was absorbed by alumina, a soil sample was added to the column. The soil was covered with 3-5 mm thick cotton wool, and carbon tetrachloride was poured in. An eluate of oil-contaminated soil dripped at the rate of 0.1-0.2 mL/min into a graduated cylinder mounted under the column. The first 3 mL of the eluate was discarded (to avoid errors) and ССl4 was poured into the column to obtain 10 mL of the eluate. The resulting eluate was poured into the cell of an IKH-025 IR spectrophotometer to determine the amount of mineral oil in the eluate at the wavelength of 3.42 nm. During the measurements, the instrument reading C was taken. C corresponded to the hydrocarbons content in the eluate (mg dm-3).
The mineral oil concentration in a sample (X gkg-1) was calculated by the formula
X = CVη/m∙1000,
where C is the concentration of mineral oil in a sample according to the instrument readings, mg dm-3;
V is the eluate volume, cm3;
m is the soil sample, g; and
η is the dilution ratio η = V2/V1, where
V1 is the eluate volume, cm3, and
V2 is the volume of ССl4 taken for the dilution, cm3.
Statistical analysis
All quantitative estimations of toxicity parameters were based on three replications, and the values were given as arithmetic average ± standard error. Software package STATISTICA 10 was used for statistic analysis of the material. Normality testing for quantitative characteristics distribution was carried out with the help of the Kolmogorov-Smirnov (K-S t), Lilliefors and Shapiro-Wilk (W) tests. The information was additionally processed with the methods of descriptive statistics. To evaluate the variance significance, Kruskal-Wallis (H) test and Mann-Whitney (U) test for independent groups were used. The relations were evaluated by correlation analysis with calculation of Spearman's rank correlation coefficient (rs).
Table 1 contains the list of the samples contaminated by oil, petroleum, and diesel fuel. Oil was introduced as a contaminant in the amount of 20-100 g/kg, petroleum was added in the amount of 20-60 ml/kg, and diesel fuel was in the amount of 20-40 ml/kg. Samples 1 and 2 were control ones and had no organic contaminants. For soil treatment 10 earthworms Dendrobena veneta and biopreparation Baykal-EM in the amount of 1 ml/kg were introduced into each sample.
Research Results
Experiment 1: Laboratory earthworm oil toxicity test
Total population of D veneta
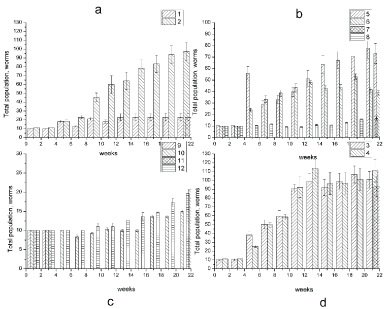
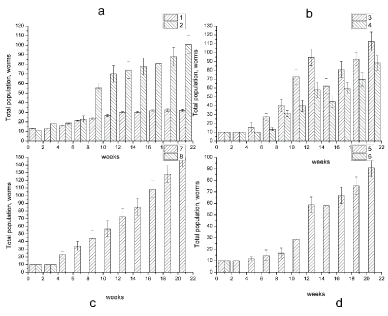
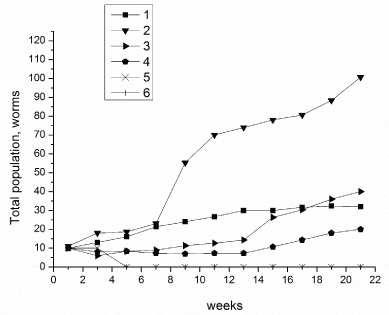
Figure 1 and Table 1 shows the changes in earthworm D. veneta amount in soil samples, contaminated with crude oil at concentration of 20-100 g/kg, after soil samples having been incubated for 22 weeks at 15-17 ℃. The numeration of the curves corresponds to the numbers of the samples in Table 1. As seen in Figure 1, after 22 weeks of incubation, control samples 1 and 2 containing 10 clitellets earthworms D. veneta (sample 1) and 10 clitellets earthworms D. veneta + microorganisms (biopreparation Baykal- EM) (sample 2) showed an increase in the total population of the earthworms with 33 species for sample 1 and 95 species for sample 2. As one can see in Figure 1, the addition of biopreparation Baykal EM to the soil (sample 2) accelerated the growth of earthworm's numbers in comparison with sample without biopreparation Baykal EM. The introduction of crude oil in to the soil at a concentration of 20-40 g/kg (samples 3-6, Figure 1) resulted in 80-100% survival of D. Veneta and a stable growth of the earthworm's population both in the presence and in the absence of the microbiological preparation. The earthworm's population after 22 weeks of incubation increased to 93 species for sample 3 and to 114 species for the sample with crude oil and biopreparation Baykal EM, i.e., by a factor of 10. As the petroleum content in the soil was increased to 40 g/kg (samples 5 and 6), the earthworm amount increase becomes lower than in samples 3 and 4 and equals 73 species in sample 5 and 40 species in sample 6 after a 22-weeks incubation. An increase in the petroleum content in the soil to 60-100 g/kg (samples 7,9,11, Figure 1), led to 100% death of all species in 3 days, which may be caused by chemical burns of the earthworms residing on the surface of contaminated substrate. To increase survivability of earthworms in the soils containing oil at a concentration of 60, 80 and 100 g/kg, soil samples 7, 9, and 11 were supplemented with 1 ml of biopreparation Baikal EM; after that, the oil-contaminated soil samples were held for 4 weeks. As seen in Figure 1, after a 4-week incubation the survivability of D. veneta rose to 60%. A further extension of incubation time from 7 to 22 weeks (Figure 1b) increased the number of earthworms in soil samples. According to Figure 1 an increase in oil content of the soil decreased the activity of earthworms, and after a 22-week incubation their number in soil samples 8, 10 and 12 containing 60, 80 and 100 g/kg of crude oil and biopreparation Baikal EM, respectively, was 20, 20 and 24 species.
Total productivity
As seen in Table 1, an increase in oil content of the soil resulted in the changes of D. veneta total productivity (the number of cocoons per container) in the samples of soil contaminated with crude oil at a concentration of 20-100 g/kg during the entire period of incubation, i.e. 22 weeks. In control sample (sample 1) the total productivity was 3.4 cocoons per container, after biopreparation introduction it increased to 15.4 cocoons per container. High total productivity of 15.3 cocoons was noticed for sample 3, containing crude oil in concentration of 20 g/kg. In sample 4 with oil concentration of 20 g/kg and biopreparation Baykal- EM the total productivity was lower and equaled 13.4 cocoons per container. The further increase of oil concentration to 40 g/kg (sample 5) resulted in a decrease of the total productivity дo 10.7 cocoons per container, and in soil with the biopreparation (sample 6) total productivity increased slightly and 6.8 cocoons were found. Further increase of crude oil content (Table 1) up to 60, 80 and 100 g/kg (samples 7, 9 and 11) total productivity lowered to 0, caused by the death of all adult earthworms. In samples 8,10, and 12, containing 60, 80 and 100 g/kg of crude oil and biopreparation Baykal-EM total productivity was 3.2, 2.5 and 2.5 cocoons per container, correspondingly. Data on the total and individual productivity of earthworms in oil contaminated soils after 22-week incubation are listed in Table 1.
Oil hydrocarbons decomposition
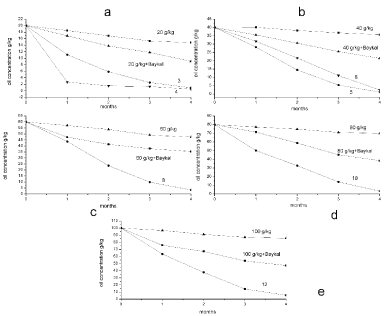
Figure 2 shows the dependence of oil content changes on the incubation time in contaminated soil samples with earthworms D. veneta and biopreparation Baykal- EM at the temperature of 15-17 ℃. Maximal incubation time was 22 weeks for all samples. Hydrocarbons content was determined with colorimetric method monthly. To analyze oil content changes in the course of incubation due to light hydrocarbons natural evaporation within 22 weeks, control samples of oil contaminated soil with hydrocarbons amount of 20, 40, 60, 80 and 100 g/kg (without worms) were prepared and investigated. Five control samples with oil concentration of 20-100 g/kg and biopreparation Baykal-EM (1 ml/kg) and without the earthworms were prepared. The petrochemicals content in contaminated samples with the earthworms was analyzed as well. The numeration of the samples corresponds to the sample numbers in Table 1.
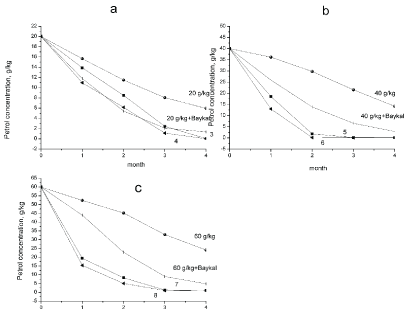
As Figure 2 shows, in control samples contaminated with oil, the petrochemicals content was changing slightly within the entire incubation period. Addition of biopreparation Baykal-EM to the soil samples results in more pronounced hydrocarbons removal. After 22 weeks of incubation, oil content decreased almost twice in the control sample with oil concentration of 20 g/kg (Figure 2) and biopreparation Baykal-EM. Addition of 20 g oil per 1 kg soil resulted in no significant concentration decrease in the control sample. After 5 months the concentration lowered to 16.73 g/kg. Soil bioremediation proceeds more actively in oil-contaminated samples containing the earthworms D. veneta with oil concentration of 20 g/kg. Hydrocarbons concentration in the soil samples with the earthworms lowered by 97-99% and was equal to 0.95 g/kg. Microbiological preparation amendment resulted in concentration decrease to 9.09 g/kg. Addition of biopreparation Baykal-EM with the earthworms D. veneta to the soil samples with 20 g/kg oil amendment resulted in concentration decrease to 0.5 g/kg. The addition of 40,000 g/kg oil decreased the TPH concentration to 35.7 g/kg while the addition of biopreparation Bayka-EM TPH concentration decreased it to 21.5 g/kg after 22 weeks. In the version with D. veneta, oil concentration lowered to 1.14 g/kg (97% efficiency), and in the sample with the biopreparation it lowered to 2.45 g/kg (93% efficiency).
The addition of 60 g/kg oil decreased the TPH concentration to 47.4 g/kg after 22 weeks. In the soil sample containing biopreparation Baykal-EM and 60 g/kg oil, the TPH concentration decreased to 33.5 g/kg after 22-week incubation, while in the soil sample with D. veneta and biopreparation Baykal-EM, the TPH concentration decreased by 3.3 g/kg (Figure 2d). The addition of 80 g/kg oil decreased the TPH concentration to 69.8 g/kg. Upon incubation of the soil sample containing 80 g/kg oil and Baykal-EM, the TPH concentration decreased to 38.7 g/kg. As for the samples with oil concentration of 80 g/kg and earthworms D. veneta along with biopreparation Baykal-EM, oil content decreased by 99% and equaled 3.6 g/kg (Figure 2c). The addition of 100 g/kg oil decreased the TPH concentration to 85.7 g/kg. Upon incubation of the soil sample containing 100 g/kg oil and Baykal-EM, the TPH concentration decreased to 47.7 g/kg (Figure 2e). An increase in the oil content to 100 g/kg hindered the soil sample bioremediation in the presence of earthworms and biopreparation Baykal-EM in soil the TPH concentration decreased by 86% and was equal to 5.5 g/kg.
Experiment 2: Laboratory earthworm petroleum toxicity test
Total population of D. veneta
As seen in Figure 3 and Table 2 after 22 weeks of incubation, control samples 1 and 2 containing 10 clitellets earthworms D. veneta (sample 1 and Figure 3) and 10 adult earthworms D. veneta + microorganisms (biopreparation Baykal- EM) (sample 2) showed an increase in the total population of the earthworms with 33 specimens for sample 1 and 95 species for sample 2. The introduction of petroleum into the soil at concentrations of 20 (samples 3-6, Figure 3) resulted in 100% survival of D. veneta and a stable growth of the clitellets earthworm's population both in the presence and in the absence of the microbiological preparation. As for sample 3 containing 20 g/kg of petroleum, the earthworms number increased to 112 species, and as for the sample with the petroleum and the biopreparation it rose to 88 specimens after a 22-week incubation. As the petroleum content in the soil was increased to 40 g/kg (sample 5), an increase in the number of earthworms was 126 after the incubation for 22 weeks. But introduction of the biopreparation Baykal- EM resulted in the death of all specimens in sample 6. An increase in the petroleum content in the soil to 60 g/kg (samples 7, Figure 3) led to a decrease in the total number of D. veneta. The total population was 146 specimens in soil sample 7 with the petroleum concentration of 60 g/kg, and with the microbiological preparation (sample 8) all earthworms died. Therefore, the presence of preparation Baykal- EM enhances the petroleum adsorption in the earthworm's digestive tract and causes an acute toxic effect.
Total productivity of D. veneta
As seen from Table 2, low total productivity was noticed in the control sample (sample 1) without petroleum, which was 3.4 cocoons per container. The introduction of the microbiological preparation (sample 2) increased the cocoon laying to 15.4 cocoons per container. The introduction of petroleum (20-60 g/kg) into soil enhanced the cocoon laying. The highest total productivity of 36 cocoons was registered in sample 7 containing petroleum in concentration of 60 g/kg without the microbiological preparation. For sample 3 (with petroleum concentration of 20 g/kg) and sample 4 (with petroleum and the biopreparation) the total productivity was 27 and 20 cocoons per container, correspondingly. The introduction of petroleum in concentration of 40 g/kg (sample 5) enhanced the cocoon laying and the total productivity became 23.8 cocoons per container. Although in samples 6 and 8 with the biopreparation Baykal- EM all earthworm specimens died.
Petroleum hydrocarbons decomposition
As Figure 4 shows, in control samples contaminated with petroleum, the petrochemicals content was changing slightly within the entire incubation period. Light petroleum fractions evaporation was registered in the soil without the earthworms. As a result, petrochemicals concentration decreased by 70% after 22 weeks of the incubation and was equal to 6 g/kg in the control sample with petroleum concentration of 20 g/kg and with biopreparation - 1.35 g/kg. Petrochemicals concentration decreased by 97% after 22 weeks of the incubation and was equal to 0.06 g/kg in the soil samples with petroleum concentration of 20 g/kg and the earthworms D. veneta, and in the presence of the microbiological preparation it was 0.02 g/kg (Figure 4a). As for the control sample with 40 g/kg petroleum (Figure 4b), petroleum content decreased by 62% and was 15 g/kg after 22 weeks of the recultivation and with biopreparation - 2.8 g/kg. The introduction of earthworm D. Veneta resulted in the decrease of hydrocarbons concentration by 97%, and it was equal to 0.16 g/kg and in the presence and in the absence of the microbiological preparation petroleum content decreased up to 0.09 g/kg. In the control sample, upon introduction of 60 g/kg petroleum, light fractions evaporation was 60%, resulting in the decrease of hydrocarbons concentration to 24 g/kg. Introduction of D. veneta earthworms led to the decrease of petroleum concentration to 1.7 g/kg (95% effectiveness), and after the microbiological preparation introduction it lowered to 0.9 g/kg (99% effectiveness).
Experiment 3: Laboratory earthworm diesel fuel toxicity test
Total population of D. veneta
Figure 5 and Table 3 shows the changes in earthworm D. veneta amount in the diesel-contaminated soil samples with the concentration of 20-40 g/kg, after the soil samples having been incubated for 22 weeks at 15-17 ℃. The numeration of the curves corresponds to the numbers of the samples in Table 1. As seen in Figure 5, the introduction of diesel fuel into the soil at concentration of 20 g/kg (samples 3, 4, Figure 5) resulted in 50% survival of D. veneta and stable growth of the earthworm's population. The earthworm's population after 22 weeks of incubation increased 3.5-fold and was equal to 36 specimens for sample 3, and it was equal to 18 specimens for the sample with diesel fuel (20 g/kg) and biopreparation Baykal- EM. An increase in the diesel fuel content in the soil to 40 g/kg (samples 5) led to the death of all specimen of D. veneta.
Total productivity
In sample 3 with diesel fuel concentration of 20 g/kg the total productivity was 6.2 cocoons per container, and after biopreparation Baykal-EM introduction (sample 4) the productivity decreased to 2.5 cocoons per container. The increase in diesel concentration to 40 g/kg (sample 5) caused the death of all earthworms. Therefore, one may conclude that D. veneta are not resistant to diesel fuel contamination and cannot be used for recultivation of diesel-contaminated soil. Data on the total and individual productivity of earthworms in diesel fuel contaminated soils after 22-week incubation are listed in Table 3.
Diesel fuel hydrocarbons decomposition
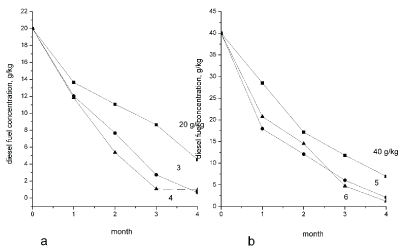
Figure 6 shows that hydrocarbons content changed significantly in control samples contaminated with diesel fuel during the entire incubation time. Light petroleum fractions evaporation was registered in the soil without the earthworms and with diesel fuel concentration of 20 g/kg. As a result, petrochemicals concentration decreased by 77% after 22 weeks of incubation and was equal to 4.5 g/kg. Petrochemicals concentration decreased by 97% after 22 weeks of incubation and was equal to 0.7 g/kg in the soil samples with earthworms D. veneta and diesel fuel concentration of 20 g/kg. The introduction of biopreparation Baykal-EM had no significant effect on soil bioremediation process. Petrochemicals content lowered to 1.01 g/kg and recultivation efficiency was 95%. As for the control sample with 40 g/kg diesel fuel (Figure 6), the hydrocarbons content decreased by 82% and was 7 g/kg. Soil bioremediation proceeds more intensely in the samples with the earthworms and diesel fuel concentration of 40 g/kg. With earthworms D. veneta introduction diesel fuel concentration lowered to 2.1 g/kg (95% effectiveness), and with the microbiological preparation the concentration decreased to 3.24 g/kg (92% effectiveness).
Discussion
High survivability of the earthworms was registered in our study at oil concentrations up to 50 g/kg (90-97%), probably caused by the soil substrate composition, including nitrogen (NH4 + NO3) content of 150 mg/l, phosphorus (P2O5) content of 270 mg/l, and potassium (K2O) content of 300 mg/l. Concentrations of oil and petroleum up to 50 g/kg had no adverse effect of survivability of D. veneta and stimulate cocoon laying and total population growth. Oil concentration more than 50 g/kg results in the death of earthworms D. veneta in 3-7 days. Microbiological preparation introduction improved the earthworms survival in oil-contaminated substrate. Consequently, microbiological preparation introduction allows to use D. veneta in recultivation of oil-contaminated oil at higher concentrations of oil, that being impossible so far.
Petroleum hydrocarbons concentration was considerably lowered in the soil with the earthworms in contrast to the soils without the earthworms. The efficiency and the rate of the oil degradation depend on the oil concentration. At introduction of low oil concentrations (20-40 g/kg), soil recultivation took 4 months, during which oil concentration decreased by 97-99%. The addition of the microbiological preparation had no significant effect on the oil degradation process. At introduction of high oil concentrations (60-100 g/kg), the oil degradation process took 5-6 months and called for compulsory addition of microbiological preparation. In vermicultivation process the oil content decrease by 90%.
Light petroleum fractions evaporation was registered in the soil without the earthworms. As a result, petrochemicals concentration decreased by 60-70% in 5 months. In all versions recultivation process took 3 months, and HC concentration decreased by 98-99%. Therefore, one may conclude that oil (at low concentrations) is not a toxic substance for the earthworms and it simulates the population growth of D. veneta. Geissen, et al., 2008 marked an increased death of E. fetida up to 70%-90% in soil contaminated with 2% of petroleum [41].
Diesel fuel has more acute toxic effect on D. veneta. The introduction of diesel fuel at concentration of 20 g/kg led to the death of 50% earthworms after 14 days of the experiment. The addition of microbiological preparation intensified the toxic effect of the diesel fuel. Diesel fuel concentration of 40 g/kg had an acute toxic effect and caused the death of 100% species after 14 days. Microbiological preparation increases the toxic effect of diesel fuel and resulted in 100% death after 14 days. Isabella Gandolfi and Matteo Sicolo noticed vast toxic effect of diesel light hydrocarbons on the earthworms, but the compost presence enhanced HC degradation. Compost introduction to diesel-contaminated soil resulted in diesel fuel remediation increase by four rings of PAHs and soil toxicity decrease, its effect on soil genotoxicity being relatively low [33].
We carried out statistical analysis of the obtained results. Verification of normality of distribution of quantitative traits was performed using the Kolmogorov-Smirnov, Lilliefors and Shapiro-Wilk (W). We are using normality tests (Lilliefors and Shapiro-Wilk tests) the resulting value was less (p < 0.05), therefore, an alternative hypothesis was formulated, i.e. the characteristic distribution differs from the normal one. At the initial stage of the investigation, the relations between soil contamination and the number of D. veneta were evaluated on all stages with Spearman's correlation analysis. When evaluating the results by the significance with Kruskal-Wallis criterion in several independent groups at soil contamination of 20 g/kg and 40 g/kg with and without the biopreparation, no statistically significant differences were found. The differences were found between the following samples: the control and the one with 20 g/kg oil and biopreparation Baykal-EM (H (3, N = 48) = 10.97 p = 0.011): the control and the one with 40 g/kg oil (H (3, N = 48) = 9.637 p = 0.0219); the control and the one with 40 g/kg and the biopreparation Baykal- EM (H (3, N = 48) = 11.29 p = 0.01). At oil concentration of 60 g/kg-100 g/kg there are differences between the control sample and the one with 100 g/kg oil (Н) H (4, N = 60) = 15.44 p = 0.0001, while in other cases the decrease in the earthworms' amount was registered at concentration increase.
When evaluating the dependence of the earthworms' numbers in the samples without the biopreparation incubated for 22 weeks, the dependence of the numbers on the oil concentrations was noticed, i.e. the increase in oil concentration resulted in the decrease of the earthworms' number. The earthworms died at increased oil concentrations of 60-100 g/kg without the biopreparation. The contribution of soil contamination was more than 70%. The obtained model is highly significant, R = -0.85. When evaluating the dependence of the earthworms' numbers on the samples with the biopreparation incubated for 22 weeks, the dependence of the numbers on the contamination level was noticed, i.e. the increase in soil contamination results in the decrease of the earthworms' amount. At increase in oil concentration to 60-100 g/kg and the addition of biopreparation Baykal EM, the earthworms' survival was 50%. The contribution of soil contamination with oil was more than 78%. The obtained model is highly significant, R = -0.82.
In evaluating the results of significance of differences criterion Kruskal -Wallis several independent groups of soil contamination with petrol 20-60 g/kg in introducing microbial drug, and without him was not statistically significant differences (Kruskal-Wallis (N) (7, N = 80) = 11.446 p = 0.1203) (median test = 62.00; Chi-square = 6,400 df = 7, p = 0.4939).
Conclusions
The method of oil contaminated soil bioremediation with oil concentrations up to 100 g/kg, petrol concentrations up to 60 g/kg and diesel fuel concentrations up to 40 g/kg in the presence of bacteria Paenibacillus pabuli, Azotobacter vinelandii, Lactobacillus casei, Clostridium limosum, Cronobacter sakazakii, Rhodotorula mucilaginosa, Cryptococcus albidus, yeast Saccharomyces, Candida lipolytica, Candida norvegensis, Candida guilliermondii, fungi Aspergillus and Penicillium as well as Actinomycetales (KOE UFC) = 2*1011 per mL) and D. veneta is proposed. Decontamination and the ecological functions recovery of the oil- and petrochemicals-contaminated substrates is carried out due to the following method: the substrate is treated with biopreparation, ploughed and put under the steam for 1 month for recultivation; afterwards, the earthworms are added in the amount of 1000 per 1 m2 of the soil; and then, cow dung is added as a nutritional medium in the amount of 1 t per 1 ha. During the experiment lasting for 5 months, a significant lowering (by 95-97%) in hydrocarbons content was registered in the soil with the earthworms and the biopreparation. The advantages of the method are as follows:
1. It has high efficiency compared to the other methods. Petrochemicals concentration in soil lowered by 97-98% when both the biopreparation and the earthworms were used, which was proven by the laboratory tests.
2. Petrochemicals concentration lowered by 90-98% after 120 days of vegetational season.
3. Hydrocarbons bioremediation proceeds at a wide temperature range (from +10 to +30 ℃).
4. The earthworms can function properly at the temperature range of +5 ... +30 ℃.
5. Unlike mechanical methods, it has no negative impact on natural ecosystems and no soil stirring or mixing is needed.
6. It can be used to decontaminate the soil onsite.
7. Both the biopreparation and the method are harmless for environment and human beings.
The disadvantages of the method are as follows
1. Recultivation period lasts for 120 days.
2. It depends on the weather conditions.
3. It cannot be applied in a climate of Extreme North.
References
- Eijsackers H, van Gestel C, De Jonge S, et al. (2001) Polycyclicaromatic hydrocarbons-polluted dredged peat sediments and earthworms: A mutual interference. Ecotoxicology 10: 35-50.
- Jacobo Rodriguez-Campos, Luc Dendooven, Dioselina Alvarez-Bernal, et al. (2014) Potential of earthworms to accelerate removal of organic contaminants from soil: A review. Applied Soil Ecology 79: 10-25.
- Hamdi H, Benzarti S, Manusadzianas L, et al. (2007) Bioaugmentation and biostimulation effects on PAH dissipation and soil ecotoxicity under controlled conditions. Soil Biology and Biochemistry 39: 1926-1935.
- Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Reviews in Environmental Science and Bio/Technology 9: 215-288.
- Sinha RK, Bharambe G, Ryan D (2008) Converting wasteland into wonderland by earthworms a low-cost nature's technology for soil remediation: A case study of vermiremediation of PAHs contaminated soil. The Environmentalist 28: 466-475
- Binet F, Kersanté A, Munier-Lamy C, et al. (2006) Lumbricid macrofauna alter atrazine mineralization and sorption in a silt loam soil. Soil Biology and Biochemistry 38: 1255-1263
- Contreras-Ramos SM, Álvarez-Bernal D, Dendooven L (2008) Removal of polycyclic aromatic hydrocarbons from soil amended with biosolid or vermicompost in the presence of earthworms (Eisenia fetida). Soil Biology and Biochemistry 40: 1954-1959.
- Ma WC, Immerzeel J, Bod J (1995) Earthworms and food interactions on bioaccumulation and disappearance in soil of polycyclic aromatic hydrocarbons: Studies on phenanthrene and fluoranthene. Ecotoxicol Environ Saf 32: 226-232.
- Schaefer M, Filser J (2007) The influence of earthworms and organic additives on the biodegradation of oil contaminated soil. Applied Soil Ecology 36: 53-62.
- Hickman ZA, Reid BJ (2008) Increased microbial catabolic activity in diesel contaminated soil following addition of earthworms (Dendrobaena veneta) and compost. Soil Biology and Biochemistry 40: 2970-2976.
- Eijsackers H (2010) Earthworms as colonisers: Primary colonisation of contaminated land, and sediment and soil waste deposits. Sci Total Environ 408: 1759-1769
- Whitfield-Aslund ML, Simpson AJ, Simpson MJ (2011) 1H NMR metabolomics of earthworm responses to polychlorinated biphenyl (PCB) exposure in soil. Ecotoxicology 20: 836-846.
- Edwards CA, Bater JE (1992) The use of earthworms in environmental management. Soil Biology and Biochemistry 24: 1683-1689.
- Viuoen SA, Reinecke AJ, Hartman L (1992) The influence of temperature on the life-cycle of Dendrobaena veneta (Oligochaeta). Soil Biology and Biochemistry 24: 1341-1344.
- Ireland MP (1979) Metal accumulation by the earthworms Lumbricus rubellus, Dendrobaena veneta and Eiseniella tetraedra living in heavy metal polluted sites. Environmental pollution 19: 201-206.
- Marinussen MPJC, Van Der Zee SEATM, De Haan FAM, et al. (1996) Heavy metal (copper, lead, and zinc) accumulation and excretion by the earthworm, Dendrobaena veneta. Journal of Environmental Quality Abstract 26: 278-284
- Elisabeth E, Loibner AP, Romana K, et al. (2013) Distillation fraction-specific ecotoxicological evaluation of a paraffin-rich crude oil. Environ Pollut 174: 236-243.
- Hickman ZA, Reid BJ (2008) Earthworm assisted bioremediation of organic contaminants. Environment International 34: 1072-1081.
- Hickman ZA, Reid BJ (2008) The co-application of earthworms (Dendrobaena veneta) and compost to increase hydrocarbon losses from diesel contaminated soils. Environ Int 34: 1016-1022.
- Rorat A, Kacprzak M, Vandenbulck F, et al. (2013) Soil amendment with municipal sewage sludge affects the immune system of earthworms Dendrobaena veneta. Applied Soil Ecology 64: 237-244.
- Kanaly RA, Bartha R, Watanabe K, et al. (2000) Rapid mineralization of benzo[a]pyrene by a microbial consortium growing on diesel fuel. Appl Environ Microbiol 66: 4205-4211.
- Grishchenkov VG, Slepenkin AV, Boronin AM (2002) Anaerobic degradation of biphenyl by the facultative anaerobic strain Citrobacter freundii BS2211. Applied Biochemistry and Microbiology 38: 125-128.
- Shuttleworth KL, Cerniglia CA (1995) Environmental aspects of PAH biodegradation. Appl Biochem Biotechnol 54: 291-301
- Singleton DR, Hendrix PF, Coleman DC, et al. (2003) Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta). Soil Biology and Biochemistry 35: 1547-1555.
- Cerniglia C (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3: 351-368.
- Johnsen AR, Wick LY, Harms H (2005) Principles of microbial PAH -degradation in soil. Environ Pollut 133: 71-84.
- Pizl V, Nováková (2003) A Interactions between microfungi and Eiseniaandrei (Oligochaeta) during cattle manure vermicomposting: The 7th international symposium on earthworm ecology. Cardiff Wales 2002. Pedobiologia 47: 895-899.
- Hong SW, Kim IS, Lee JS, et al. (2011) Culture-based and denaturing gradient gel electrophoresis analysis of the bacterial community structure from the intestinal tracts of earthworms (Eisenia fetida). J Microbiol Biotechnol 21: 885-892
- Khalid A, Arshad M, Crowley DE (2008) Decolorization of azo dyes by Shewanella sp under saline conditions. Appl Microbiol Biotechnol 79: 1053-1059.
- Tiquia SM (2010) Salt-adapted bacteria isolated from the Rouge River and potential for degradation of contaminants and biotechnological applications. Environ Technol 31: 967-978.
- Wu X, Monchy S, Taghavi S, et al. (2011) Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida. FEMS Microbiol. Rev 35: 299-323.
- Gandolfi I, Sicolo M, Franzetti A, et al. (2010) Influence of compost amendment on microbial community and ecotoxicity of hydrocarbon-contaminated soils. Bioresour Technol 101: 568-575
- Kasinathan V, Drake T, Williams C, et al. (2017) Validation of MALDI-TOF MS based analysis of bacteria and east for rapid identification using Vitek MS System with SARAMIS database.
- (2018) GOST R 51105-97 Fuel for internal combustion engines. Unleaded gasoline.
- GOST R 52368-2005 (EN 590:2009). Diesel fuel EURO.
- Heimbach F (1984) Correlation between three methods for determining the toxicity of chemicals to earthworms. Pestic Sci 15: 605-611.
- SOIL SAMPLING GOST 28168-89.
- GOST 17.4.3.01-83 nature Protection. Soil. General requirements for sampling.
- (2017) GOST of Russia 17.4.4.02 Nature protection. Soils. Methods for sampling and preparation of soil for chemical, bacteriological, helmintological analysis.
- GOST of Russia 54039-2010 Soil quality. Quick method for the determination of oil products by NIR spectroscopy.
- Geissen V, Gomez-Rivera P, Lwanga EH, et al. (2008) Using earthworms to test the efficiency of remediation of oil-polluted soil in tropical Mexico. Ecotoxol and Environ Saf 71: 638-642.
Corresponding Author
SB Chachina, Department of Chemical Technology and Biotechnology, Petrochemical Institute, Omsk State Technical University, 11 Mira str, 644050 Omsk, Russian Federation.
Copyright
© 2018 Chachina SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.