Tillage and Coated-Urea Effects on Nitrous Oxide Emissions from Direct-Seeded, Delayed-Flood Rice Production in Arkansas
Abstract
Rice (Oryza sativa L.) is a key component of the diet of billions of humans, thus rice is a main agricultural product in many regions, particularly in eastern Arkansas, USA. Rice production is known to be a source of greenhouse gases, namely methane (CH4), but, under certain conditions, nitrous oxide (N2O) as well. The objective of this study was to evaluate the effects of tillage practice [conventional tillage (CT) and no-tillage (NT)] and urea fertilizer type [N-(n-butyl) thiophosphoric triamide (NBPT)-coated and non-coated urea] on N2O fluxes, season-long N2O emissions, and the global warming potential (GWP) from rice grown in eastern Arkansas in a direct-seeded, delayed-flood production system on a silt-loam soil. Gas samples were collected from enclosed chambers at 20-min intervals for 1 hr on a weekly basis between establishment of a full-season flood and four days after end-of-season flood release. Nitrous oxide fluxes were unaffected (P > 0.1) by tillage practice, urea fertilizer type, or time throughout the 2017 rice growing season. Nitrous oxide emissions ranged from 0.27 to 0.50 kg N2O-N ha-1 season-1 from the NT/NBPT-coated urea and NT/non-coated urea treatment combinations but were unaffected (P > 0.1) by tillage practice or fertilizer type. Total global warming potential (GWP) ranged from 1324 to 2204 kg CO2-equivalent ha-1 season-1 from the CT/NBPT-coated urea and NT/non-coated urea treatment combinations but was also unaffected (P > 0.05) by tillage practice or urea fertilizer type. However, though not significant (P > 0.05), averaged across tillage treatments, GWP was 14.2% numerically lower from NBPT-coated than from non-coated urea. With limited studies in Arkansas evaluating the impacts of tillage practice or urea fertilizer type on N2O emissions, it is important to quantify and evaluate potential agronomic factors affecting N2O emissions from rice production to properly determine if changing to alternative management practices for improved soil health (i.e., no-tillage) or to limit ammonia volatilization (i.e., NBPT-coated urea) will impact N2O emissions.
Keywords
Conventional tillage, No-tillage, Greenhouse gas emissions, Silt-loam
Introduction
The uncommon use flood irrigation during the growing season for rice production, compared to upland grain crops [i.e., soybean (Glycine max L.), corn (Zea mays L.), and wheat (Triticum aestivum L.)], can be viewed as both beneficial and detrimental to the environment. Anaerobic soil conditions in flood-irrigated rice production can increase soil nutrient availability and facilitate weed management, but at the same time can lead to the unintended production of greenhouse gases (GHG), such as methane (CH4) and nitrous oxide N2O).
Nitrous oxide, along with CH4, is a common and potent GHG produced and emitted from soils used for rice production as a consequence of water management practices. Between 1700 and the present, atmospheric N2O and CH4 concentrations have increased globally by 20 and 150%, respectively [1]. It is expected that, from rice production alone, non-carbon-dioxide (CO2) emissions (i.e., N2O and CH4) will increase 2% by 2030 [2]. Nitrous oxide and CH4 are more potent in the atmosphere than CO2, with global warming potentials (GWP) that are 298 and 34 times, respectively, greater than that of CO2 [3]. The magnitudes of GHG emissions are known to differ among a variety of agronomic practices.
Tillage is a frequent soil management practice in many crops and is used extensively in rice production. However, the preparation of crop fields with CT prior to planting can lead to detrimental effects on the environment because CT removes residue from the soil surface, leaving a bare soil surface to potentially increase soil erosion [4]. Other tillage practices, such as conservation tillage and no-tillage (NT), have been utilized to reduce soil erosion. No-tillage practices minimize soil erosion and support soil health by improving soil structure and increasing water infiltration and retention [5,6]. No-tillage also generally increases soil organic matter (SOM), which not only provides nutrients to crops, but also supplies an increased amount of carbon (C) substrate to microbial communities that are known to facilitate the production of N2O in partially saturated-soil conditions [7,8].
Research studies evaluating the impacts of differing tillage practices (i.e., CT, reduced/conservation tillage, and/or NT) on N2O fluxes and/or emissions from row crops [i.e., barley (Hordeum vulagre L.), corn, wheat, and rice) have been inconclusive. A few studies have reported a significant difference between CT and NT [7,8], while others reported no effect of tillage practice on N2O emissions [9,10]. The potential effects that tillage practices have on N2O emissions are inconsistent, but, when a significant difference has been reported, N2O emissions tended to be greater from NT than CT. In addition to extensive CT being a common pre-plant agronomic activity associated with rice production, N management is also a careful consideration for optimal rice production.
Nitrogen is an essential plant macronutrient that most crops become deficient with due to many soils' limited N-mineralization and N-supplying capacity relative to N requirements for optimal production [11]. For rice specifically, sufficient N is most important during vegetative growth before panicle differentiation, which ultimately influence grain yield [12]. To compensate for N deficiencies in soils, synthetic N fertilizers are used. The two most regularly used N fertilizers in rice production are uncoated urea (46% N) and N-(n-butyl) thiophosphoric triamide (NBPT)-coated urea (46% N) because of their large N concentration [12,13].
Urea has two amine groups, instead of nitrate groups, which help reduce N loss through denitrification. However, when urea is applied to a dry soil surface and not flooded within a day or two to limit aerobic conditions (i.e., nitrification), significant loss of mineral N through ammonia (NH3) volatilization can occur [14,15]. Urease enzymes, which commonly exist in the soil, are the catalyst for NH3 volatilization, therefore inhibitors are used to limit activity of the urease enzyme and reduce N-volatilization losses. The compound NBPT is a urease inhibitor that has been reported to reduce NH3 volatilization by as much as 30% [13]. If N is not lost as NH3, the N is hydrolyzed to ammonium (NH4+) and is either taken up by the plant or is adsorbed to the surrounding soil colloids. Nitrogen from non-treated urea is lost rapidly in the field through volatilization, thus rending that lost N unusable by the plants and can result in less growth (i.e., aboveground biomass and/or yield). However, the urease-inhibitor-coated urea is reported to provide a more slow release of N [13], thereby allowing the plant to use more of the applied N to potentially result in more biomass and/or yield.
Field studies evaluating NBPT-coated-urea effects on N2O emissions from rice production are absent, however, studies of seasonal N2O emissions from NBPT-coated urea have been conducted in corn (Zea mays L.) and pasture land, where seasonal N2O emissions either increased from non-coated urea compared to NBPT-coated urea [16,17] or no difference was reported [18]. The general trend of increased N2O emissions from non-coated urea is likely related to the ability of urea to hydrolyze more in a wet to nearly saturated soil profile than in dry-soil conditions.
There are no known studies evaluating effects of tillage practice and NBPT coating of urea on N2O emissions in Arkansas. Therefore, the objective of this study was to evaluate the effects of tillage practice (i.e., NT and CT) and urea fertilizer type (NBPT-coated and non-coated) on N2O fluxes, season-long N2O emissions, and GWP (i.e., N2O and CH4 combined) from rice grown on a silt-loam soil from a drill-seeded, delayed-full-season-flood production system in Arkansas. It was hypothesized that N2O fluxes, season-long N2O emissions, and GWP would be greater from the NT/non-coated-urea than from any other tillage/fertilizer-type treatment combination because NT will increase the C concentration near the soil surface more than CT, therefore increasing microbial activity and the non-coated urea will supply a more labile form of N.
Materials and Methods
Site description
Research was conducted between May and October 2017 at the University of Arkansas, Division of Agriculture's Rice Research and Extension Center (RREC) near Stuttgart, AR (34.46°N, 91.46°W). A Dewitt silt loam (fine, smectitic, thermic Typic Albaqualfs) with < 1% slope was present throughout the research site [19,20]. Replicate, large research plots under long-term NT management for at least 10 years [21] and an adjacent area that has been under CT management for over 75 years were used for this study. The NT plots used in this study were sub-areas of larger NT plots that were part of an on-going, long-term NT phosphorous (P) fertilization study [21].
The area surrounding Stuttgart, AR is classified as humid subtropical, which includes warm weather with periodic precipitation year-round [22]. The average monthly air temperature is 16.5 ℃, ranging from a minimum of -1.1 ℃ in January to a maximum of 33.3 ℃ in July, and annual precipitation is 125.6 cm [23]. The 2017 growing season (i.e., May-September) had an average daily temperature of 25.0 ℃, which was similar to the 30-year (i.e., 1981 to 2010) average of 25.1 ℃ for the same months [24]. Precipitation during the 2017 growing season was 55.0 cm, 1.3 times larger than the 30-year average of 43 cm [24].
Treatments and experimental design
A randomized complete block (RCB) design replicated four times was used with a factorial arrangement of each tillage (CT and NT)-fertilizer type [NBPT-coated urea (NBPT-U) and non-coated urea (NC-U)] treatment combination. Two long-term NT plots, 4.57 m wide by 7.62 m long, were used with the placement of two, 30 cm-diameter, gas sampling chamber base collars (described in more detail below) fertilized with NBPT-U and two for NC-U in each of two NT plots. Conventional tillage plots, 1.6 m wide by 4.6 m long with 18 cm row spacing, established immediately adjacent to the long-term NT plots, had one gas sampling chamber base collar placed per plot, with four chamber base collars associated with the NBPT-U and four chamber base collars associated with the NC-U treatment. No-tillage and CT plots were situated in two full-season-flood rice bays that were immediately adjacent to one another and separated by a levee. There was a total of 16 field plots encompassing the four tillage-fertilizer-type treatment combinations (i.e., NT/NBPT-U, NT/NC-U, CT/NBPT-U, CT/NC-U).
The tillage and fertilizer-type treatments were arranged as a split-plot, where tillage was the whole-plot factor and fertilizer type was the split-plot factor, while time (i.e., gas measurement date) was a split-split-plot factor for gas flux analyses. For measured parameters without a time component, a split-plot design was used, with tillage as the whole-plot factor and fertilizer type as the split-plot factor.
Plot management
Conventionally tilled plots were disked with one pass then floated (i.e., smoothed to prepare for planting) with two passes on 20 November 2016 and 25 April, 2017, respectively. Pre-plant fertilization of 29.4 kg P ha-1 as triple superphosphate, 83.8 kg K ha-1 as muriate of potash, and 11.2 kg Zn ha-1 as zinc sulfate were applied to CT plots on 22 March 2016. No-tillage plots were pre-plant fertilized only with 83.8 kg K ha-1 as K2O on 22 March 2017 and seeds were pre-treated with Zn. No-tillage plots were cropped to soybean, while the CT plot area was left fallow during the 2016 growing season. The pure-line cultivar 'CL172', which is a long-grain, semi-dwarf cultivar that was bred by the University of Arkansas, was planted on 9 May in the NT plots and on 11 May 2017 in the CT plots. An Obey (FMC Corp., Philadelphia, PA), which is a mixture of clomazone (2-[(2-chlorophenyl)methyl]-4,4-dimethyl-3-isoxazolidinone) and quinclorac (3,7-dichloro-8-quinolinecarboxylic acid), and Permit Plus [halosulfuron-methyl, methyl 3-chloro-5-(4,6-dimethoxypyrimidin-2-ylcarbamoylsulfamoyl)-1-methylpyrazole-4-carboxylate; Gowan Co., Yuma, AZ] herbicide mixture was sprayed pre-emergence on 9 May 2017 for weed control, while no additional herbicide applications were made the remainder of the season.
Two separate bays for full-season-flood water management were created with a levee that was established around the NT and CT plot areas after planting and two to three weeks prior to flooding. A recommended single, pre-flood N application at the rate of 118 kg N ha-1, determined according to the N-Soil Test for Rice (N-STaR; [13]) in the NT portion of the study area, was broadcast manually within each gas sampling chamber to dry soil in both CT and NT plots on 12 June 2017. The N-STaR fertilizer-N recommendation scheme was based on soil samples to a depth of 46 cm and refined based on cultivar selection and soil textural class [13]. The delayed, full-season flood was established at the 5-leaf rice stage on 13 June 2017, after which the flood was maintained at a 10 cm depth with periodic water additions made on an as-needed basis until two weeks prior to harvest when the flood was released.
Soil sampling and analyses
On 30 May 2017, two weeks before flood establishment, soil samples were collected from the top 10 cm in each plot prior to N fertilization and flooding. Soil samples were collected for bulk density determinations using a 4.8 cm-diameter, stainless-steel core chamber and slide hammer. Eight additional soil cores per plot were collected from the top 10 cm prior to N fertilization and flooding using a 2 cm-diameter, stainless-steel push probe that were combined and used for particle-size and chemical analyses. Soil samples were dried at 70 ℃ for at least 48 hr and weighed. Dried soil samples were sieved to pass a 2 mm mesh screen. A modified 12 hr hydrometer method was used to determine particle-size distribution [25]. A 1:2 soil mass:water volume suspension was used to determine soil pH and electrical conductivity (EC) potentiometrically. Mehlich-3 extractable nutrient concentrations (i.e., P, K, Ca, Mg, Fe, Mn, Na, S, Cu, and Zn) were determined using inductivity coupled, argon-plasma spectrophotometry after extraction in a 1:10 soil mass-to-solution-volume ratio [26]. Total carbon (TC) and nitrogen (TN) concentrations were determined by high-temperature combustion with a VarioMax CN analyzer (Elementar Americas Inc., Mt. Laurel, NJ; [27]). Soil organic matter (SOM) concentration was determined by weight-loss-on-ignition [27]. Using the measured bulk density and 10 cm sample depth, measured elemental concentrations (g kg-1) were converted to contents (kg or Mg ha-1).
Soil redox potential and temperature
Soil oxidation-reduction (redox, Eh) potential sensors (Model S650KD-ORP, Sensorex, Garden Grove, CA) and thermocouples (Type E, chromel-constantan) were installed adjacent to two NT/NBPT-U and two NT/NC-U gas sampling chambers in the NT plots and adjacent to two chambers in the CT/NBPT-U and CT/NC-U plots at a depth of 7.5 cm the day of flooding and at a depth of 4 cm a day prior to flooding, respectively. The flood bays were oriented east-to-west with the prevailing slope, with three sets of sensors positioned (i.e., CT/NBPT-U, and CT/NC-U) on the east end and three sensors on the west end of the CT bay. Soil Eh sensors and thermocouples were placed at depth in the soil vertically and horizontally, respectively. Each sensor was connected to a CR1000 datalogger (Campbell Scientific, Inc., Logan, UT) to record data at 15 min intervals and output averaged data at 1 hr intervals throughout the flooded portion of the growing season and for several additional days after the flood was released to prepare for harvest. There was one datalogger in the NT bay and two in the CT bay to accommodate the length of the bay. Soil Eh measurements from the silver/silver-chloride reference electrodes were adjusted by adding 199 mV to each sensor to convert to the standard hydrogen electrode Eh measurement [29]. Recorded sensor data were collected weekly, at which time all sensors were checked for proper functioning. Soil Eh and soil temperature data were summarized based on the values recorded at 0900 hrs on each gas sampling date, except for 89 days after flooding (DAF) since sensors had been removed prior to that date.
Trace gas sampling and analyses
Using procedures described in detail in Rogers, et al. [30] and Smartt, et al. [31,32], 30 cm-tall by 30 cm-diameter, polyvinyl chloride (PVC) base collars [33] were installed in both NT and CT plots prior to pre-flood fertilization. Chamber extensions, 30 cm in diameter by either 40 or 60 cm in length made out of PVC, were used to accommodate the growing rice throughout the season. Extensions were outfitted with a rubber flap to connect to the base collar or each other. Wooden boardwalks were constructed between field plots and over adjacent levees to limit soil disturbance next to the chambers during gas sampling.
A 10 cm-tall, 30 cm-diameter PVC cap was placed on top of the upper-most extension immediately prior to sampling and sealed with a rubber flap to create an enclosed headspace chamber that traps gases for sampling [34]. Sampling occurred between 0930 and 1030 hours (i.e., a comparable time to previous studies; [30-32,35]). A 2.5 cm2 fan (MagLev GM1202PFV2-8, Sunon Inc., Brea, CA), powered by a 9-V battery, was installed on the bottom side of the cap and used to circulate the entrapped gas in the headspace during sampling.
Gas sample collection was accomplished by inserting a 20 mL syringe [Beckton Dickson and Co (B-D), Franklin Lakes, NJ] into a septa (part #73828A-RB, Voigt Global, Lawrence, KS) at the top of the cap. The gas sample was transferred from the syringe into a pre-capped (20 mm headspace crimp cap; part #700-181, SUN-SRi, Rockwood, TN) and pre-evacuated, 10-mL glass vial (part #405-134, SUN-SRi, Rockwood, TN). Samples were collected in 20-min intervals for 1 hr (i.e., at 0, 20, 40, 60 min) once the caps were placed over the chambers. Sampling occurred on a weekly basis from after the establishment of the full-season flood until four days after flood release at the end of the season in preparation for harvest. Air temperature, relative humidity, and barometric pressure were recorded adjacent to the chamber at the time of sampling. Chamber height was measured from the top of the floodwater, or soil surface if no standing flood was present, to the bottom of the cap for chamber volume determinations.
Gas samples were stored at room temperature and analyzed as soon after collection in the field as possible, which was typically within 48 hours. However, several sets of weekly samples had to be stored for several weeks due to instrument malfunction. Gas samples were analyzed on a Shimadzu GC-2014 gas chromatograph (Shimadzu North America/Shimadzu Scientific Instruments Inc., Columbia, MD) using a flame-ionization detector for CH4 detection and an electron-capture detector for N2O. Nitrous oxide and CH4 fluxes were determined based on the change in concentration in a chamber over the 1-hr sampling interval [30-32]. The concentration at each 20 min interval (mL L-1) was plotted against the time interval (min) and fitted with a linear regression equation to determine the change in concentration over time (i.e., slope of the regression line; [30-32]) to determine the flux (µL m-2 min-1; [33]). Total season-long N2O and CH4 emissions were calculated by linear interpolation between consecutive sample dates on a chamber-by-chamber basis (i.e., area-scaled emissions). Total seasonal emissions were also converted to CO2-equivalent global warming potential (GWP) for each treatment combination using the climate-carbon-feedback, 100-yr GWP conversion rates of 298 and 34 for N2O and CH4, respectively [3]. Hereafter, all comparative studies have had GWP conversion rates for CH4 adjusted from 25 to 34.
Plant sampling
Aboveground biomass in each base collar was collected by harvesting rice plants 2 cm above the soil surface on 10 September 2017 (i.e., four days after flood release). Biomass samples were dried at 55 ℃ for 3 weeks and weighed to determine aboveground dry matter. Yield was determined by clipping panicles on a chamber-by-chamber basis, then weighing and adjusting the panicle masses to 12% grain moisture. Season-long N2O emissions were also divided by the rice panicle yield on a chamber-by-chamber basis to calculate an emissions efficiency (i.e., yield-scaled emissions).
Statistical analyses
A two-factor analysis of variance (ANOVA) was performed using SAS 9.4 (SAS Institute, Inc., Cary, NC) to determine the effects of tillage practice, pre-assigned N-fertilization type, and their interaction on initial soil properties in the top 10 cm. A three-factor ANOVA was performed to evaluate the effects of tillage, N-fertilizer type, time, and their interactions on N2O fluxes. A two-factor ANOVA was performed to evaluate the effects of tillage practice, N-fertilizer type, and their interaction on panicle yield, pre- and post-flood-release area-scaled N2O emissions, area- and yield-scaled, season-long N2O emissions, and GWP. When appropriate, means were separated by least significant difference (LSD) at the α = 0.1 level for N2O fluxes and emissions due to the low expected magnitudes and large expected variability. All other data sets had means that were separated by LSD at the α = 0.05 level.
Results and Discussion
Pre-flooding soil physical and chemical properties
Pre-flooding soil properties were measured to evaluate field plot uniformity among pre-assigned urea-fertilizer and tillage treatment combinations. Soil bulk density and extractable soil K differed (P < 0.05) among tillage-fertilizer treatment combinations, while soil pH and extractable soil P, Mg, Na, Fe, Mn, and Zn differed (P < 0.05) between tillage treatments (Table 1). All other soil properties measured in the top 10 cm before flooding (i.e., sand, silt, and clay; EC; extractable soil Ca, S, and Cu; TN, TC, C:N ratio; and SOM) were unaffected (P > 0.05) by tillage or fertilizer treatment (Table 1).
Pre-flood soil bulk density did not differ between fertilizer treatments under CT; however, bulk density in the CT/NBPT-U and CT/NC-U treatment combinations (1.38 and 1.37 g cm-3, respectively) were 11 and 19% greater (P < 0.05) than bulk density in the NT/NBPT-U and NT/NC-U treatment combinations (1.23 and 1.15 g cm-3, respectively), where bulk density in the NT/NBPT-U was 7% greater than that in the NT/NC-U treatment combination. Pre-flood extractable soil K content only differed between NT/NBPT-U (156 kg ha-1) and NT/NC-U (135 kg ha-1) treatment combinations and did not differ between fertilizer treatments under CT (143 kg ha-1). However, all treatment combinations had extractable soil K concentrations in the top 10 cm of soil that fell within the "Medium' (91 to 130 mg K kg-1) soil-test category for fertilizer recommendations for rice grown in Arkansas [13].
Pre-flood soil pH was 13% greater in the CT (pH = 6.1) than in the NT treatment (pH = 5.4), but soil pH in both tillage treatments were within the optimal pH range for rice production (~5.0 to 6.75; [11]; Table 1). Pre-flood extractable soil P, Mg, Na, Mn, and B contents were 12, 60, 45, 24, and 18%, respectively, greater under CT than under NT, while extractable soil Fe and Zn contents were 1.2 and 2.1 times, respectively, greater under NT than under CT (Table 1). Extractable soil Zn concentrations were 2.1 and 5.1 mg kg-1 under CT and NT, respectively, with CT having a soil-test category of "Low" and NT "Optimum" [13]; however, a yield response would not have been expected from the application of a small amount of additional Zn. Unlike extractable soil K and Zn, the extractable soil P concentration under both tillage treatments were in the "Very Low" (i.e., ≤ 15 mg kg-1) soil-test category [13]; however, like for Zn, based on experience at this particular research location, a yield response would not have been expected from additional P. Furthermore, the NT study plots were part of a long-term P study, which superseded the adjustment of the soil-test P to a more optimum level. Mean sand, silt, and clay fractions (0.14, 0.71, and 0.15 g g-1, respectively) in the top 10 cm confirmed a silt-loam soil surface texture for both tillage treatments (Table 1). Though several soil physical and chemical properties differed prior to flooding, differences were relatively minor and generally non-agronomically significant, and it was reasonably assumed that any measured differences in N2O fluxes and/or emissions were the result of imposed treatment effects rather than due to large, inherent differences among plots prior to flooding.
Nitrous oxide fluxes
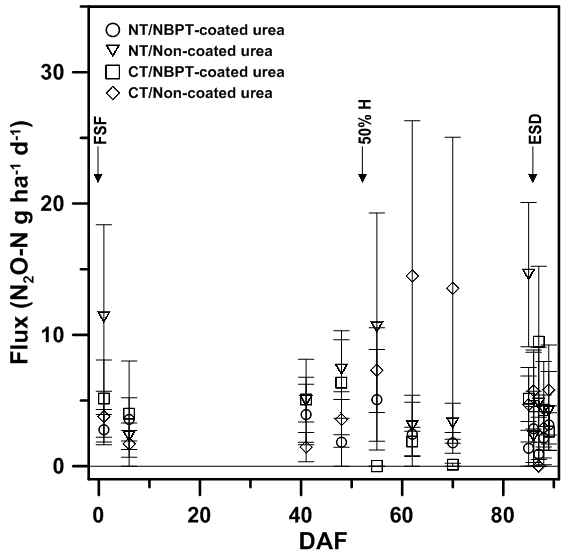
Unlike previous reports for CH4 [30-32], N2O fluxes during the 2017 growing season (i.e., establishment of full-season flood to four days after end-of-season flood release) did not show any discernable trend over time. Mean N2O fluxes did not exceed 15 N2O-N g ha-1 d-1 at all during the 2017 growing season, with peak numeric N2O fluxes ranging in occurrence from 62 DAF from the CT/NC-U treatment combination (14.5 g N2O-N ha-1 d-1) to 85 DAF from the NT/NC-U treatment combination (14.6 g N2O-N ha-1 d-1) (Figure 1). All treatment combinations had peak numeric N2O fluxes that occurred after 50% heading. However, the NT/NC-U, CT/NC-U, and NT/NBPT-U (5.1 g N2O-N ha-1 d-1) treatment combinations had peak numeric N2O fluxes that occurred prior to the end-of-season drain, while the CT/NBPT-U (9.5 g N2O-N ha-1 d-1) treatment combination had a peak numeric flux after the end-of-season drain.
In contrast to that hypothesized, neither tillage practice nor fertilizer type affected (P > 0.1) N2O fluxes throughout the 2017 growing season (Table 2). Similarly, averaged across field treatments, N2O fluxes did not differ over time throughout the 2017 growing season (Table 2). A multi-week gap occurred between 6 and 41 DAF, where no N2O fluxes were measured due to analytical difficulties, may have impacted the ability to ascertain field treatment and/or time effects on N2O fluxes. However, though non-significant due to large variability associated with flux measurements, N2O fluxes from non-coated-urea treatment combinations tended to be numerically greater than fluxes from NBPT-coated urea treatment combinations (Figure 1). It was somewhat expected that there was no difference in N2O fluxes over time because there was no split N-fertilizer application made. Rice grown in the delayed-flood system are known to take up available N efficiently [13]. Furthermore, both tillage treatments had a full-season flood that minimized fluctuations of soil Eh that would have promoted N2O production and release.
Limited research has been conducted evaluating individual or combined effects of tillage practice and urease-inhibiter-coated urea on N2O fluxes. Studies investigating NT and/or CT effects on N2O fluxes are few and inconclusive. Based on a 3 yr, wheat-rice rotation study in China on a silty-clay-loam soil, Zhang, et al. [26] documented no numerical trends or difference in N2O fluxes among tillage practice [i.e., NT or reduced tillage (RT) and CT] in rice production. In contrast, based on a 1 yr study in China on a silty-clay-loam soil, Ahmad, et al. [8] reported NT produced greater peak N2O fluxes than CT, with peaks occurring after application of N fertilizer. Zhang, et al. [26] measured a peak flux at ~24 g N2O-N ha-1 d-1, while Ahmad, et al. [8] measured a peak N2O flux at 240 g N2O-N ha-1 d-1, with both peak N2O fluxes 1.6 to 16 times greater than the peak flux measured in this study (14.6 g N2O-N ha-1 d-1). Liu, et al. [7] conducted a 1 yr corn study in Colorado on a clay-loam soil, while Chatskikh and Olesen [9] conducted a 1yr barley study in Denmark on loamy-sand soil, with both study results supporting a numerical trend of NT producing greater N2O fluxes than CT.
The limited research on NBPT-coated urea and its effect on N2O fluxes are more consistent, where generally lower numeric peak N2O fluxes have been reported from treatments using urease inhibitors (i.e., NBPT; [16-18]). Ding, et al. [17] conducted a 1 yr study in corn on a sandy-loam soil in China and reported a peak N2O flux (120 g N2O-N ha-1 d-1) that was 1.5 times greater from a NC-U than from a NBPT-U treatment, where both treatments had peak fluxes after N-fertilizer application. Sanz-Cobena, et al. [18] conducted a 2 yr study, also in corn, on a sandy-clay-loam soil in Spain and reported peak N2O fluxes from a NC-U treatment that ranged from 80 to 160 g N2O-N ha-1 d-1, which were 60% greater than peak N2O fluxes from a NBPT-U treatment. Dawar, et al. [16] conducted a 1 yr study in New Zealand, mainly in silt-loam soils, under grazed pasture landuse that excluded cattle one year prior to initiating the study and reported a peak N2O flux (20 g N2O-N ha-1 d-1) from a NC-U treatment that was two times greater than the peak N2O flux from a NBPT-U treatment, where all peak fluxes occurred during large rainfall or irrigation events.
Aboveground dry matter and yield
Aboveground dry matter for CL172 was unaffected (P = 0.61) by fertilizer treatment but was affected by tillage practice (P 0.01; Table 3). Aboveground biomass was 18% greater from CT (19.4 Mg ha-1) than from NT (16.5 Mg ha-1). No-tillage had a lower initial soil-test P than CT and did not receive additional P fertilizer, which could have impacted biomass production.
Similar to aboveground biomass, rice panicle yield for CL172 was unaffected (P = 0.54) by fertilizer treatment but differed (P = 0.01) between tillage practices (Table 3). Panicle yield was 15% greater from CT (8.9 Mg grain ha-1) than from NT (7.8 Mg grain ha-1). Rice yields measured in this study were slightly lower than the multi-location mean yield for CL172 (9.2 Mg grain ha-1) based on Arkansas yield trials [36]. In this study, the same quantity of fertilizer N was applied to plots in both tillage treatments, but, unlike CT, the NT treatment was not fertilized with additional P due to the long-term nature of P-fertilization treatments the NT plots were a part of that were used in this study.
Nitrous oxide emissions
Area-scaled N2O emissions for the pre-flood-release (i.e., establishment of flood to end-of-season drain) portion of the 2017 growing season ranged from 0.26 kg N2O-N ha-1 period-1 in the NT/NBPT-U to 0.49 kg N2O-N ha-1 period-1 in the NT/NC-U treatment (Table 4). However, area-scaled N2O emissions for the pre-flood-release portion of the 2017 growing season were unaffected (P > 0.10) by tillage practice or fertilizer treatment (Table 2). There was also no discernable trend in pre-flood-release N2O emissions among treatment combinations (Table 4). Though not significant, treatments with NBPT-U (0.47 kg N2O-N ha-1) had pre-flood-release, area-scaled N2O emissions that were nearly twice that from the NC-U treatments (0.28 kg N2O-N ha-1, Table 4).
Area-scaled N2O emissions for the post-flood-release (i.e., end-of-season drain to harvest) portion of the 2017 growing season ranged from < 0.01 kg N2O-N ha-1 period-1 in the NT/NBPT-U to 0.02 kg N2O-N ha-1 period-1 in the CT/NBPT-U treatment (Table 4). However, area-scaled N2O emissions for the post-flood-release portion of the 2017 growing season were also unaffected (P > 0.10) by tillage practice or fertilizer treatment (Table 2). Similar to pre-flood-release emissions, there was no discernable trend in post-flood-release N2O emissions among treatment combinations (Table 4). Post-flood-release N2O emissions accounted for less than 7% of total season-long emissions across all treatment combinations (Table 4). No known studies have reported differences between pre- and post-flood-release N2O emissions among tillage treatments or among differing urea-fertilizer treatments. However, Zhao, et al. [37], who evaluated the effects of water management practice on N2O emissions, and Adviento-Borbe, et al. [38], who evaluated the effects of N-fertilizer rate on N2O emissions, determined that emissions during the post-flood-release period could contribute between 0 and 82% of total season-long N2O emissions.
Season-long, area-scaled N2O emissions ranged from 0.27 kg N2O-N ha-1 season-1 in the NT/NBPT-U to 0.50 kg N2O-N ha-1 season-1 in the NT/NC-U treatment (Table 5). However, similar to pre- and post-flood-release N2O emissions, season-long, area-scaled N2O emissions were unaffected (P > 0.10) by tillage practice or fertilizer treatment (Table 2). Nitrous oxide emissions averaged 0.39 kg N2O-N ha-1 season-1 across all tillage-fertilizer treatment combinations, which was 11% greater than the expected 0.35 kg N2O-N ha-1 season-1 based on the Intergovernmental Panel on Climate Change's N2O emissions factor of 3 g N2O-N (kg N-input)-1 for a N input of 118 kg ha-1 season-1 [39].
Few studies have evaluated the effect of tillage practice and/or urease-inhibitor-coated urea, such as NPBT, on season-long N2O emissions. However, studies investigating NT and/or CT practices in various crops either reported significantly greater N2O emissions from NT than from CT [8,40] or reported only a numerical difference [7,10]. Ahmad, et al. [8] reported a 32% increase in season-long N2O emissions from NT (7.4 kg N2O-N ha-1) compared to CT (5.6 kg N2O-N ha-1) from rice. Venterea, et al. [40] conducted a 2 yr corn study in Minnesota on a silt-loam soil and reported greater N2O emissions from NT than from CT. Liu, et al. [7] reported N2O emissions that were more than two times numerically greater from NT (0.90 kg N2O-N ha-1) than from CT (0.44 kg N2O-N ha-1). During a 3 yr wheat-rice rotation study, Zhang, et al. [10] reported numerically greater season-long N2O emissions from CT (0.15 kg N2O-N ha-1) than from NT (0.12 kg N2O-N ha-1) during rice production.
In contrast to tillage effects, the few studies evaluating NBPT-coated urea and its effect on N2O emissions were more consistent, with NBPT-coated urea resulting in significantly lower N2O emissions than non-coated urea from corn production [16,18] and from pastureland [17]. During a 2-yr study, Sanz-Cobena, et al. [18] reported N2O emissions that were two times greater from NC-U than from NBPT-U; however, there was no difference in N2O emissions between NC-U and NBPT-U treatments in one of two years. Ding, et al. [17] also reported greater N2O emissions from NC-U than from NBPT-U, while Dawar, et al. [16] measured an 8% increase in N2O emissions from NC-U than from NBPT-U.
Similar to season-long, area-scaled N2O emissions, yield-scaled N2O emissions were also unaffected (P > 0.10) by tillage practice or fertilizer treatment (Table 2). Yield-scaled N2O emissions averaged 0.05 kg N2O-N (Mg grain)-1 across all tillage-fertilizer treatment combinations (Table 5). Sanz-Cobena, et al. [18] also report yield-scaled N2O emissions did not differ between NBPT-U and NC-U treatments.
Soil Eh and temperature
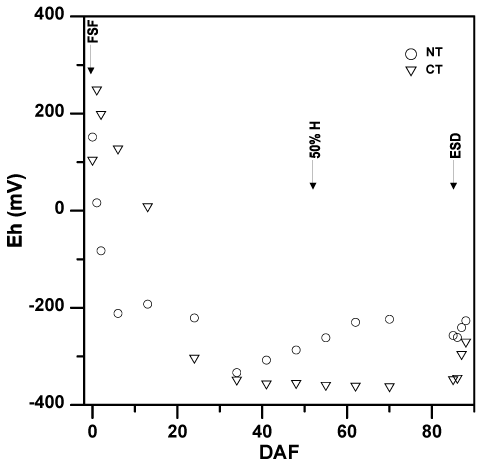
The reduction of NO3- occurs efficiently when soil reduction-oxidation (redox) potential (Eh) ranges from 220 to 280 mV, therefore increasing the likelihood of N2O production [41]. Soil Eh at the 7.5 cm depth differed between tillage practices over the growing season (P < 0.01) and differed between tillage-fertilizer treatment combinations (P < 0.01; Table 3). However, means separation could not specifically identify which sample dates soil Eh differed between tillage treatments, but differences in soil Eh between tillage treatments tended to be greater early than late in the growing season (Figure 2). Optimal (246 mV) or near optimal (195 mV) soil Eh for NO3- reduction was only measured 1 and 2 DAF in NT, while there were no sample dates under CT that were within or near the optimal soil Eh range for NO3- reduction. Soil Eh fell below 0 mV between 13 and 24 DAF and did not increase above 0 mV throughout the remainder of the rice growing season (Figure 2). Averaged over time, soil Eh among tillage-fertilizer treatment combinations [NT/NC-U (-55.6 mV), NT/NBPT-U (-340 mV), CT/NC-U (-199 mV), and CT/NBPT-U (-183 mV)] were variable, but specific differences were also unable to be identified with means separation.
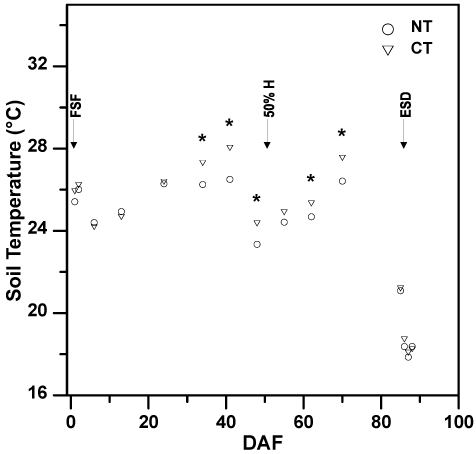
Soil temperatures at the 7.5 cm depth fluctuated throughout the growing season, where soil temperature started around 26 ℃, increased to around 28 ℃ mid-season (41 DAF), and decreased to below 20 ℃ after the end-of-season drain (86-88 DAF; Figure 3). Similar to soil Eh, soil temperature at the 7.5 cm depth differed between tillage practices throughout the season (P < 0.01) and differed between tillage-fertilizer treatment combinations (P = 0.03; Table 3). The soil temperature was significantly greater under CT than under NT on numerous dates during the middle of the flooded portion of the growing season (i.e., 34, 41, 48, 55, 62, and 70 DAF), but did not differ by more than 2 ℃ on any given date (Figure 3). Averaged across measurement dates, the mean soil temperature was significantly warmer in the CT/NBPT-U (24.5 ℃) and CT/NC-U (23.8 ℃), which did not differ, than in the NT/NBPT-U (23.5 ℃) and NT/NC-U (23.6 ℃) treatment combinations, which did not differ. Brye, et al. [42] documented an impact of day and nighttime air temperatures on CH4 emissions from silt-loam soils in Arkansas, but there is currently no known study that has evaluated the potential impact of day and/or nighttime air temperatures on N2O emissions.
Total global warming potential
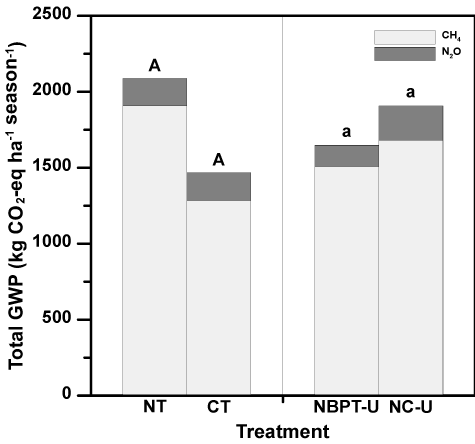
Total GWP (i.e., the combination of CO2-equivlent CH4 and N2O emissions) during the 2017 growing season (i.e., flood establishment to harvest) ranged from 1324 to 2204 kg CO2 equivalent ha-1 season-1 from the CT/NBPT-U and NT/NC-U treatment combinations, respectively (Table 5). However, total GWP was unaffected (P > 0.05) by tillage practice or fertilizer treatment (Table 2). Nitrous oxide accounted for ≤ 12% of the total GWP across all treatments (Figure 4).
Few studies have reported total GWP of non-CO2 emissions (i.e., N2O + CH4) among tillage practices. Methane emissions from rice under a full-season flood can contribute over 90% of the total GWP [35,43]. Ahmad, et al. [8] reported a significant difference in CH4 emissions in rice between CT (180 kg CH4-C ha-1) and NT (140 kg CH4-C ha-1) tillage practices, consequently, explaining the significantly greater total GWP in CT (24097 kg CO2 equivalent ha-1) than NT (19781 kg CO2 equivalent ha-1) despite N2O emissions being numerically greater from NT (4.9 kg N2O-N ha-1) than from CT (3.6 kg N2O-N ha-1). However, Zhang, et al. [10] reported greater CH4 emissions from NT than from CT, which could be inferred that an increase in CH4 emissions could result in an increase in total GWP, since it is commonly understood that CH4 emissions are the dominant driver of GWP for non-CO2 emissions.
Considering only a few studies have evaluated the effects of NBPT-coated urea on N2O emissions, there are no known studies that have evaluated NBPT-coated urea effects on non-CO2 total GWP. Malla, et al. [44] evaluated the impact of the urease inhibiter hydroquinone (HQ) and reported no change in GWP from rice production when HQ was used with urea compared to urea not used with a urease inhibiter. Unlike Malla, et al. [44], Xu, et al. [45] reported a significant decrease in CH4 emissions from urea mixed with HQ compared to non-treated urea for rice production. Considering the potential for NBPT-coated urea to reduce N2O emissions [16-18] and the lack of consensus among studies on the potential to reduce GWP, further studies examining the context dependency of NBPT-coated urea effects on GWP are still needed.
Environmental implications
Averaged across all treatment combinations, N2O accounted for only 10% of the total GWP, with the remainder of the total GWP due to CH4. Methane as the key driver of GWP for rice production is supported by other studies evaluating GHG emissions from rice grown on silt-loam soils in the Lower Mississippi River Delta region of eastern Arkansas [43,46].
Nitrous oxide emissions, in combination with CH4, from agricultural sources are expected to rise through the next decade and beyond [2]. Even more concerning is that N2O and CH4 are more potent as GHGs than CO2 in the atmosphere. Therefore, in order to make responsible decisions regarding crop production, N2O emissions need to be quantified and the effects of traditional and alternative production practices on N2O emissions need to be evaluated. Expanding research on N2O emissions is of increasing importance because the human population continues to grow, which will require a similar increase in crop production, especially commonly consumed grains such as rice. A proper understanding of N2O emissions can help mitigate or offset detrimental environmental impacts resulting from increased crop production.
Conclusions
Contrary to that hypothesized, neither N2O fluxes nor season-long emissions (i.e., area- or yield-scaled) (P > 0.1) or total GWP were affected (P > 0.05) by tillage practice (CT or NT) or type of urea fertilizer (i.e., NBPT-coated and non-coated) from rice grown under a direct-seeded, delayed-flood production system during the 2017 growing season on a silt-loam soil in eastern Arkansas. However, though not significant, averaged across tillage treatments, GWP was 14.2% numerically lower from NBPT-coated than from non-coated urea, which supports the theoretical expected effect of using a urease-inhibitor-coated urea as the N source for rice production. Despite the lack of statistically significant differences, this study has contributed valuable information to the limited knowledge of N2O emissions from rice production. The global importance of rice production makes it imperative to quantify GHG emissions and evaluate potential traditional and alternative agronomic and environmental factors that may affect N2O production and emissions.
Acknowledgments
This study was supported by the University of Arkansas System Division of Agriculture. Planning and field assistance provided by Donna Frizzell ad Eddie Castaneda-Gonzalez were greatly appreciated.
References
- https://www.ipcc.ch/pdf/assessment-report/ar5/syr/AR5_SYR_FINAL_All_Topics.pdf.
- Smith P, Bustamante M, Ahammad H, et al. (2014) Agriculture, forestry and other land use (AFOLU). Climate change 2014: Mitigation of climate change. In: O Edenhofer, R Pichs Madruga, Y Sokona, et al. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, NY, USA, 811-922.
- Myhre G, Shindell D, Breon FM, et al. (2013) Anthropogenic and natural radiative forcing. Climate change 2013: The physical science basis. In: TF Stocker, D Qin, GK Plattner, et al. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
- Pittelkow CA, Linquist BA, Lundy ME, et al. (2015) When does no-till yield more? A global analysis. Field Crops Res 183: 156-168.
- Seta AK, Blevins RL, Frye WW, et al. (1993) Reducing soil erosion and agricultural losses with conservation tillage. J Environ Qual 22: 661-665.
- Turmel MS, Speratti A, Verhulst N, et al. (2014) Crop residue management and soil health: A systems analysis. Agr Syst 134: 6-16.
- Liu XJ, Mosier AR, Halvorson AD, et al. (2006) The impact of nitrogen placement and tillage on NO, N2O, CH4, and CO2 fluxes from a clay loam soil. Plant Soil 280: 177-188.
- Ahmad S, Li C, Dai G, et al. (2009) Greenhouse gas emission from direct seeding paddy field under different rice tillage systems in central China. Soil Tillage Res 106: 54-61.
- Chatskikh D, Olesen JE (2007) Soil tillage enhanced CO2 and N2O emissions from loamy sand soil under spring barley. Soil Tillage Res 97: 5-18.
- Zhang Y, Sheng J, Wang Z, et al. (2015) Nitrous oxide and methane emissions from Chinese wheat-rice cropping system under different tillage practices during the wheat-growing season. Soil Tillage Res 146: 261-269.
- Havlin JL, Tisdale SL, Nelson WL, et al. (2014) Soil Fertility and Fertilizers: An Introduction to Nutrient Management. (8th edn), Pearson Education Inc, Upper Saddle River, NJ, USA, 516.
- Norman RJ, Wilson CE, Slaton NA (2003) Soil fertilization and mineral nutrition in U.S. mechanized rice culture. In: CW Smith, RH Dilday, Rice: Origin, history, technology, and Production. John Wiley & Sons, Inc, Hoboken, NJ, USA, 331-412.
- Norman RJ, Slaton NA, Roberts T (2013) Soil fertility. In JT Hardke, Rice Production Handbook. University of Arkansas, Division of Agriculture, Cooperative Extension Service, Fayetteville, AR, USA, 69-101.
- Norman RJ, Wilson CE, Slaton NA, et al. (2009) Nitrogen fertilizer sources and timing before flooding dry-seeded, delayed-flood rice. Soil Sci Soc Am J 73: 2184-2190.
- Dillon KA, Walker TW, Harrell DL, et al. (2012) Nitrogen sources and timing effects on nitrogen loss and uptake in delayed flood rice. Agron J 104: 466-472.
- Dawar K, Zaman M, Rowarth JS, et al. (2011) Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions. Agri Ecosys Environ 144: 41-50.
- Ding WX, Yu HY, Cai ZC (2011) Impact of Urease and nitrification inhibitors on nitrous oxide emissions from fluvo-aquic soil in the North China Plain. Biol Fertil Soils 47: 91-99.
- Sanz-Cobena A, Sanchez-Martin L, Garcia-Torres, et al. (2012) Gaseous emissions of N2O and NO and NO3- leaching from Urea applied with Urease and nitrification inhibitors to a maize (Zea mays) crop. Agric Ecosyst Environ 149: 64-73.
- http://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx.
- https://soilseries.sc.egov.usda.gov/OSD_Docs/D/DEWITT.html.
- Slaton NA, Hardke J, Roberts TL, et al. (2017) Evaluation of mehlich-3 extraction of field-moist and oven-dried soil from long-term fertilization trials. In: NA Slaton, Wayne E. Sabbe Arkansas soil fertility studies, 2016. Res Series 642: 29-33.
- Arnfield JA (2016) Koppen Climate Classification. Encyclopædia Britannica. Encyclopædia Britannica.
- http://www.ncdc.noaa.gov/cdo-web/datatools/normals.
- https://www.ncdc.noaa.gov/cdo-web/datatools.
- Gee GW, Or D (2002) Particle-size analysis. In: JH Dane, GC Topp, Method of soil analysis. Part 4: Physical methods. (1st edn), Soil Science Society of America, Madison, WI, 255-293.
- http://aesl.ces.uga.edu/sera6/PUB/MethodsManualFinalSERA6.asp.
- Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: DL Sparks, AL Page, PA Helmke, et al. Methods of soil analysis. Part 3: Chemical analysis. (3rd edn), Soil Science Society of America, Madison, WI, 961-1010.
- Schulte EE, Hopkins BG (1996) Estimation of soil organic matter by weight loss-on-ignition. In: FR Magdoff, MA Tabatabai, Edward Aloysius Hanlon, Soil organic matter: Analysis and interpretation. SSSA Spec Publ 46. SSSA, Madison, WI, USA, 21-31.
- Patrick WH, Gambrell RP, Faulkner SP (1996) Redox measurements of soils. In: DL Sparks, Methods of soil analysis. Part 3 - Chemical methods. (3rd edn), SSSA, Madison, WI, USA, 1255-1273.
- Rogers CW, Brye KR, Smartt AD, et al. (2014) Cultivar and previous crop effects on methane emissions from drill-seeded, delayed-flood rice production on a silt-loam soil. Soil Sci 179: 28-36.
- Smartt AD, Brye KR, Rogers CW, et al. (2016) Characterization of methane emissions from rice production on a clay soil in Arkansas. Soil Sci 181: 57-67.
- Smartt AD, Brye KR, Rogers CW, et al. (2016) Previous crop and cultivar effects on methane emissions from drill-seeded, delayed-flood rice grown on a clay soil. Appl Environ Soil Sci 2016: 1-13.
- https://www.ars.usda.gov/ARSUserFiles/np212/Chapter%203.%20GRACEnet%20Trace%20Gas%20Sampling%20Protocols.pdf.
- Livingston G, Hutchinson G (1995) Enclosure-based measurement of trace gas exchange: applications and sources of error. In: PA Matson, RC Harriss, Biogenic Trace Gases: Measuring Emissions from Soil and Water. Blackwell Sciences Ltd, Osney Mead, Oxford, UK, 14-51.
- Adviento-Borbe MA, Pittelkow CM, Anders M, et al. (2013) Optimal fertilizer nitrogen rates and yield-scaled global warming potential in drill seeded rice. J Environ Qual 42: 1623-1634.
- Hardke JT, Moldenhauer K, Sha X, et al. (2014) Arkansas rice performance trials, 2012-2014. Rice Information 173.
- Zhao X, Min J, Wang S, et al. (2011) Further understanding of nitrous oxide emission from paddy fields under rice/wheat rotation in south China. J Geophys Res 116.
- Adviento-Borbe MA, Padilla GN, Pittelkow CM, et al. (2015) Methane and nitrous oxide emissions from flooded rice systems following end-of-season drain. J Environ Qual 44: 1071-1079.
- http://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf.
- Venterea RT, Burger M, Spokas KA (2005) Nitrogen oxide and methane emissions under varying tillage and fertilizer management. J Environ Qual 34: 1467-1477.
- Brady NC, Weil RR (2008) The Nature and Properties of Soils. (14th edn), Pearson Prentice Hall, Upper Saddle River, NJ, USA, 975.
- Brye KR, Nalley LL, Tack JB, et al. (2016) Factors affecting methane emissions from rice production in the Lower Mississippi River Valley, USA. Geoderma Reg 7: 223-229.
- Simmonds MB, Anders M, Adviento-Borbe MA, et al. (2015) Seasonal methane and nitrous oxide emissions of several rice cultivars in direct-seeded systems. J Environ Qual 44: 103-114.
- Malla G, Bhatia A, Pathak H, et al. (2005) Mitigating nitrous oxide and methane emissions from soil in rice-wheat system of the Indo-Gangetic plain with nitrification and Urease inhibitors. Chemosphere 58: 141-147.
- Xu X, Boeckx P, van Cleemput O, et al. (2002) Urease and nitrification inhibitors to reduce emissions of CH4 and N2O in rice production. Nutr Cycl Agroecosyst 64: 203-211.
- Linquist BA, Anders MM, Adviento-Borbe MAA, et al. (2015) Reducing greenhouse gas emissions, water use, and grain arsenic levels in rice systems. Global Change Biol 21: 407-417.
Corresponding Author
Dr. Kristofor R Brye, Department of Crop, Soil, and Environmental Sciences, 115 Plant Sciences Building, University of Arkansas, Fayetteville, AR 72701, USA.
Copyright
© 2018 Rector C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.