Approaches to the Study of Plant-Phytopathogen Interactions: In Vivo and In Vitro Assay Systems of Phytobacterial Pathogenesis
Abstract
Plant-phytopathogen interactions, such as rice-Xanthomonas oryzae pv. oryzae (Xoo) interactions, are important for the fate of both the host plant and invading phytopathogen, particularly in the early stage of infection. Thus far, many in vivo and in vitro systems have been developed to study the plant-phytopathogen interactions to cause disease or resistance in plant and each system has its own merits and limits. In vivo system is easy to monitor the effector translocation from phytopathogen to plant and has been used to study the resistance mechanism of plant like Hypersensitivity Response (HR). In vitro system is useful to study the pathogenic mechanism of phytopathogen such as pathogenic gene expression. Recently, new in vitro system was developed, which enables us to monitor the time-dependent gene expression of phytopathogen upon the interaction with host plant. The in vivo and in vitro assay systems will be useful to study the mechanism of phytobacterial pathogenesis and plant resistance.
Keywords
Plant-phytopathogen interactions, Rice, Xanthomonas oryzae Pv. oryzae (Xoo), Phytobacterial pathogenesis, Plant resistance
Introduction
Hosts and pathogens have competed throughout the whole evolutionary history of life. In many cases, pathogenic mechanism between plant and phytopathogen is well conserved with that between animal and animal pathogen [1-3]. At the early stage of infection, plant-phytopathogen interactions are important for the fate of interaction to cause diseases on susceptible plants or to elicit Hypersensitive Reactions (HR) on resistant plants [4]. Rice is the most widely consumed staple food worldwide, especially in Asian countries. The gram-negative plant pathogen Xanthomonas oryzae pv. oryzae (Xoo) is the causal agent of bacterial blight on rice [5], of which outbreak easily reduces rice yields by as much as 50% [6].
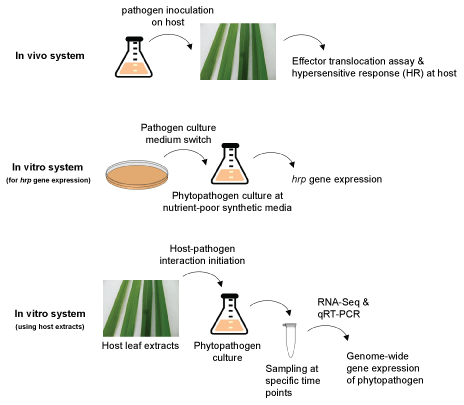
In vivo and in vitro systems have been successfully developed to study plant-phytopathogen (rice-Xoo) interactions and each system has its own merits and limits (Figure 1). The resistance mechanisms of host rice could be studied better with the in vivo systems [7], whereas for the pathogenic mechanism of phytopathogen the in vitro systems are generally more suitable. Recently, a new in vitro system, mimicking both the in vivo and in vitro systems, was developed, which initiates and activates the Xoo pathogenicity by adding fresh rice leaf extracts into Xoo culture medium [8]. The in vitro system was also combined with RNA-Seq to study the genome-wide gene expression of the phytopathogen Xoo.
The Type III Secretion System (T3SS) is a well conserved protein translocation system in Gram-negative phytopathogenic bacteria which infect plants and animals [9-11], of which components are encoded by Hypersensitive Response and Pathogenicity (hrp) genes including hrpG and hrpX genes [12]. The T3SS delivers bacterial effector proteins into the host cells to modulate host defense signaling pathways and cause diseases. Currently, the T3SS, hrp genes, and effectors are crucial molecules to study the pathogenic mechanism of phytopathogen and resistance of host plant.
In vivo assay system for effector translocation and HR
Basically, in vivo assay system consists of phytopathogen inoculation on plant and disease or resistance mechanism study in plant [13]. The translocation of effector proteins from phytopathogenic bacteria to plants via T3SS has been successfully monitored in the in vivo system with the activity of reporter protein attached to the effectors [14]. Calmodulin-Dependent Adenylate Cyclase (cya) domain from Bordetella pertussis has been used as a reporter protein, which produces cAMP depending on the existence of eukaryotic plant calmodulin [14-17]. Accordingly, it is active only when it translocates from the prokaryotic cell into the eukaryotic host cell. For example, in Xanthomonas campestris pv. vesicatoria, as early as 3 h after inoculation, the translocation of effector protein AvrBs2 (avrBs2) to host pepper plants was confirmed with the in vivo assay system [14]. Because the in vivo system uses intact plant, we could monitor HR-like plant responses resulting from the phytopathogen infection.
In vitro assay system for hrp gene expression
In the initial interaction with plant, the quick response of pathogenic signal activation in phytopathogen is important for successful infection. The pathogenic signaling pathway is a good target to develop pesticides against the plant disease. However, heterogenous phytopathogen population at in vivo infection site makes it hard to study the pathogenicity signals such as pathogenicity-related gene expressions. Simplified in vitro assay system could be more useful to monitor the pathogenic gene expressions in phytopathogen. The hrp genes in Gram-negative phytopathogenic bacteria including Xoo play important roles for pathogen's pathogenicity on host plants [12]. The expression of hrp genes is highly controlled and usually up-regulated in certain nutrient-poor synthetic media compared to nutrient-rich complex media [18-21]. The nutrient-poor synthetic media has been used to activate the hrp gene expression in plant pathogens xanthomonas such as the synthetic minimal medium of XOM2 and XVM2, which is known to mimic the apoplast plant environment to activate the pathogenic signal of phytopathogen [22-25]. Among the components of the synthetic minimal medium, specific carbohydrate sources are known to be important. Xoo propagates in rice xylem vessels, of which 60% are xylan (xylose) [26] and the xylose concentration in the synthetic minimal medium is critical to regulate the expression of hrp genes in the in vitro assay system [22]. The in vitro system lacks any rice-derived factors and could be the minimal condition that could activate the hrp gene expression.
In vitro assay system using host extracts
Recently, a new in vitro system for rice-Xoo interactions was developed, which activates Xoo pathogenicity by adding Rice Leaf Extract (RLX) into Xoo culture [8]. The in vitro system showed the upregulation of effector gene expression and T3SS-dependent effector protein secretion after RLX treatment on Xoo [8,27,28]. The in vitro system was successfully combined with RNA-Seq to analyze the time-resolved genome-wide gene expressions of Xoo upon the interactions with RLX. The new in vitro system could synchronize the pathogenicity activation signal in the RLX-treated Xoo cells, which enables to monitor the pathogenic signal of Xoo in a time-dependent way and the signal to noise ratio of RNA-Seq data was high. Because it is possible to turn on the pathogenic signal of Xoo at any specific time point, we could study the pathogenic signal pathways in the same genetic background of wild-type Xoo without making single gene-knockout mutants for comparison. The RNA-Seq results provided the expression of many pathogenicity-related genes of Xoo was initiated within 5 min upon the contact with RLX. The hrpG gene was transcribed at the maximum level within 10 min and hrpX gene expression reached the maximum level in 15 min.
Conclusion
Xanthomonas genus includes many pathogenic organisms like Xoo, Xanthomonas oryzae pv. oryzicola, Xanthomonas albilineans, Xanthomonas axonopodis pv. phaseoli, Xanthomonas axonopodis pv. manihotis, Xanthomonas campestris pv. campestris, Xanthomonas campestris pv. armoraciae, Xanthomonas campestris pv. musacearum, Xanthomonas campestris pv. vasculorum, Xanthomonas citri pv. citri, Xanthomonas euvesicatoria, and Xanthomonas fuscans subsp. aurantifolii, which infect diverse crops like rice, sugarcane, beens, cassava, crucifers, banana, citrus, tomato, and pepper [29]. Many pathogenicity-related genes in Xanthomonas are well conserved in other plant pathogens and even human pathogens [3].
We reviewed most commonly used and newly developed in vivo and in vitro assay systems to study the plant-phytopathogen interactions. The in vivo system uses intact plant and phytopathogen for assay and is useful to study the plant responses to the phytopathogen infection. However, it is hard to study the pathogenesis mechanism on the side of phytopathogen due to the heterogeneity of phytopathogen populations in the infection site. The in vitro system using minimal medium is the simplest system to upregulate the expression of pathogenicity-related genes, such as hrp genes, in phytopathogens. The upregulating mechanism is still unclear and needs to be further studied. Newly developed in vitro system activates phytopathogen pathogenicity by using host leaf extracts instead of minimum medium and enables us to study the time-dependent pathogenic responses of phytopathogen upon the interaction with plant in the same genetic background. The assay systems will help us to understand the mechanism of pathogenesis in phytopathogens and resistance in plants and crops.
Acknowledgements
This study was supported by the Next-Generation BioGreen 21 Program (No.PJ01103102), Rural Development Administration, Republic of Korea and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2015R1A2A2A01004375).
References
- Cornelis GR (2002) The Yersinia Ysc-Yop 'type III' weaponry. Nat Rev Mol Cell Biol 3: 742-752.
- Ideses D, Gophna U, Paitan Y, et al. (2005) A degenerate type III secretion system from septicemic Escherichia coli contributes to pathogenesis. J Bacteriol 187: 8164-8171.
- Mudgett MB (2005) New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu Rev Plant Biol 56: 509-531.
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323-329.
- Lee BM, Park YJ, Park DS, et al. (2005) The genome sequence of Xanthomonas oryzae pathovar oryzae KACC10331, the bacterial blight pathogen of rice. Nucleic Acids Res 33: 577-586.
- Suh JP, Jeung JU, Noh TH, et al. (2013) Development of breeding lines with three pyramided resistance genes that confer broad-spectrum bacterial blight resistance and their molecular analysis in rice. Rice (N Y) 6: 5.
- Yoshimura S, Yamanouchi U, Katayose Y, et al. (1998) Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc Natl Acad Sci U S A 95: 1663-1668.
- Kim S, Cho YJ, Song ES, et al. (2016) Time-resolved pathogenic gene expression analysis of the plant pathogen Xanthomonas oryzae pv. oryzae. BMC Genomics 17: 345.
- Anderson DM, Fouts DE, Collmer A, et al. (1999) Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc Natl Acad Sci U S A 96: 12839-12843.
- Galan JE, Collmer A (1999) Type III secretion machines: Bacterial devices for protein delivery into host cells. Science 284: 1322-1328.
- Cornelis GR, Van Gijsegem F (2000) Assembly and function of type III secretory systems. Annu Rev Microbiol 54: 735-774.
- Alfano JR, Collmer A (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: Trafficking harpins, Avr proteins, and death. J Bacteriol 179: 5655-5662.
- Roden JA, Belt B, Ross JB, et al. (2004) A genetic screen to isolate type III effectors translocated into pepper cells during Xanthomonas infection. Proc Natl Acad Sci U S A 101: 16624-16629.
- Casper-Lindley C, Dahlbeck D, Clark ET, et al. (2002) Direct biochemical evidence for type III secretion-dependent translocation of the AvrBs2 effector protein into plant cells. Proc Natl Acad Sci U S A 99: 8336-8341.
- Cunnac S, Occhialini A, Barberis P, et al. (2004) Inventory and functional analysis of the large Hrp regulon in Ralstonia solanacearum: identification of novel effector proteins translocated to plant host cells through the type III secretion system. Mol Microbiol 53: 115-128.
- Schechter LM, Vencato M, Jordan KL, et al. (2006) Multiple approaches to a complete inventory of Pseudomonas syringae pv. tomato DC3000 type III secretion system effector proteins. Mol Plant Microbe Interact 19: 1180-1192.
- Sory MP, Cornelis GR (1994) Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol 14: 583-594.
- Brito B, Marenda M, Barberis P, et al. (1999) prhJ and hrpG, two new components of the plant signal-dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum. Mol Microbiol 31: 237-251.
- Schulte R, Bonas U (1992) A Xanthomonas Pathogenicity Locus Is Induced by Sucrose and Sulfur-Containing Amino Acids. Plant Cell 4: 79-86.
- Wengelnik K, Marie C, Russel M, et al. (1996) Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol 178: 1061-1069.
- Xiao Y, Lu Y, Heu S, et al. (1992) Organization and environmental regulation of the Pseudomonas syringae pv. syringae 61 hrp cluster. J Bacteriol 174: 1734-1741.
- Tsuge S, Furutani A, Fukunaka R, et al. (2002) Expression of Xanthomonas oryzae pv. oryzae hrp Genes in XOM2, a Novel Synthetic Medium. J Gen Plant Pathol 68: 363-371.
- Seo YS, Sriariyanun M, Wang L, et al. (2008) A two-genome microarray for the rice pathogens Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola and its use in the discovery of a difference in their regulation of hrp genes. BMC Microbiol 8: 99.
- Guo Y, Figueiredo F, Jones J, et al. (2011) HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol Plant Microbe Interact 24: 649-661.
- Astua-Monge G, Freitas-Astua J, Bacocina G, et al. (2005) Expression profiling of virulence and pathogenicity genes of Xanthomonas axonopodis pv. citri. J Bacteriol 187: 1201-1205.
- Takeuchi Y, Tohbaru M, Sato A (1994) Polysaccharides in primary cell walls of rice cells in suspension culture. Phytochemistry 35: 361-363.
- Kim S, Nguyen TD, Lee J, et al. (2013) Homologous expression and T3SS-dependent secretion of TAP-tagged Xo2276 in Xanthomonas oryzae pv. oryzae induced by rice leaf extract and its direct in vitro recognition of putative target DNA sequence. J Microbiol Biotechnol 23: 22-28
- Kim SH, Lee SE, Hong MK, et al. (2011) Homologous expression and quantitative analysis of T3SS-dependent secretion of TAP-tagged XoAvrBs2 in Xanthomonas oryzae pv. oryzae induced by rice leaf extract. J Microbiol Biotechnol 21: 679-685.
- Ryan RP, Vorholter FJ, Potnis N, et al. (2011) Pathogenomics of Xanthomonas: Understanding bacterium-plant interactions. Nat Rev Microbiol 9: 344-355.
Corresponding Author
Lin-Woo Kang, Department of Biological Sciences, Konkuk University, Seoul 05029, South Korea, Tel: 82-2-450-4090, Fax: 82-2-444-6707.
Copyright
© 2017 Seunghwan KIM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.