Long-Term Preservation of Cultures of Phytophthora Species Causing Black Pod Disease on Cacao
Abstract
Theobroma cacao L. (cacao) is the main raw material in the production of chocolate which is enjoyed worldwide. One of the most important diseases affecting cacao is Phytophthora pod rot and losses up to 100% have been reported. Significant amount of research work is conducted annually on the pathogen but preserving Phytophthora species in culture to support research is always problematic. In our study, we assessed suitability of six storage media namely sterilized distilled water (SDW), sterilized and unsterilized soil suspension (SSS and USS), vegetable 8 juice broth (V8JB), OMA slant at 4℃ under mineral oil and empty (unfilled) tube for long-term preservation of five isolates each of P. palmivora (Pp) and P. megakarya (Pm) at room temperature. We also described fungal structures responsible for long-term survival and maintenance of Phytophthora species during the storage. Our study showed the possibility of preserving Phytophthora cultures in SDW and SSS at room temperature for more than 5 years. In the empty tube, Pm was viable for 15 months while Pp survived 6 months. Cultures kept on agar slants at 4℃ did not store well. Other least effective media for long term storage of Phytophthora spp. was V8J broth and USS. The effects of storage media on viability, pathogenicity, virulence and growth characteristics of the Phytophthora isolates were specie specific. Although, morphological characteristics of the cultures were maintained relative to the originals, there was reduction in the aggressiveness and virulence of the cultures. Survival mechanism of the Phytophthora species during storage was production of spherical, thin-walled chlamydospores. In view of our study, SDW and SSS at room temperature are recommended for long-term preservation of Pp and Pm cultures. The storage room temperature should not exceed 28℃. Reduction in aggressiveness and virulence may be restored with successive passages through cacao pod but this requires further verification. Zoospore's production was similar to those recorded prior to storage. This is significant to support on-going works of screening for new environmentally friendly bio-fungicides and resistant cacao varieties to reduce impact of Phytophthora pod rot disease on cacao industry.
Keywords
Phytophthora cultures, Pod rot, Survival, Virulence, Water storage
Introduction
Theobroma cacao L. (cacao) is a major cash crop in Ghana and the main raw material for the chocolate industry. One of the most important diseases affecting the crop worldwide is known as Phytophthora pod rot (commonly called black pod disease). There are at least four economically important Phytophthora species (P. megakarya, P. palmivora, P. citrophthora and P. tropicalis/capsici) causing the disease on cacao [1]. In Ghana, cacao pod rot disease is caused by P. megakarya (Pm) and P. palmivora (Pp). Pod losses up to 100% have been reported [2]. As a result, significant amount of research work is conducted annually on these species [3]. This is to better understand the biology, genetic diversity, population dynamics, adaptation and host-pathogen interrelationships for efficient management of the cacao disease. Preserving Phytophthora species in culture for a long period to maintain genetic and morphological stability is difficult as the fungus losses viability rapidly. The classical way of preserving fungal culture has been to keep it on agar slant. This requires frequent transfer onto fresh agar medium. Continuous sub-culturing over a period of time leads to reduction in pathogenicity, virulence and growth rate of isolate [4,5]. Maintaining an isolate on a host tissue is probably the best way to preserve the pathogenicity as reported for P. infestans [6]. However, the necessary procedure of re-inoculation of isolate to fresh tissue is very laborious and not feasible for long-term storage. In some instances, preserving a culture on silica gel, in soil or sand is useful but there is still the risk of serial transfers with the accompanying loss of some morphological characters and pathogenicity [7,8]. Other efforts of preserving Phytophthora species are based on slowing down fungal metabolism at low temperature and under limited oxygen access. Cultures in our laboratory are maintained as such, under mineral oil at 4℃, for a maximum period of six months. The increasing number of Phytophthora collections requires large amount of space and work on refreshing the cultures continuously. Lyophilization and liquid nitrogen storage ensure long-term viability of most large collections with distinct advantages of labour saving and preserving fungus in its original genetic state [9,10]. However, the method requires special equipment and it is expensive to maintain.
Phytophthora species have been maintained in sterilized distilled water successfully for a long period. Ko [11] reported that cultures of P. parasitica, P. palmivora and P. cinnamomi were viable in SDW at room temperature for 6 to 23 years. This simple and inexpensive method was also used to preserve P. cactorum and P. megasperma var sojae for 3-8 years at room temperature [12]. In-spite of these successes, some species of Phytophthora including P. parasitica which were kept at 5℃ survived one year in water while isolates of P. infestans and P. colocasia survived only 2-6 months [13]. This suggests that low temperature may not be suitable for water storage of Phytophthora. Hence, in this study we assessed the suitability of six different storage media for long-term preservation of Pp and Pm at room temperature. We identified and described fungal structures responsible for long-term survival and maintenance of morphological and pathological stability of the Phytophthora species.
Materials and Methods
Fungal isolate
We obtained five isolates of each species of P. palmivora (Pp-BAR133; Pp-ER340; Pp-WR411; Pp-VR093; Pp-CR 243) and P. megakarya (Pm-AR334; Pm-CR255; Pm-BAR326; Pm-ER 335; Pm-VR316) from collections at Cacao Research Institute of Ghana. The pathogens were originally isolated from cacao pods showing black pod disease symptoms and maintained on oatmeal agar (OMA) slants in a cold room (4℃). We selected the isolates of Phytophthora based on their unique morphological and pathological characteristics. The species were revived by sub-culturing on 20% clarified V8 juice agar (V8JA) plates containing antimicrobial amendments pimaricin, ampicillin, rifampicin, pentachloronitrobenzene (PARP) [14]. They were subsequently inoculated twice, successively, to cacao pod (Mocorongo clonal variety). The re-isolated fungal isolates were cultured on Oxoid corn meal agar (CMA) prepared according to manufacturer instructions. They were allowed to grow at 25℃ for 7 days under total darkness and subsequently transferred into continuous light (1,400 lux, SunLite, China) for 3 days before use.
Storage methods
We evaluated six storage media: Sterilized distilled water (SDW), sterilized and unsterilized soil suspension (SSS and USS), vegetable 8 juice broth (V8JB), OMA slant and empty (unfilled) glass tube at room temperature. In the SDW medium, a clear glass tube (25 mm diam × 82 mm long) containing 10 mL distilled water was sterilized by autoclaving at 121℃ for 15 minutes. Mycelial agar plugs (5-mm) were aseptically taken from the margins of 10-day-old pure culture of Phytophthora isolate growing on CMA into the tube and capped tightly. For soil suspensions, we added 15 g of sieved (2 mm mesh) soil from uncultivated field to 1 liter distilled water and stirred overnight with magnetic stirrer (Fisher Scientific, Penn USA). We allowed the soil particles to settle out of suspension after 5-hrs and 10 mL of aqueous portion transferred to glass tubes. The tubes were either sterilized or unsterilized and used to store mycelial plugs as sterilized soil suspension (SSS) or unsterilized soil suspension (USS). We transferred fungal plugs aseptically into 10 mL of V8JB tube containing 10% clarified V8 juice (Campbell, USA) and 0.02% CaCO3. We made similar transfers into empty tube and OMA slant. The OMA slant contained 10 mL filtrate from 4% oat flakes soaked overnight and 2% agar. We allowed the plugs to grow on the slant as previously for 10 days. Sterile mineral oil (Sigma, molecular biology grade) was added to the tube until fungal colony was completely covered. The slant was kept in an upright position and the oil level checked periodically. In all the tests, ten 5-mm diameter mycelial plugs were placed into each tube and 20 replicate tubes (4 tubes/isolate) kept for each storage medium per species. All the tubes were sealed with Parafilm (Bemis flexible packaging, Neenah, Wisconsin) prior to storage in the dark at room temperature (26 ± 2℃; alternating day light and night). Plugs on OMA slants were stored at 4℃ in the dark for comparison. We assessed viability and morphological characteristics of fungus from each storage treatment at 3 months interval for 18 months and subsequently once a year up to five years from 15/3/2014 to 17/03/2019. Prior to storage of these cultures, we determined their mycelial growth rates on VJ8A plates and lesion sizes on cacao pods (Mocorongo clonal variety).
Viability test
To test for viability of the stored cultures, we selected five replicate tubes (per isolate) from each treatment, then one plug from each tube was blotted-dry on tissue paper and placed on a fresh V8JA medium. A mycelium colony formed from each treatment on V8JA at 25℃ was scored viable. In a non-viable, no colony growth was observed but the test was repeated for the other mycelial plugs until all the tubes were exhausted for that treatment.
Detached pod test
Mocorongo cacao genotype with sufficient flowers was hand-pollinated to obtain pods of uniform age. We selected five trees and 40 seemingly healthy, hand-pollinated pods from them were harvested at 4 months after pollination. We sterilized the pods in 10% (v/v) commercial bleach (Clorox, Ghana) for 1 minute and rinsed twice in SDW. A 7-mm diameter, 6-mm deep wounds were made on the pods and test plugs from each treatment was placed into the wounds and covered with the excised tissues. We arranged the pods in a completely randomized design with five replications inside an aluminum tray (72 × 62 × 10 cm) lined with moist plastic foam (Latex foam, Ghana Ltd). Beakers of SDW were placed inside the trays, covered and sealed to maintain humidity. We kept the trays on a laboratory bench under ambient conditions (26 ± 2℃; alternating day light and night) and examined after 7 days for infection and re-isolation of the test fungus. We scored mycelial plugs causing typical Phytophthora pod rot lesion as virulent. We also traced the disease lesion sizes on transparent paper and assessed them from brown-paper cut-outs which we trimmed to the size of each lesion. These cut-outs were measured with a leaf area meter (WinDIAS, Delta-T device Ltd, Cam, England). We compared the pod lesion sizes as ratio to the initial lesions recorded prior to the storage of the cultures. Lesion ratios less than 1 indicated loss of fungal aggressiveness.
Tetrazolium chloride test
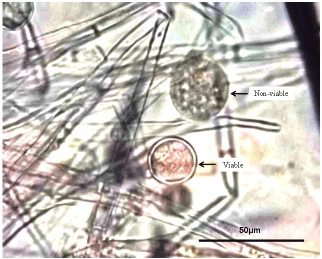
To examine viable fungal structures including chlamydospores in the stored Phytophthora cultures, a staining test was conducted on them using 2, 3, 5-triphenyl tetrazolium chloride (TTC) solution. Mycelium was teased out from stored plug onto a microscope slide. The fungal tissue was soaked in droplets of freshly prepared 1% TTC solution and the slide incubated on laboratory bench for 4-hrs. The stained tissue was observed and photographed with a Nikon Eclipse E600 fluorescent microscope (Nikon, Inc., 1300 Walt Whitman Road, Melville, NY, USA) mounted with Nikon Digital Camera (DXM1200). The TTC solution stained viable Phytophthora tissues pink (or violet) while non-viable tissues remained clear or black after incubation.
Inoculum preparation and leaf disc test
For the zoospore production, we incubated cultures from each storage treatment growing on fresh V8JA plates at 25℃ in the dark and continuous light for a total of 10 days as previously described. Each plate (9 cm diameter) was flooded with 10 mL chilled SDW (4℃) and kept in a refrigerator (4℃) for 45 minutes. We transferred the plates into a dark incubator (28℃) for 30 minutes and the zoospores liberated were harvested. Zoospore's concentration was determined for each culture using hemocytometer (KOVA International Inc., USA). About 1-2 drops of 70% ethanol were added to 1 ml suspension to immobile the zoospore counted under light microscope (Leica) at × 10 magnification. The total number of zoospores after 5 repeated counts was calculated as: (Number of zoospores/5) × 104. For the leaf disc preparation and inoculation test, we hand-washed healthy cacao leaves (3-months old) from Mocorongo clonal trees under running water, rinsed twice in SDW and wiped-dry with tissue paper. Ten leaf discs of 1.5 cm diameter were cut with a cork borer and inoculated at the center of the abaxial surface with approximately 10 μl zoospore suspension (20 × 104 zoospore/ml) from each treatment per species. We arranged the inoculated leaf discs in a completely randomized design and incubated inside aluminum tray as previously described. The leaf discs were scored at 6 days after inoculation for infection using a 0-5 point scale as described by Nyassé, et al. [15] (0: Absence of symptoms; 1: Initiation of tiny necrotic spots; 2: Large number of small necrotic spots; 3: Coalescence of brown necrotic spots; 4: Non-expanding brown lesions and 5: Large, uniform-brown lesions and expanding). The entire experiment was terminated after 60 months (5-years) when viable, mycelial plugs of some storage treatments became exhausted.
Data Analysis
Initial analysis of the effect of storage medium on the Phytophthora isolates showed homogeneity of response and was species-specific. Data from the five isolates of each species were then pooled for further analyses. Analysis of variance was used to assess the effect of storage medium on virulence and aggressiveness of the Phytophthora spp. using GenStat 11.1 (2008). The differences in the means were compared at p < 0.05 using Duncan multiple range test. Data on aggressiveness was arcsine transformed (Sin -1√X, where X= aggressiveness value) before analysis.
Results
After 60 months (5-years) of storage, Phytophthora cultures from SDW and SSS media were still viable and grew actively on freshly prepared V8JA (Table 1). Survival of cultures in these two storage media was similar for both species of Phytophthora. The next most effective medium for long-term storage was the empty (unfilled) tube where the Pm cultures remained viable for more than one year (15 months). In contrast, Pp cultures were viable in the glass tube for 6 months. Isolates on oatmeal agar slant kept at 4℃ as routinely practiced in our laboratory were viable for 3-6 months. Other least effective media for the long term storage of Phytophthora were V8J broth and USS (Table 1). Generally, more viable cultures were detected using the Tetrazolium chloride test (TTC) than the agar plate test (Table 2). There were instances where mycelial plugs were found viable in the TTC but the plugs failed to grow on V8JA plate. This was particular among the Pm isolates. The main survival structure of the Phytophthora species during storage was identified as thin- and thick-walled chlamydospores (Figure 1). On agar medium, the thin-walled chlamydospores germinated and produced new colonies readily than the thick-walled ones.
There was significant effect of the storage medium on virulence and aggressiveness of the Phytophthora cultures. Virulence, measured in terms of the necrosis sizes on cacao pod, was lower after 5 years of storage (Table 2). Among the treatments, however, cultures from SSS storage medium produced significantly (P < 0.05) higher necrosis on cacao pod than the SDW-stored cultures (Table 2). Also, there was reduction in the Phytophthora species aggressiveness after 5-years. Yet, the Pm isolates maintained higher (P < 0.05) aggressiveness than the Pp (Table 2). All the stored cultures were capable of initiating lesions on cacao leaves. Unusually, the Pp cultures from SSS storage produced larger, brown, coalescing, necrotic spots compared to the small, non-coalescing necrotic spots produced prior to storage. Generally, SDW and SSS media did not affect zoospores production of the cultures relative to initial production (Table 2). Overall growth of the Phytophthora species was consistent with the original descriptions in terms of colony, mycelial and sporangial characteristics.
Discussions
Phytophthora pod rot disease of cacao has remained one of the major constraints of the chocolate industry worldwide and this is due to poor understanding of the molecular and biology of host-pathogen interaction. Long term preservation of the causal agents (Phytophthora species) to support research has always been problematic. In our study however, we were able to preserve cultures of Phytophthora species, Pp and Pm, successfully in SDW for 5 years. Sterile water medium has been used widely to store fungal cultures either at room temperature or at lower temperatures between 4℃ and 5℃ [11-13,16-22]. Most of the studies have affirmed that keeping the cultures at room temperature is more appropriate for long term storage than lower temperature. Ko [11] reported 23 years viability of Phytophthora species kept in SDW at room temperature. Meanwhile, Sutton [12] had earlier reported 6-year storage of some cultures of Phytophthora species in SDW at room temperature. These supported our choice of room temperature for the long term preservation of Pp and Pm cultures. There are seasonal fluctuations in room temperature and high room temperature can be detrimental to Phytophthora isolates in storage. Puig [23] observed that cultures of Pp and Pm lost their viability after 3 days at temperatures of 36℃ and 32℃ respectively. In our study, the ambient room temperature during the long term storage was 26℃ (± 2℃). Based on these observations, temperatures recommended for long term storage of Pp and Pm in SDW should not exceed 28℃.
Besides, when we kept the Phytophthora cultures on agar slants at lower temperature (4℃) under mineral oil as used routinely in our laboratory, they did not store well. Another drawback to this method is the additional cost involved in maintaining low temperature during storage. Currently in our laboratory, the agar slant under mineral oil method is replaced with water storage at room temperature. Also, we preserved cultures of Phytophthora species in empty tubes for long term storage. Although, morphological growth of the cultures was preserved relative to the original ones, the Pp isolates survived 6 months. Contrarily, viability of Pm isolates was maintained up to 15 months. The difference in storage between the two Phytophthora species is expected because in cacao plantations, Pm generally survives longer in soil, even in the dry season than Pp [24,25]. Similarly, in a fiberglass matrix (free of plant material), Ward & Griffin [26] found out that Pm survived for 18 months while Pp survived for 10 months. The main problem with the empty glass tube storage was contamination and drying-up of the mycelial plugs. Probably, the airtightness of the tube is affected by fluctuations in the environmental temperature.
Generally, sterile water medium is an easy to use and a cheaper way of preserving Phytophthora and other oomycete cultures for a longer period. In this study, we modified the sterile water by replacing it with sterile soil suspension (SSS). The influence of the SSS medium on viability, growth characteristics and virulence of Phytophthora cultures were similar to the SDW. There were similar morphological characteristics of the cultures before and after 5-years of storage, indicating that the medium (SSS or SDW) did not affect mycelium growth of the fungi. This is desirable for laboratories maintaining large collections of Phytophthora isolates to support on-going research. In most instances, there were reduction in virulence and aggressiveness of the Phytophthora cultures after storage in SSS or SDW when compared to the original growth. Although the exact cause of this reduction is not known, it may be attributed to long period of fungal inactivity or lack of a host stimulus for growth. The only means of restoring aggressiveness and virulence of fungal species is by successive passages through the host plant. Sobkowiak, et al. [10] reported significant increase in aggressiveness of Phytophthora infestan after many times passage through potato tuber slice in comparison to one time passage. It is therefore necessary to determine the number of passages required for Pp and Pm isolates through cacao pod in a future study.The main survival mechanism of the Phytophthora species during storage was production of spherical chlamydospores. Ko [11] reported a similar mechanism of maintaining Phytophthora species during 23 years of storage in SDW at room temperature. In our study, both thick- and thin-walled chlamydospores were produced in SDW and SSS but the thin-walled chlamydospores developed new colonies readily on fresh V8JA media. Moreover, sporangial production and subsequent release of zoospores was similar to those recorded prior to storage. This is significant to support the on-going screening of new environmentally friendly fungicides and development of resistant cacao varieties to reduce the impact of Phytophthora species on the global cacao industry.
Conclusions
The SDW and SSS media proved suitable for long term preservation of Phytophthora cultures. Both methods maintained morphological characteristics of the species. The survival mechanism of the Phytophthora species was production of spherical chlamydospores with thin and thick walls. After 5-years of storage, there was reduction in the aggressiveness and virulence of the Phytophthora cultures. However, this may be restored with re-inoculation of the cultures a number of times to cacao pod. This should, however, be verified in further studies. Our study was terminated after 60 months (5-years) when viable cultures in storage SDW and SSS were exhausted for further assessments. We will continue this study to conclusively determine the exact length of time Phytophthora cultures in SDW and SSS at room temperature can be stored.
Acknowledgements
We are grateful to the staff of the mycology laboratory of Cocoa Research Institute of Ghana (CRIG) for the technical assistance. This paper (CRIG/2020/00) is published courtesy the Executive Director of CRIG, Akim Tafo.
References
- Surujdeo Maharaj S, Sreenivasan TN, Motilal LA, et al. (2016) Black pod and other phytophthora induced diseases of cacao: History, Biology, and Control. In: Bailey BA, Meinhardt LW, Cacao Diseases: A history of old enemies and new encounters, Springer International Publishing, Switzerland, 213-266.
- Dakwa JT (1988) A serious outbreak of the black pod disease in a marginal area of Ghana. In: Cocoa Producers' Alliance. Proceedings of the tenth international cocoa research conference santa domingo: Dominican Republic, 447-451.
- Bailey BA, Ali SS, Akrofi AY, et al. (2016) Phytophthora megakarya, a causal agent of black pod rot in Africa. In: Bailey B, Meinhardt L, Cacao Diseases. Springer, Cham, 267-303.
- Losch A, Hutwimmer S, Strasser H (2010) Carbon utilization pattern as a potential quality control criterion for virulence of Beauveria brongniartii. J Invertebr Pathol 104: 58-65.
- Ansari MA, Butt TM (2011) Effects of successive sub culturing on stability, virulence, conidial yield, germination and shelf-life of entomopathogenic fungi. J Appl Microbiol 110: 1460-1469.
- Borba Cde M, da Silva AM, de Oliveira PC (1992) Long-time survival and morphological stability of preserved Sporothrix schenckii strains. Mycoses 35: 185-188.
- Swiszczewska J, Osinska M, Piotrowski W (1971) Pathogenicity of Phytophthora infestans Mont. de Bary races in dependence on the biotype, substrate and season. biul inst ziemn 8: 21-27.
- Windels CE, Burnes PM, Kommedahl T (1993) Fusarium species stored on silica gel and soil for ten years. Mycologia 85: 21-23.
- Granke LL, Quesada-Ocampo LM, Hausbeck MK (2012) Differences in virulence of Phytophthora capsici isolates from a worldwide collection on host fruits. Eur J Plant Pathol 132: 281-296.
- Ryan MJ, Smith D, Jeffries P (2000) A decision-based key to determine the most appropriate protocol for the preservation of fungi. World J Microbiol Biotechnol 16: 183-186.
- Sobkowiak S, Zarzycka H, Sliwka J (2012) The influence of long-term storage in liquid nitrogen on survival and pathogenicity of Phytophthora infestans isolates. J Plant Prot Res 52: 479-485.
- Ko WH (2003) Long-term storage and survival structure of three species of Phytophthora in water. J Gen Plant Pathol 69: 186-188.
- Sutton W, Reeser P, Hansen E (2007) Long-term storage of Phytophthora cultures in water. Phytophthoras in Forests and Natural Ecosystems 26-31.
- Marx DH, Daniel WJ (1976) Maintaining cultures of ectomycorrhizal and plant pathogenic fungi in sterile water cold storage. Can J Microbiol 22: 338-341.
- Ferguson A, Jeffers S (1999) Detecting multiple species of Phytophthora in container mixes from ornamental crop nurseries. Plant Dis 83: 1129-1136.
- Nyasse S, Cilas C, Herail C, et al. (1995) Leaf inoculation as an early screening test for cocoa (Theobroma cacao L.) resistance to Phytophthora black pod disease. Crop Protection 14: 657-663.
- Hartung De Capriles C, Mata S, Middelveen M (1989) Preservation of fungi in water (Castellani): 20 years. Mycopathologia 106: 73-79.
- Burdsall Jr HH, Dorworth EB (1994) Preserving cultures of wood decaying Basidiomycotina using sterile distilled water in cryovials. Mycologia 86: 275-280.
- Borman AM, Szekely A, Campbell CK, et al. (2006) Evaluation of the viability of pathogenic filamentous fungi after prolonged storage in sterile water and review of recent published studies on storage methods. Mycopathologia 161: 361-368.
- Elliott ML (2005) Survival, growth and pathogenicity of Gaeumannomyces graminis var. graminis with different methods of long-term storage, Mycologia, 97: 901-907.
- Roy CB, Srinivas P, Jacob CK (2014) Relative efficacy of long-term storage methods on survival and virulence of Corynespora cassiicola and Phytophthora meadii pathogenic on rubber (Hevea brasiliensis). Rubber Sci 27: 202-214.
- Cui H, Ren X, Yun L, et al. (2018) Simple and inexpensive long-term preservation methods for Phytophthora infestans. J Microbiol Methods 152: 80-85.
- Puig AS, Ali S, Strem M, et al. (2018) The differential influence of temperature on Phytophthora megakarya and Phytophthora palmivora pod lesion expansion, mycelia growth, gene expression, and metabolite profiles. Physio Molecular Plant Patho 102: 95-112.
- Prabha PK, Chandramohanan R (2014) Integrated management of black pod disease of cocoa caused by Phytophthora palmivora. Internat J Plant Protec 7: 107-110.
- Akrofi AY (2015) Phytophthora megakarya: A review on its status as a pathogen on cacao in West Africa. Afr Crop Sc J 23: 67 -87.
- Ward M, Griffin M (1981) Soil phase of cocoa Phytophthora. In: Gregory PH, Maddison AC (Eds.), Epidemiology of Phytophthora on cocoa in Nigeria Kew: Commonwealth Mycological Institute 25: 50-61.
Corresponding Author
Ishmael Amoako Attah, Department of Crop Science, University of Ghana, Legon P. O. Box 8, Akim Tafo, Ghana.
Copyright
© 2021 Attah AI. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.