Congenital Melanocytic Nevi of the Lower Limb: The Rule of Thumb for Surgical Excision in Children
Summary
Background: Congenital melanocytic nevi (CMN) of the lower limb display the highest projected adult size. Surgical management includes simple excision and also more complex interventions with tissue expansion, skin graft, or flap, depending on the CMN size and patient age. Here, we present our experience in CMN of the lower limb management and used these data to identify CMN size thresholds to guide the choice of surgical approach.
Methods: This retrospective study included patients with CMN (n=182) of the lower limb who underwent surgery at the Department of Pediatric Surgery, Montpellier, France, from January 2003 to September 2020. Patients were divided in three age groups: 0 to 2.5 years (group A), 2.6 to 10 years (group B), and 10.1 to 18 years (group C). The size threshold value corresponded to the maximum Youden index.
Results: The mean CMN width was 21 mm and 79% of patients underwent simple surgery. No complication was reported in 82% of patients during the follow-up. The CMN width thresholds for complex surgery were 45, 30, and 15 mm for the A, B, and C group, respectively. The most sensitive and specific threshold for all ages was a width of 26 mm and a surface area of 1.25 cm2.
Conclusions: Our study identified CMN size thresholds that might help surgeons to decide between simple and complex techniques for the management of CMN of the lower limb. To minimize surgery, we suggest considering excision as early as possible, and in multiple operating steps, if necessary.
Keywords
Congenital Melanocytic Nevi, Lower Limb, Local Flap
Introduction
Congenital melanocytic nevi (CMN) occur in 1% of newborns [1,2] and the giant form in 1/20.000 newborns [3,4]. CMN are the result of monoclonal cell proliferation probably caused by an in uteri somatic mutation [5]. The risk of malignant transformation is very low, but increases with the CMN size [6]. There is no consensus about a threshold size for prophylactic surgical resection [7,8]. However, the family is often strongly in favor of CMN removal for aesthetic and psychosocial reasons [9,10]. CMN can have different sizes and histological features, and the lack of an universal classification [9] complicates the comparison of CMN removal techniques. The most consensual classification (Krengel et al, [11] is based mainly on the projected CMN size in adulthood, which varies in function of their location, and on additional features, such as color heterogeneity, surface rigidity, presence of nodules. The growth projection for CMN of the lower limb is greater compared with those of trunk, head and upper limb. Therefore, if required, early surgery is recommended to take advantage of the child's skin elasticity [12], to reduce the need of complex surgery and reconstruction for large CMN, and also to limit post-surgery and cosmetic complications [13]. Techniques vary from a simple single-step excision, followed by suture; to Complex approaches that include skin expanders, skin grafts and flaps in one or more steps. The choice depends on the CMN size and location. Generally speaking, larger CMN require more complicated surgery.
To help the surgeon determine the choice of technique and to better inform the family, we reviewed data on patients with CMN of the lower limb who underwent surgery from January 2003 to September 2020 to propose a CMN size threshold for the simple excision-suture technique in function of the patient's age.
Patients and methods
This study retrospectively analyzed the data, including surgical procedure report and nevus size, of 209 children hospitalized for the management a CMN of the lower limb in the Department of Pediatric Surgery, Montpellier, France, between 2003 and 2016. Patients with more than one nevus in the lower limb were excluded. This retrospective study was approved by the local ethic committee (IRB ID: 202000329) and conforms to the World Medical Association Declaration of Helsinki. The lower limb was divided in two surgical units: lower skin elasticity region (foot, ankle, leg, knee, political area) and higher skin elasticity region (thigh, gluteal area). As the CMN size before surgery was not always reported in the medical record, the size of the surgical sample that contained the nevus and 1-2 millimeters of margins was used. After resection, the sample was fixed in four percent formaldehyde and sent to the pathologist who measured the CMN size. This parameter represents the estimation of the skin loss (ESL) that should be reconstructed after the resection phase.
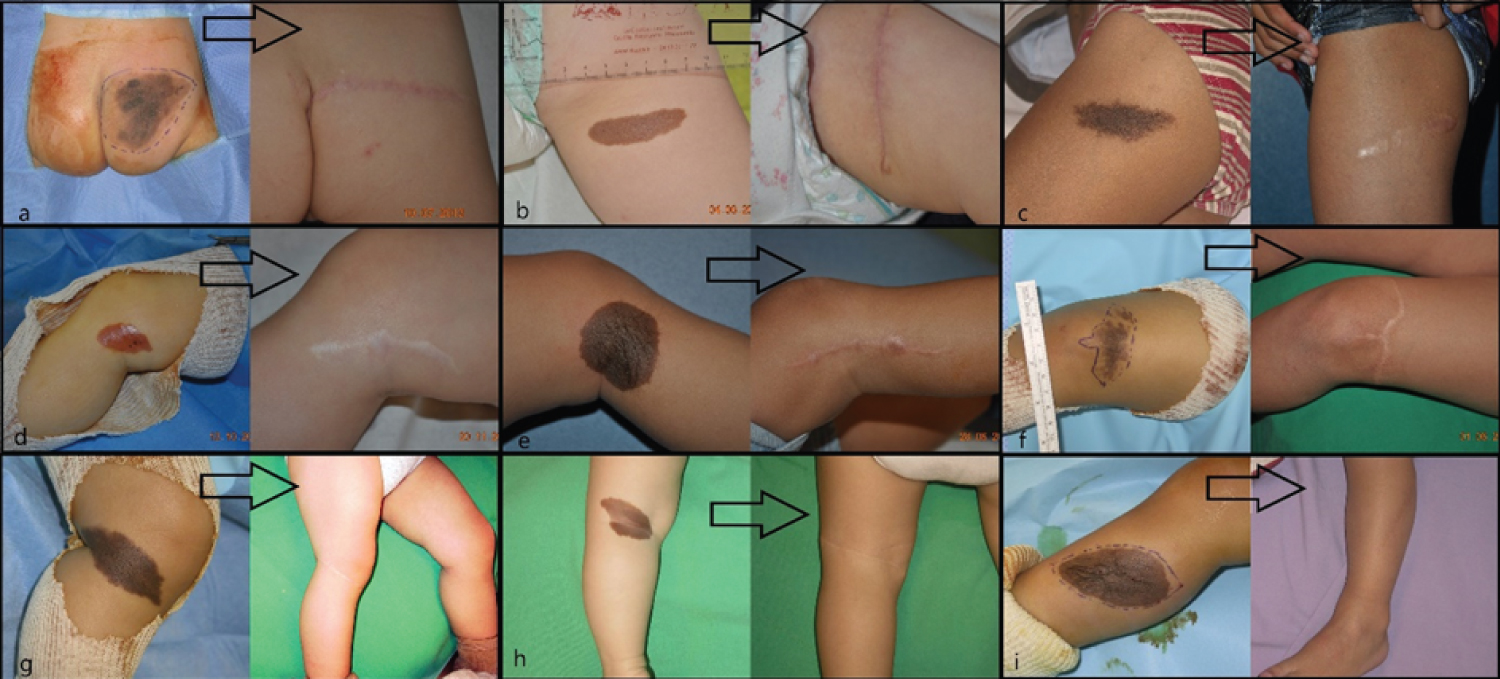
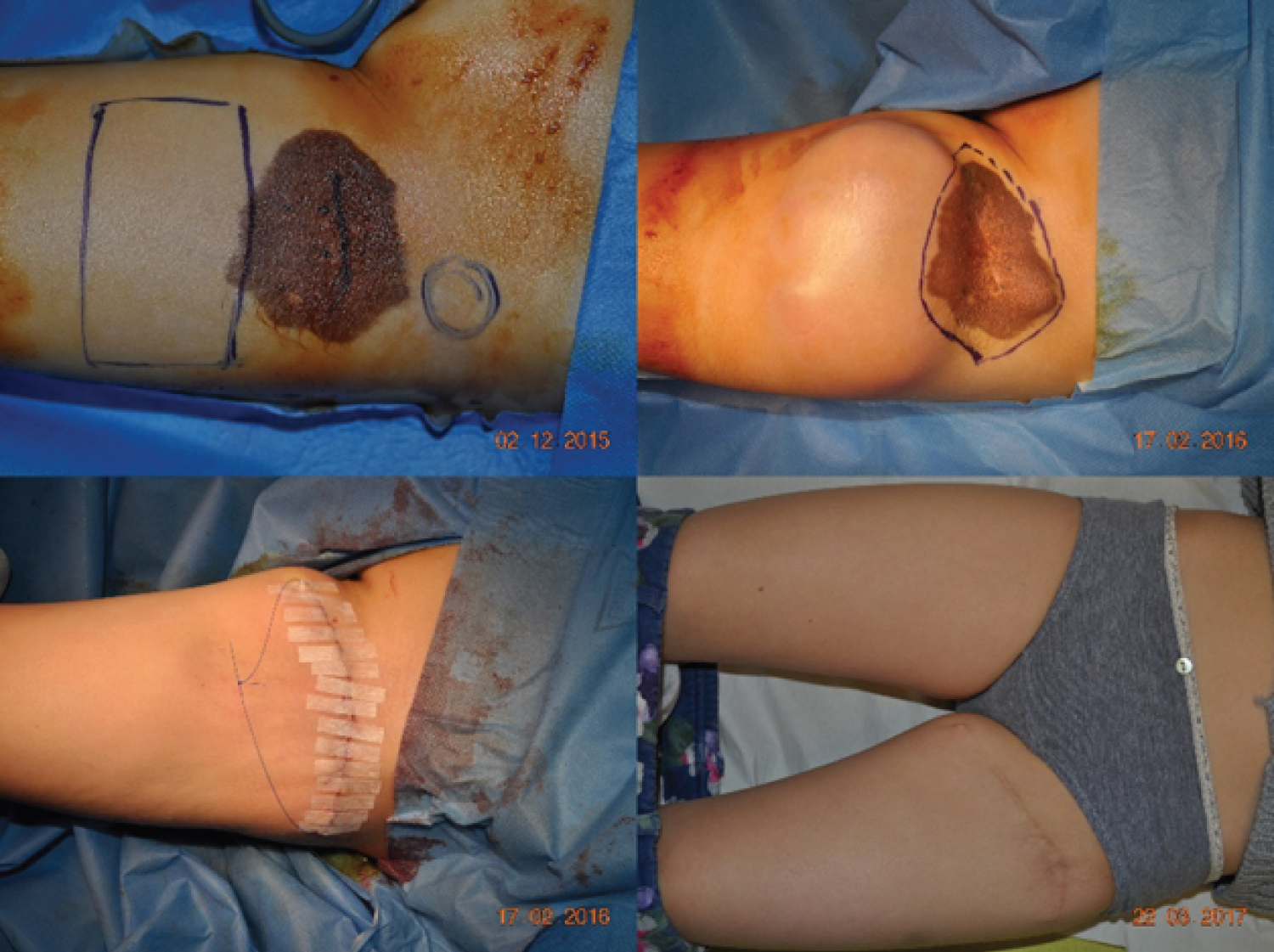
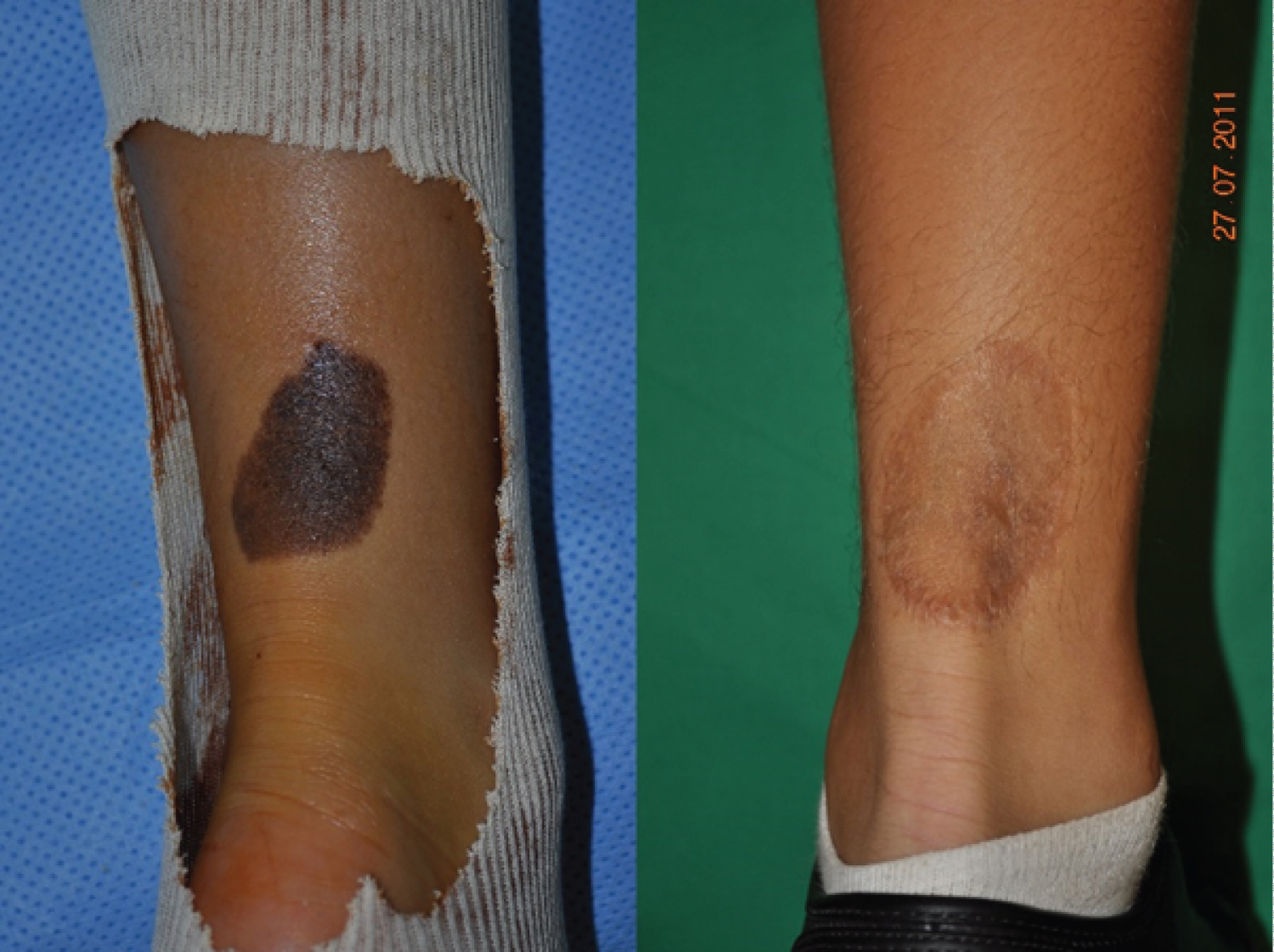
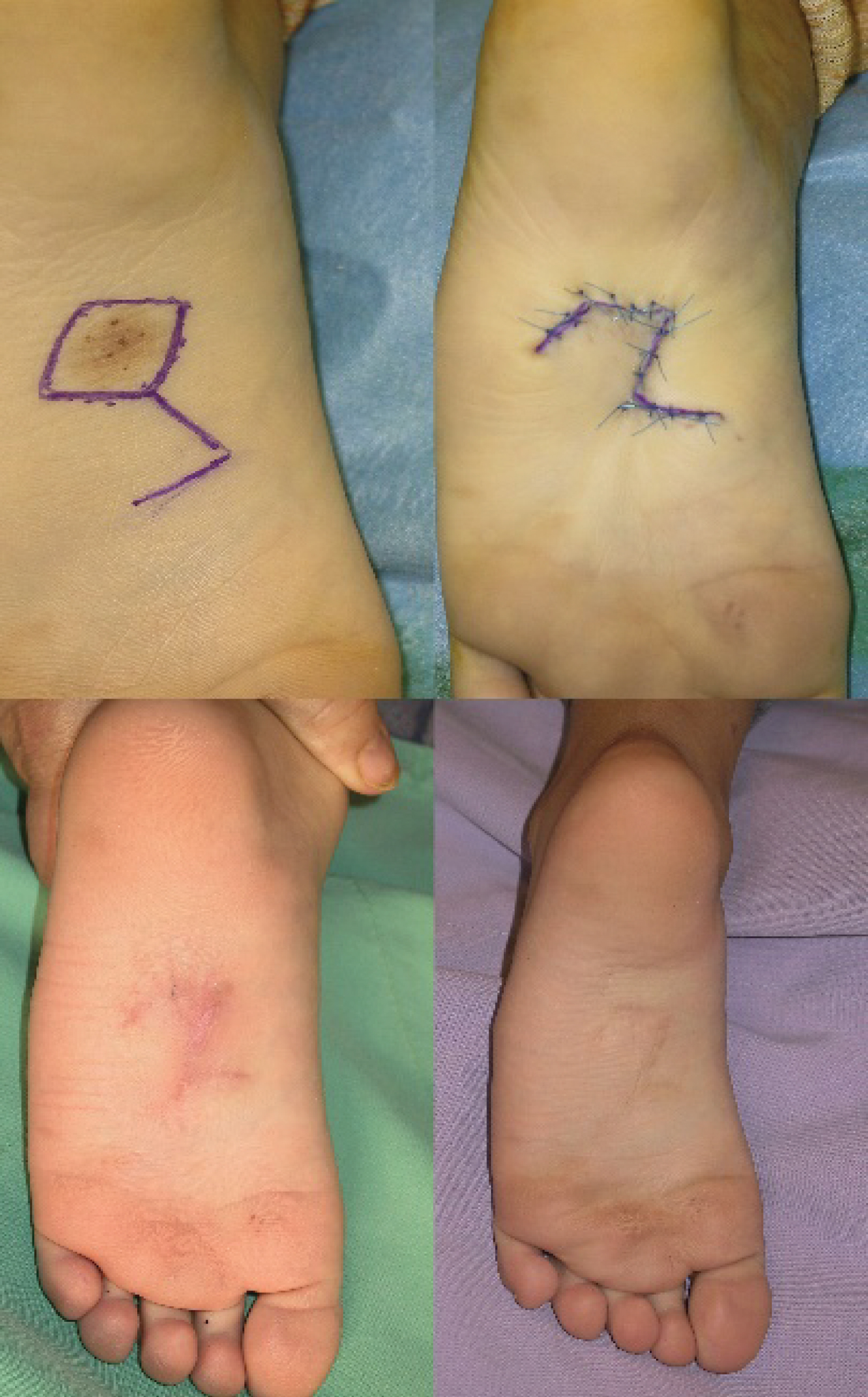
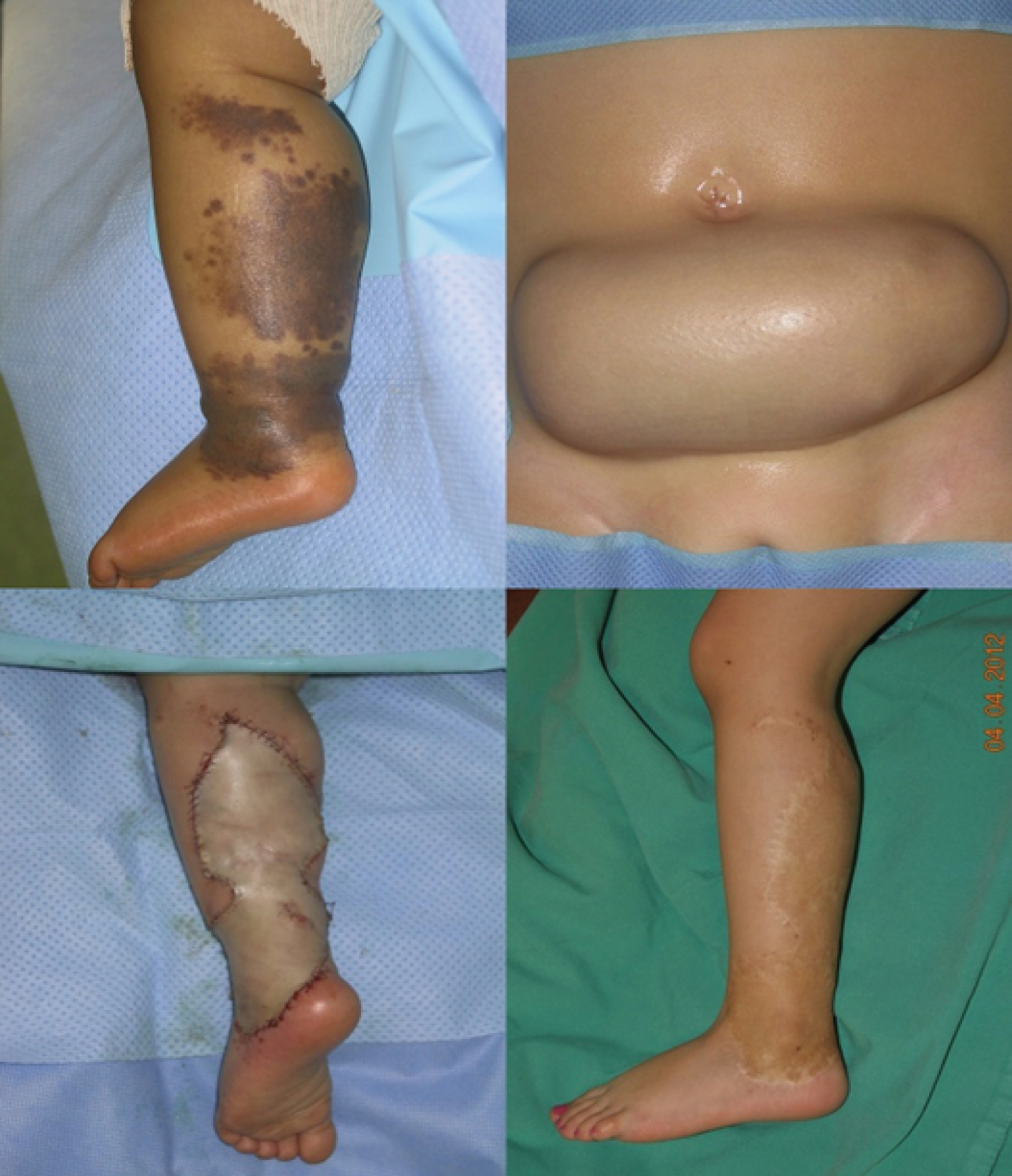
Age, sex, CMN area, surgical procedure, early and late follow-up visits, and final cosmetic result were extracted from the medical records. Surgical interventions were performed by several senior surgeons and the results were analyzed independently of the surgeon. Surgical procedures were divided in simple surgery (excision, subcutaneous and subcuticular suture, in one intervention) (Figure1) and complex surgery. This second group included any procedure that required several interventions and the use of skin expanders (Figure2), full-thickness skin grafts (Figure3), split-thickness skin grafts, local flaps (Figure4), artificial dermis, or multi-stage simple surgery. Some complex procedure has been combined (Figure 5). Full-thickness skin grafts were taken from the inguinal or abdominal region. Split-thickness skin grafts were taken from the scalp or close to the CMN location. In all cases, surgeons removed both the CMN and subcutaneous tissue to avoid recurrences, while preserving the underlying deep fascia. Margins were 1-2 millimeters of normal skin around the CNM. The early surgical complications were classified according to the Clavien-Dindo [14] criteria. The cosmetic results of the scar were classified as satisfactory or not.
Statistical analysis
Qualitative variables were described using frequencies and percentages, and quantitative variables using median, minimum, maximum, or mean and standard deviation (SD). Unpaired samples were compared with the Wilcox on-Mann-Whitney non-parametric test. CMN size data (length, width, surface area) for threshold identification were selected according to the area under the Receiver Operating Characteristic (ROC) curve (Area under Curve method - AUC). The AUC values were compared two-by-two with the Chi-square test. The threshold values were determined using the maximum Youden index. To determine the CMN size threshold in function of the age group (0 to 2.5 years, 2.6 to 10 years, and 10.1 to 18 years), a multivariate logistic regression analysis was performed. A curve of age threshold depending on the CMN size was created. Complications after simple and complex surgery were compared with the Cochran-Armitage tendency test. All statistical tests were performed with SAS Guide Enterprise version 7.12, and the alpha risk was set at 5%.
Results
Among the 209 eligible patients, 27 were excluded due to missing data or presence of more than one nevus in the lower limb, and finally data from 182 patient with a CMN in the lower limb (37% in the lower skin elasticity zone) were selected. The demographic features and CMN location are summarized in (Table 1). Pathological analysis did not find any malignancy feature in the CMN samples analyzed. The mean age at surgery was 4.7 years. Simple surgery was used in 79% of patients (Table 2). No patient required a free flap, laser technique or dermabrasion these last two techniques have been associated with a risk of malignant transformation [15,16]. The mean follow-up was 3 months [1 month-5 years]. Surgery was without complications (graded according to the Clavien-Dindo classification) for 82% of patients, and complications were not mentioned in the medical file of 58 patients. Overall, grade I complications (8% of patients) concerning scar healing, and grade II and III complications (5% of patients/each) local infection or necrosis (Table 3). No/grade I complication was observed in68%and 54% of patient in the simple and complex surgery group, respectively (Table 4). The complication distribution (all grades) was not different in the simple and complex surgery groups. On the other hand, complication rate tend to be higher in older patients (8% in the 0 to 2.5 years, 13% in the 2.6 to 10 years, and 26% in the 10.1 to 18 years group; 27%, 35% and 32% of missing data, respectively). Overall, the cosmetic result (scar) was satisfactory for 57% of patients at 3 months after surgery (Table 5).
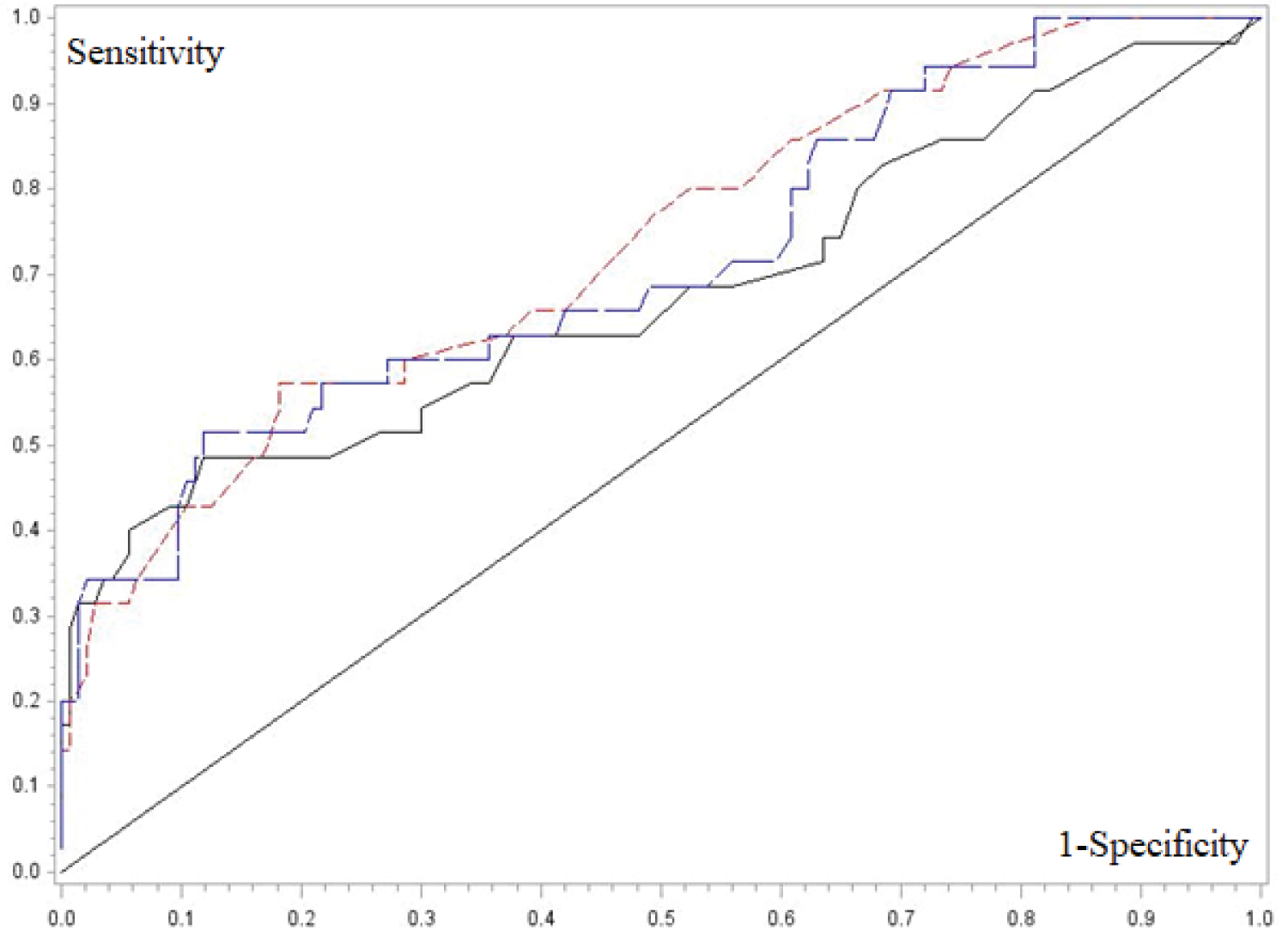
The mean (±SD) and median (range) length, width and surface area of the ESL were 36 mm (±31) and 29 mm [3-200], 21 mm (±19) and 15 mm [3-130], and 9.9 cm2 (±22.8) and 3.4 cm2 (0.7-204), respectively. Two-by-two comparison of the different ESL size parameters using the Chi-square test indicated that the surgical technique (simple vs complex) was chosen in function of the CMN size (p< 0.05) (Table 6). The ROC curves showed that the ESL width and surface area had the best sensitivity and specificity (Figure 6 and Table 7). The cutoff values of 26 mm for width and 1.25 cm2for surface area had the highest Youden index, independently from the patient's age and CMN localization (lower or higher elasticity region). When patients were divided in the three age groups, the ESL width cutoff for simple versus complex surgery differed according to the age (Table 8). For instance, an ESL width of 15 mm predicted the surgery type chosen for 10.1 to 18-year-old patients, with 60% of sensibility and 66% of specificity.
Discussion
Our study revealed a rule-of-thumb CNM size threshold of 26mmin width or 1.25cm² in surface area for using a simple removal technique, independently of the patient's age and CNM location in the lower limb. The low sensitivity of these cutoff values (< 60%) is not a problem for a clinical guide, whereas the specificity of 81% for width and 87% for surface area can be considered good enough. We did not find any other study suggesting a size threshold to help planning CMN excision. For less experimented surgeons, who may not confidently appreciate the skin elasticity, a rule-of-thumb size threshold may help to plan surgery and anesthesia (single or multiple interventions). Moreover, these results could be extrapolated to other conditions entailing skin loss (e.g. traumas).
One of the limits of the study was the use of CMN measurements made after surgery by the pathology laboratory. Indeed, the CNM size before surgery was not always recorded in the medical file. Moreover, CMN were isolated from limb regions in which skin elasticity varies depending on the location. Therefore, after surgery they retract and appear smaller, although laboratory measures should be more precise and repeatable than clinical estimation. However, compared with in vivo parameters, CMN size was underestimated. Also, in vivo, the edges of the area of skin loss retract, causing the defect to stretch after CMN excision. Similarly, the CMN spatial orientation within the Langer lines of skin tension was not taken into account. Moreover, several parameters, such as the clinical estimation and surgeon's experience, ability of the family and the patient to organize/undergo one or several interventions, social environment that can facilitate the management of an expander or a skin graft at home, could have influenced the technique choice by the surgeon, but could not be controlled in a retrospective study.
One strength of our study is that pediatric patients were classified in three different age groups. Indeed, this pediatric population presented a large age range (5months-17 years) where newborns, babies, toddlers, children and teenagers are grouped together, although limb size and skin elasticity physiologically varies with age. Moreover, patients with more than one nevus in the lower limb were excluded to obtain a more homogeneous picture, without CMN with satellite nevi. However, our rule of thumb could apply also to patients with multiple nevi, if they are distant from each other and do not interfere within skin elasticity. Finally, this study retrospectively analyzed the routine decisions by our team of surgeons, made without specific guidelines other than clinical sense. The retrospective design limits the potential classification bias between simple technique and complex techniques to cover skin loss.
In our study, most CMN were between 30 and 40mm in size, as previously reported [17]. No post-operative complication was recorded for82% of patients. As our center is the only plastic pediatric surgery department in the region, the 58 missing data for post-operative complications might indicate that the outcome was uneventful. Using the Clavien-Dindo classification,5% of grade I and 5% of grade II complications were identified, without any difference between simple and complex surgery groups and CMN width. Conversely, the complication rate tended to be lower in patients with CN Min the lower skin elasticity region and higher in older patients, which suggests the importance of the intrinsic skin parameters in complication occurrence. Moreover, in a newborn with a 20mm CMN of the leg, the projected diameter will attain almost 100mm in adulthood, according to the classification proposed by Krengel et al11. This should encourage the early resection also of little nevi to avoid a complex reconstruction if surgery is planned later in life.
To model the ability of skin loss reconstruction in one or several times, the lower limb was divided in two parts in function of the skin elasticity. At this time there is no consensual way to do so, particularly for pediatric populations. The chosen method makes clinical sense because the periarticular regions (knee, ankle, foot) are considered lower elasticity regions, and the non-particular regions (buttocks, thigh, leg) higher elasticity regions. Most of the time, CMN are localized and therefore, it is possible to perform a simple fusi form excision according to the long axis of the fibers and the skin tension lines. However, when this is not possible, a choice has to be made between simple and more complex procedures, without the support of recommendations about CNM management [18]. In this context, our rule-of-thumb size threshold according to the child's age might be taken into account during the decision-making process.
Our management for children follows the principle of early, preventive and complete surgery. Early removal is justified first by the malignancy risk and by the important CNM size augmentation during growth, especially in the lower limb. Moreover, skin elasticity and wound healing might be better before two years of age [19-21].According to some authors, operating younger children decreases CMN psychological consequences and improves the multiple surgery tolerance. Children younger than 3 years of age might not have memories of the treatment [10]. Bauer et al suggest operating from three to twelve months of age with the aim of completing CMN management by primary school initiation [22]. Moreover, nowadays the anesthesia risk in children is lower than the risk of malignant transformation. This percentage varies between studies but prophylactic removal is encouraged [8,23,24], knowing that melanoma could develop from CMN [25,26]. Also CMN of the lower leg, when large, are a challenge for reconstructive surgeons [13], thus justifying their early removal.
Acknowledgement
We would like to thank the surgeons who operated some patients in the department throughout the study period: Dr F. Bekara, Dr M. Bigorre, Dr F. Boissière, Dr J. Gibrila and ProfC. Herlin. We also would like to thank the parents who trusted the team and allowed us to take photos.
Conflicts of interest
None. Any of the authors have also no funding source to declare.
Authors contribution
Design and performed study: Prof. Guillaume Captier/Prof. Marie-Christine Picot/Dr Amin Kara/Dr Yacine Belkacemi
Collecting data: Dr Amin Kara / Dr Yacine Belkacemi.
Wrote and revised paper: Prof. Guillaume Captier/Prof. Marie-Christine Picot/Dr Amin Kara/Dr Yacine Belkacemi/Dr Federica Maggiulli
References
- Walton RG, Jacobs AH, Cox AJ, et al.(1976) Pigmented lesions in newborn infants. Br J Dermatol. 95: 389-396.
- Jugpal S Arneja, Arun K Gosain (2005) Giant congenital melanocytic nevi of the trunk and an algorithm for treatment. J Craniofac Surg 16: 886-893.
- Karvonen SL, Vaajalahti P, Marenk M, et al. (1992) Birthmarks in 4346 Finnish newborns. Acta Derm Venereol 72: 55-7.
- Castilla EE, da Graça Dutra M, Orioli-Parreiras IM et al. (1981) Epidemiology of congenital pigmented naevi: I. Incidence rates and relative frequencies. Br J Dermatol. 104: 307-315.
- Charbel C, Fontaine RH, Malouf GG, et al. (2014) NRAS Mutation Is the Sole Recurrent Somatic Mutation in Large Congenital Melanocytic Nevi. J Invest Dermatol 134: 1067-1074.
- DeDavid M, Orlow SJ, Provost N, et al. (1997) A study of large congenital melanocytic nevi and associated malignant melanomas: Review of cases in the New York University Registry and the world literature. J Am Acad Dermatol. 36: 409-416.
- Kopf AW, Bart RS, Hennessey P et al. (1979) Congenital nevocytic nevi and malignant melanomas. J Am Acad Dermatol 1: 123-130.
- Ruiz-Maldonado R, Tamayo L, Laterza AM, et al. (1992) Giant pigmented nevi: Clinical, histopathologic, and therapeutic considerations. J Pediatr 120: 906-911.
- Zaal LH, Mooi WJ, Sillevis Smitt JH, et al. (2004) Classification of congenital melanocytic naevi and malignant transformation: A review of the literature. Br J Plast Surg 57: 707-719.
- Bellier-Waast F, Perrot P, Duteille F, et al. (2008) Surgical treatment for giant congenital nevi: what are the psychosocial consequences for the child and family?. Ann Chir Plast Esthet 53: 408-414.
- Krengel S, Scope A, Dusza SW, Vonthein R, et al. (2013) New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol. 68: 441-451.
- Chuan Gu 1, Xiu-Xia Wang, Xusong Luo, et al. (2018) An alternative strategy treated giant congenital melanocytic nevi with epidermis and superficial dermis of the lesions. - PubMed - NCBI [Internet]. [cited 2020 Apr 26].
- Corcoran J, Bauer BS, et al. (2005) Management of large melanocytic nevi in the extremities. J Craniofac Surg 16: 877-885.
- Dindo D, Demartines N, Clavien P-A, et al. (2004) Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240: 205-213.
- Hori Y, Nakayama J, Okamoto M, (1989) et al. Giant congenital nevus and malignant melanoma. J Invest Dermatol. 92: 310S-314S.
- J A Zitelli, M G Grant, E Abell, (1984) et al. Histologic patterns of congenital nevocytic nevi and implications for treatment. J Am Acad Dermatol 11: 402-409.
- Alikhan A, Ibrahimi OA, Eisen DB et al. (2012) Congenital melanocytic nevi: where are we now? Part I. Clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 67 : 495.e1-17; quiz 512-4.
- Kryger ZB, Bauer BS, (2008) Surgical management of large and giant congenital pigmented nevi of the lower extremity. Plast Reconstr Surg 121: 1674-1684.
- Cousin-Verhoest S, Heusse J-L, Verhoest G, et al. (2012) Exérèse des nævus congénitaux géants : jusqu’où aller avec la chirurgie.
- Captier G (2015) Les nævus mélanocytaires de la face chez l’enfant : quoi de neuf .
- Rothfuss M, Schilling M, Breuninger H, et al. (2009) Early excision of congenital melanocytic nevi under tumescent anesthesia and skin expansion by intracutaneous double butterfly sutures. J Dtsch Dermatol Ges 7: 427-33.
- Bauer BS, Few JW, Chavez CD, et al. (2001) The role of tissue expansion in the management of large congenital pigmented nevi of the forehead in the pediatric patient. Plast Reconstr Surg. 107: 668-675.
- Rhodes AR. (1986) Melanocytic precursors of cutaneous melanoma. Estimated risks and guidelines for management. Med Clin North Am 70: 3-37.
- Quaba AA, Wallace AF (1986) The incidence of malignant melanoma (0 to 15 years of age) arising in ‘large’ congenital nevocellular nevi. Plast Reconstr Surg 78: 174-181.
- Illig L, Weidner F, Hundeiker M, et al. (1985) Congenital nevi less than or equal to 10 cm as precursors to melanoma. 52 cases, a review, and a new conception. Arch Dermatol 121: 1274-1281.
- Betti R, Inselvini E, Vergani R, et al. (2000) Small congenital nevi associated with melanoma: Case reports and considerations. J Dermatol 27: 583-590.
Corresponding Author
Prof. Guillaume Captier, Pediatric Orthopedic and Plastic Surgery, CHU Montpellier, University of Montpellier, Montpellier, France
Copyright
© 2022 Belkacemi Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.