The Effect of Pre-Operative Cabergoline on Prolactinoma Fibrosis: A Case Series
Abstract
Introduction: Prolactinomas are a frequently diagnosed intracranial neoplasm and constitute the majority of pituitary adenomas. Although patients can present with variable hormone dysregulation and symptom severity, the use of dopamine agonists (DA) often remains a first-line treatment. While bromocriptine has been found to increase tumor fibrosis, the effect of cabergoline on collagen deposition has been disputed. The aim of this paper is to understand the influence of cabergoline on tumor fibrosis prior to resection.
Case presentations: Four male patients who underwent resection of prolactinomas were included in this report. The average age was 39.8 years (range: 26 to 52 years). Pre-treatment prolactin levels ranged from 957.8 to 16,487.4 ng/mL. Three patients received cabergoline for at least one month prior to surgery (treatment range: 1 to 6 months). One patient had surgery without prior cabergoline use. Pathology reports confirmed each tumor to be of lactotroph origin. For each sample, Masson’s trichrome staining was performed and the percentage of sample fibrosis was quantified using an artificial intelligence imaging software. Among those who received pre-operative cabergoline, the extent of tumor fibrosis was in the range of 50-70%. In contrast, specimen fibrosis was approximately 15% without cabergoline use.
Conclusion: This report demonstrates that a short duration of pre-operative cabergoline can cause significant prolactinoma fibrosis. Understanding the effect of cabergoline on tumor consistency prior to surgery is essential as increased fibrosis can lead to more difficult tumor removal, reduce the extent of resection, and increase surgical complications. Considering these effects, further studies regarding the use of surgery prior to cabergoline for prolactinoma management are warranted.
Keywords
Cabergoline, Pituitary adenoma, Prolactinoma, Neurosurgery, Neuroendocrinology
Introduction
Pituitary adenomas are a commonly identified tumor of the skull base, with most being of lactotroph lineage and causing prolactin hypersecretion. Such tumors, referred to as prolactinomas, account for approximately 50% of all pituitary adenomas with an incidence of 3-5 cases/100,000/year [1, 2]. Without treatment, prolactinomas can result in amenorrhea, diminished libido, galactorrhea, erectile dysfunction, infertility, gynecomastia, and decreased bone mass [3,4]. Additionally, patients may develop symptoms of hypopituitarism, visual field deficits, cranial nerve dysfunction, and seizures as a result of the mass effect that large tumors may impose on nearby intracranial structures [4].
Currently, the preferred initial treatment of prolactinomas consists of medical management with dopamine agonists (DA), such as cabergoline. Use of this medication has proven effective at normalizing prolactin levels and reducing tumor volume in a majority of patients, with notable improvement in clinical symptoms [5]. While previous reports have documented bromocriptine use with increased tumor fibrosis, limited data exists on the relationship between cabergoline and pituitary tumor collagen content. In this report, we present three cases of prolactinomas treated with cabergoline prior to surgery, resulting in significant tumor fibrosis. Given that increased amounts of fibrotic tissue can increase the risk of surgical complications and reduce the potential for complete resection, understanding the effects of pre-operative cabergoline use on tumor consistency is imperative for surgical planning and future patient management.
Case Presentations
Case 1
A 51-year-old male with a past medical history of hyperlipidemia and hypertension presented with sudden-onset headaches, bilateral blurry vision, photophobia, and diminished libido and spontaneous erections. He denied galactorrhea, breast engorgement, heat/cold intolerance, change in ring or shoe size, fatigue, fractures, polyuria, polydipsia, weakness, or weight changes. On initial physical exam, pupils were equal and reactive to light and extraocular movements were intact, however, inferotemporal field loss in the right eye and supertemporal field loss in the left eye were present. His strength, reflexes, coordination, and gait were all normal. The remaining physical exam was unremarkable. Optical coherence tomography (OCT) revealed diminished retinal nerve fiber layer (RNFL) thickness, with a value of 70µ and 71µ in the right and left eye, respectively.
Initial endocrine work-up revealed a significantly elevated prolactin of 4,053.2 ng/mL (ref. range: < 15 ng/mL). Other laboratory results showed thyroid stimulating hormone (TSH) 1.94 uU/mL (ref. range: 0.3-4.2 uU/mL), free thyroxine (FT4) < 0.4 ng/dL (ref. range: 0.6-1.5 ng/dL), follicle-stimulating hormone (FSH) 0.5 mIU/mL (ref. range: < 15 mIU/mL for adult male), luteinizing hormone (LH) 0.2 mIU/mL (ref. range: < 10 mIU/mL for adult male), testosterone 1 ng/dL (ref. range: 250-1100 ng/dL), cortisol < 1.0 ug/dL (ref. range: 4-20 ug/dL), and insulin-like growth factor-1 (IGF-1) 18 ng/mL (ref. range: 50-317 ng/mL). Magnetic resonance imaging (MRI) of the brain demonstrated a heterogeneously enhancing sellar mass elevating the optic chiasm and bilateral inferomedial frontal lobes with invasion into the left cavernous sinus. The lesion measured 5.4 cm (craniocaudal [CC]) × 2.9 cm (transverse [TV]) × 3.7 cm (anterior-posterior [AP]).
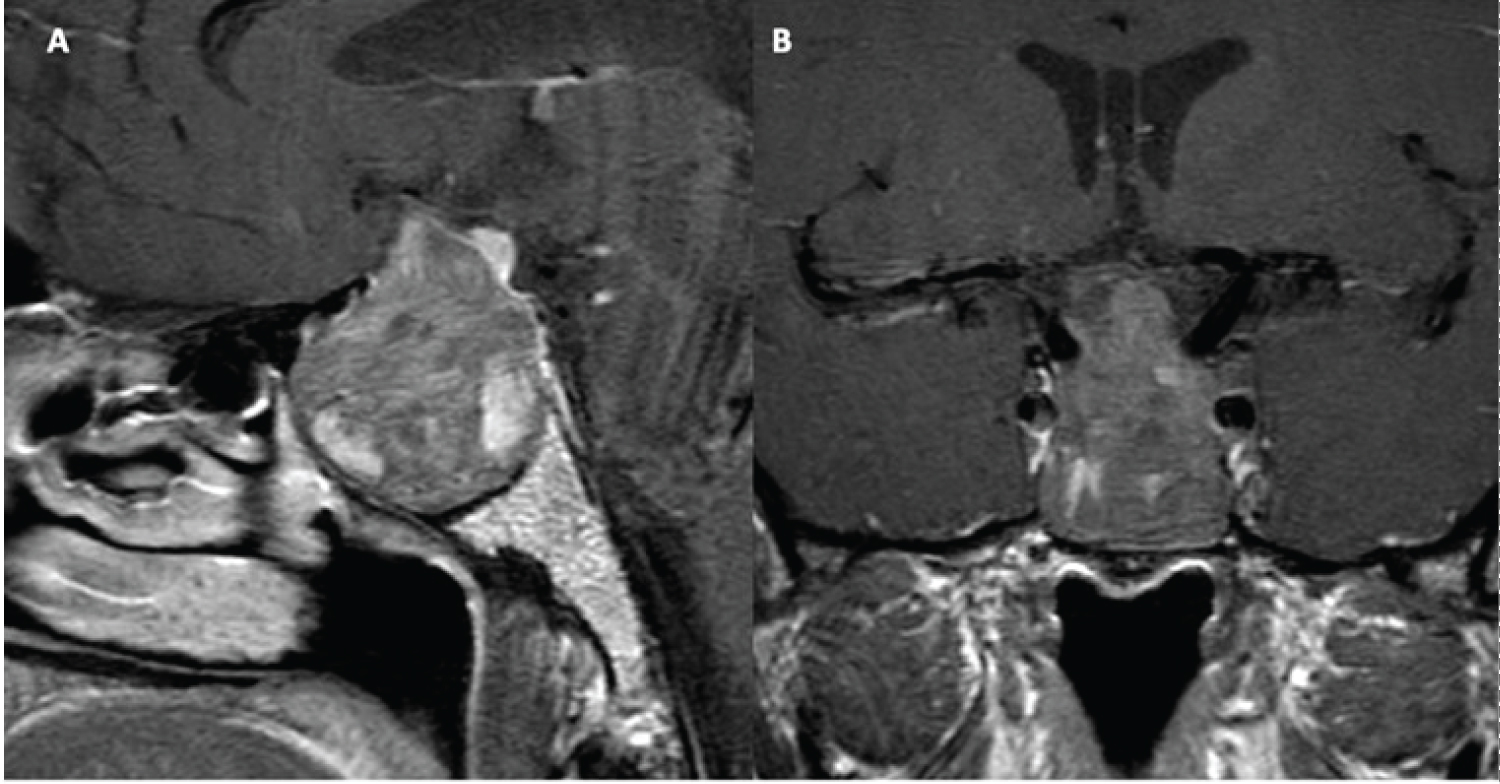
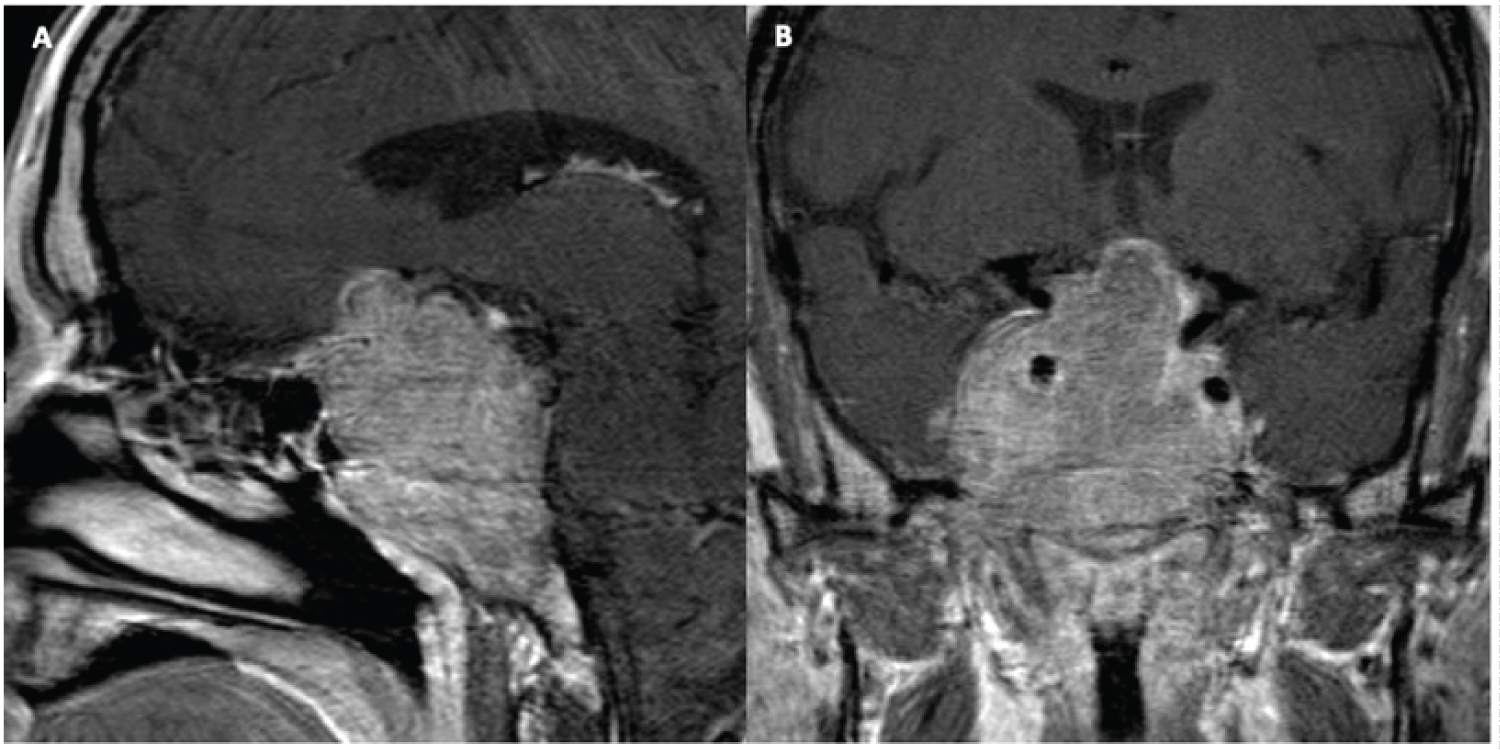
Treatment with cabergoline was initiated at 0.5 mg, twice weekly. Additionally, the patient received hydrocortisone (2x/day; dose 15/10 mg) and levothyroxine (50 ug daily). The patient noted improvement of his headaches after a few days but opted for surgical intervention after one month of treatment, despite decreasing tumor size on MRI, due to concerns of continued blurry vision and visual field deficits (Figure 1).
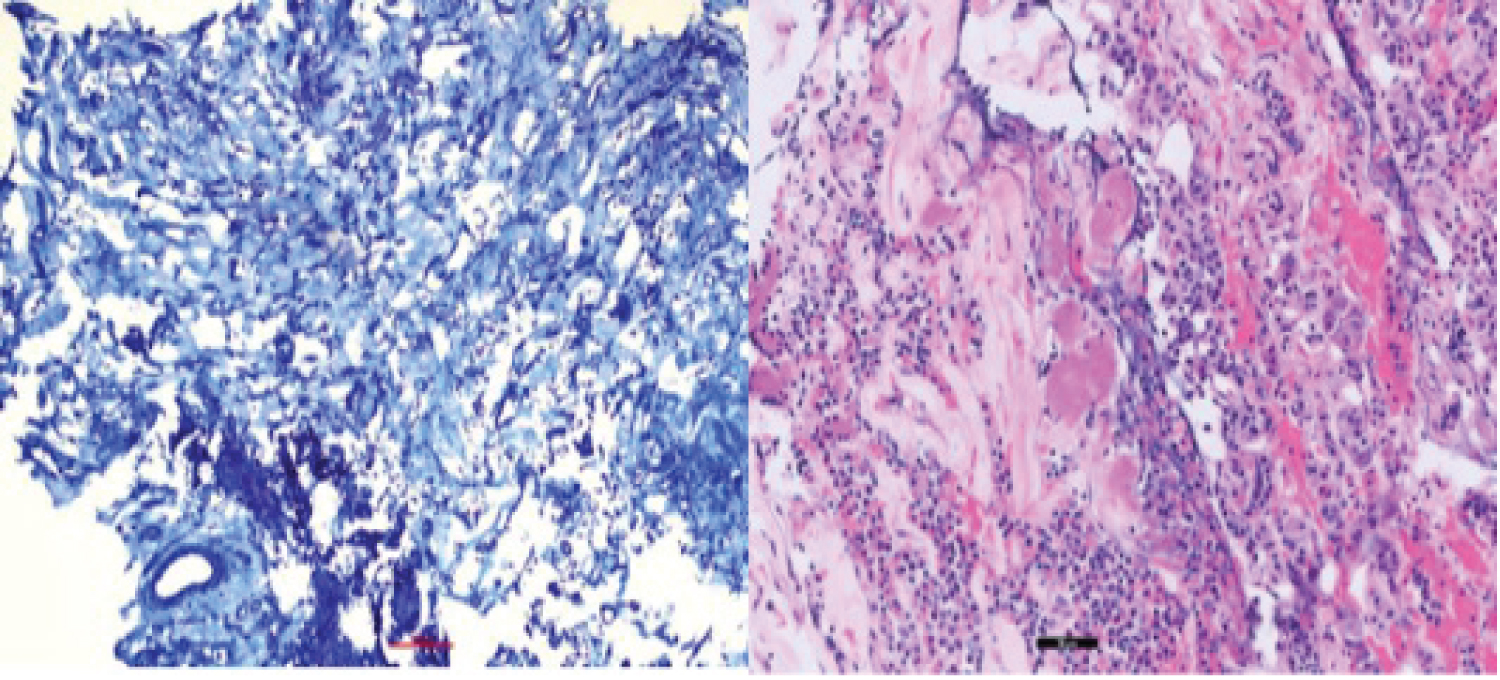
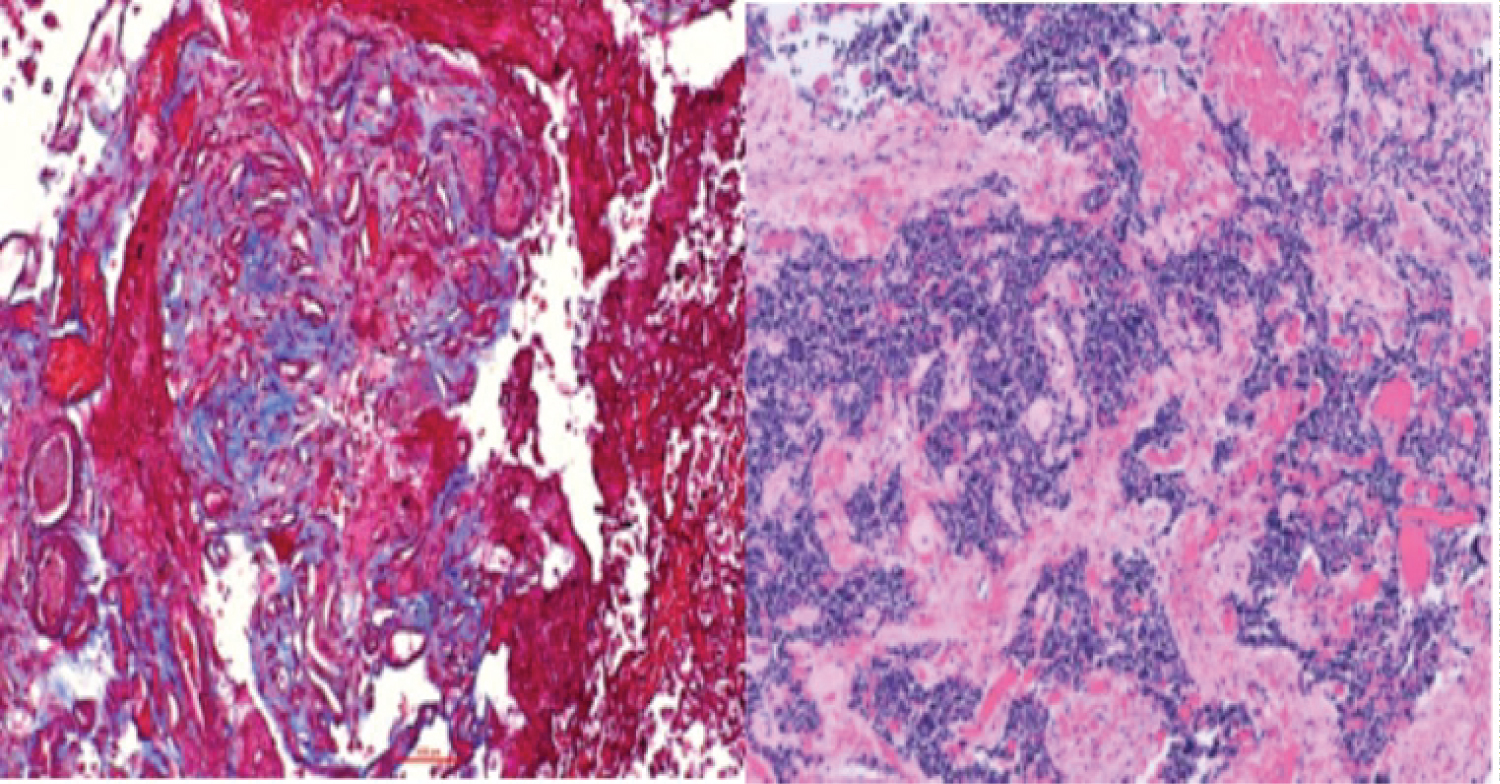
The patient underwent an unremarkable endoscopic transnasal transsphenoidal resection of the pituitary tumor. Pathology of the respected tissue revealed a lactotroph adenoma with neoplastic cells arranged in sheets, exhibiting round to oval nuclei with speckled chromatin and scant amounts of amphophilic cell cytoplasm. Significant perivascular fibrosis was present. Quantification of overall specimen fibrosis after Masson’s trichrome stain revealed a level of 60-70% on ImageJ (Figure 2).
Post-operatively, his prolactin levels normalized (< 5.0 ng/mL) and cabergoline use was discontinued. Post-operative MRI at 1-month confirmed that gross total resection was achieved. By his 3-month follow-up visit, the patient reported significant improvement in his prior visual field deficits and resolution of headaches. Endocrine labs were not completed at this time and the patient was lost to follow-up.
Case 2
A 30-year-old male with no significant past medical history presented with eight months of peripheral visual field loss, blurry vision, headaches, and decreased libido. He denied gynecomastia, galactorrhea, Change in hair distribution, polyuria, polydipsia, Change in ring or shoe size, fatigue, heat/cold intolerance, weight changes, or weakness. Physical exam was unremarkable aside from left-sided visual field deficits. Laboratory work-up revealed a prolactin level of 2,624 ng/mL, TSH 2.5 uU/mL, FT4 0.7 ng/dL, LH 1.8 mIU/mL, FSH 3.5 mIU/mL, testosterone 33 ng/dL, adrenocorticotropic hormone (ACTH) 78 pg/mL (ref. range: 6-50 pg/mL), and cortisol 1.8 ug/dL. MRI of the brain from an outside institution showed a 2.5 × 3.5 cm sellar mass.
The patient was started on cabergoline 0.5 mg, twice weekly, which was taken for one month before discontinuation by the patient per his preference. He reported improvement in vision loss and prolactin levels decreased to 369.6 ng/mL. Ophthalmology evaluation at this time revealed complete temporal hemianopsia on the left and superior temporal vision defect on the right, with diminished RNFL thickness of 79µ and 65µ in the right and left eye, respectively. After one month of no cabergoline therapy, the patient was restarted on 0.5 mg, three times per week. The patient continued the medication for an additional five months, with improvement of prolactin levels to < 5.0 ng/mL. MRI showed tumor shrinkage (2.6 cm (AP) × 3.0 cm (TV) × 3.3 cm (CC)), however, the patient did not notice any visual improvements (Figure 3). Repeat OCT values were 77µ for the right and 61µ for the left eye.
The patient underwent an uncomplicated transnasal transsphenoidal pituitary tumor resection due to progressively worsening visual field loss on cabergoline therapy. Pathology report detailed a pituitary adenoma staining positive for prolactin and growth hormone, with features of a mixed sparsely granulated lactotroph (predominant component) and somatotroph adenoma (relatively minor component). The neoplastic cells were arranged in nests and exhibited round to oval nuclei with speckled chromatin and small amounts of chromophobic to faintly eosinophilic cell cytoplasm. Significant mitotic figures were not identified. Masson’s trichrome staining revealed an overall specimen fibrosis of 50-60% (Figure 4).
The patient experienced no post-operative complications and was discharged without continued cabergoline treatment. At his three-month follow-up appointment, he reported decreased headaches with minimal vision improvement. MRI revealed subtotal resection of the adenoma with a small portion of residual tumor invading into the right cavernous sinus, surrounding the internal carotid artery. The patient was subsequently lost to follow-up.
Case 3
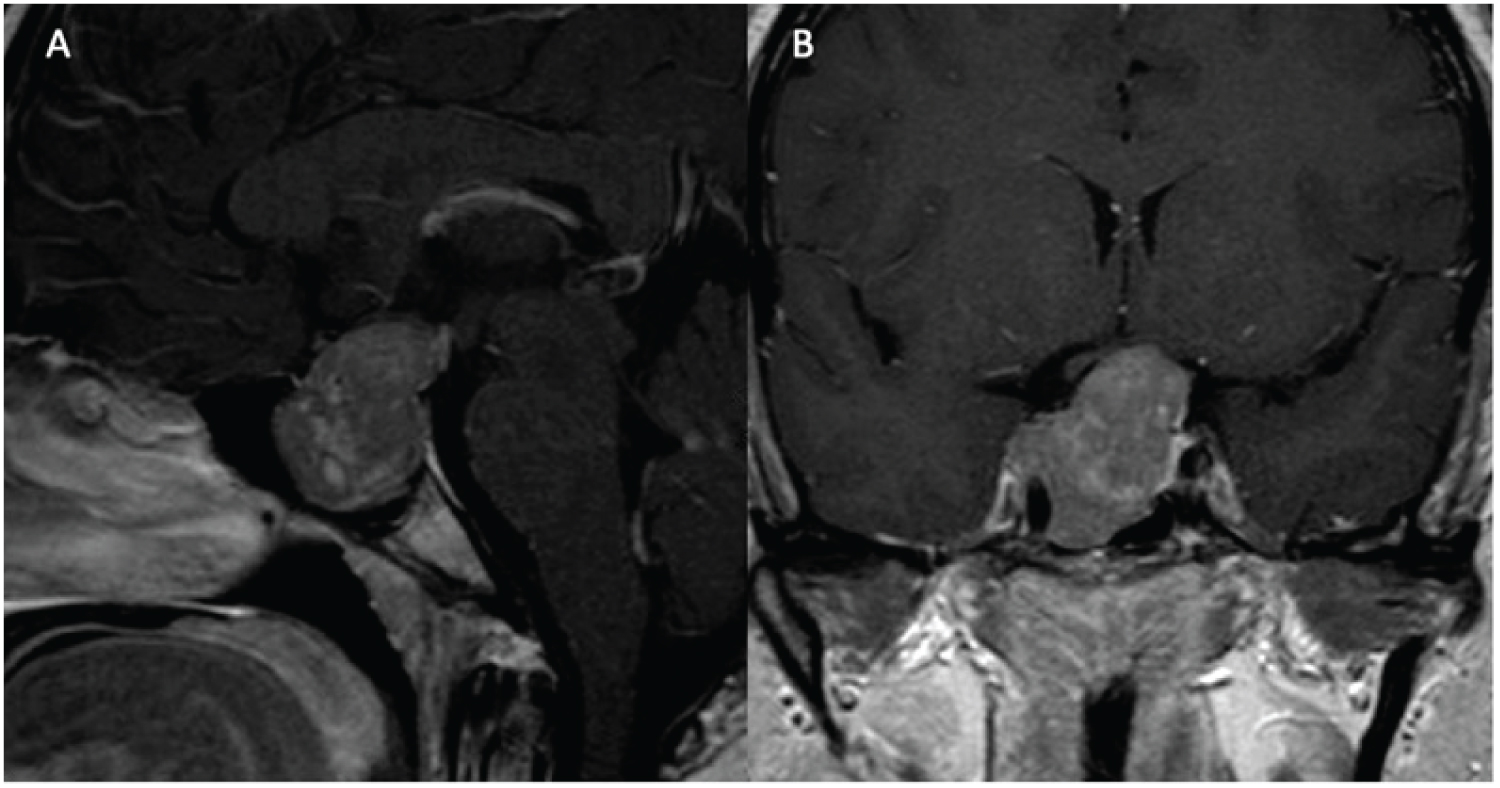
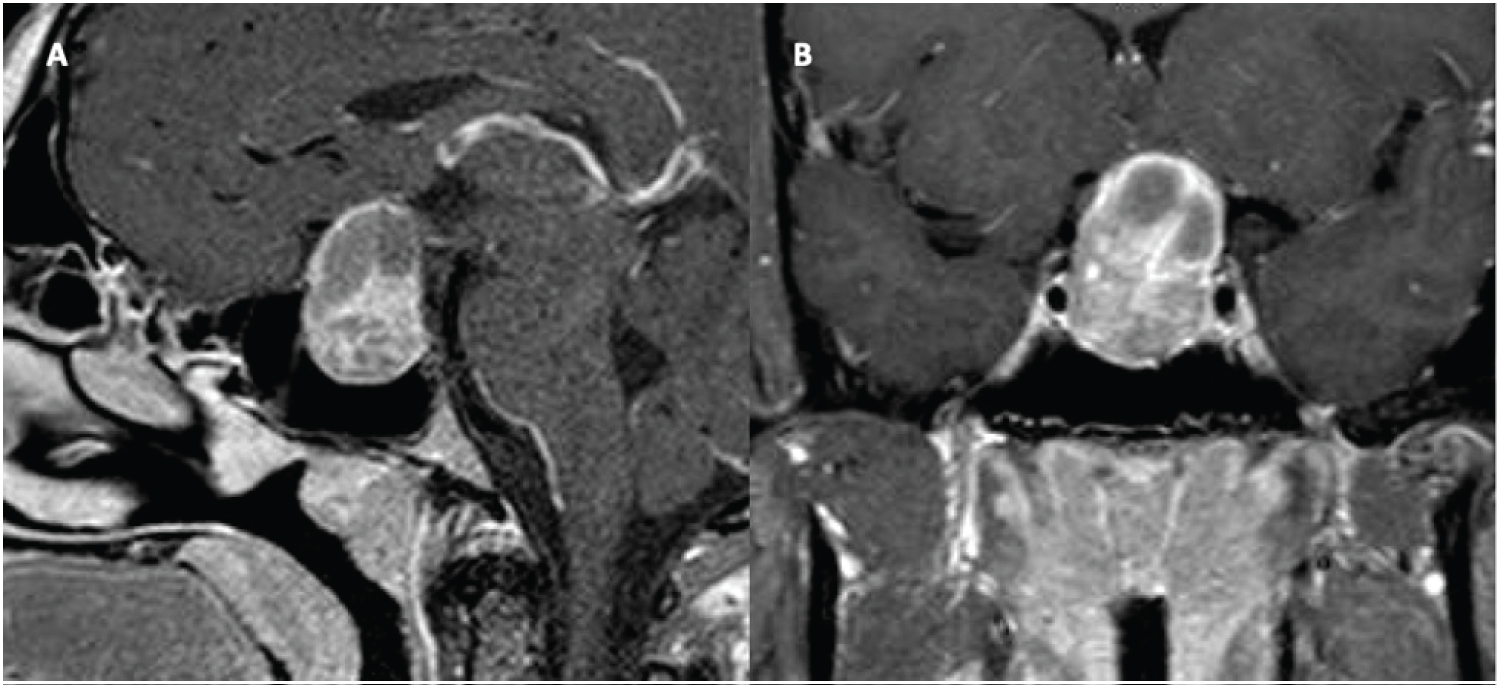
A 52-year-old male with no significant past medical history presented with headaches, decreased libido, and lack of spontaneous erections. He did not have galactorrhea, gynecomastia, weight changes, vision deficits, fatigue, Change in ring or shoe size, weakness, polydipsia, polyuria, or heat/cold intolerance. Physical exam was unremarkable aside from a right eye temporal visual field defect. Initial endocrine work-up revealed a prolactin level of 16,487.4 ng/mL, TSH 1.11 uU/mL, FT4 0.7 ng/dL, LH 0.8 mIU/mL, FSH 2.0 mIU/mL, total testosterone 64 ng/dL, and IGF-1 57 ng/mL. MRI of the brain from an outside institution demonstrated a large intra and suprasellar mass measuring 4.8 cm (TV) × 5.2 cm (CC) × 4.5 cm (AP) that extended into the bilateral cavernous sinuses, right middle cranial fossa, and inferiorly to the clivus. Significant optic chiasm compression and involvement of the inferomedial bilateral frontal lobes were noted.
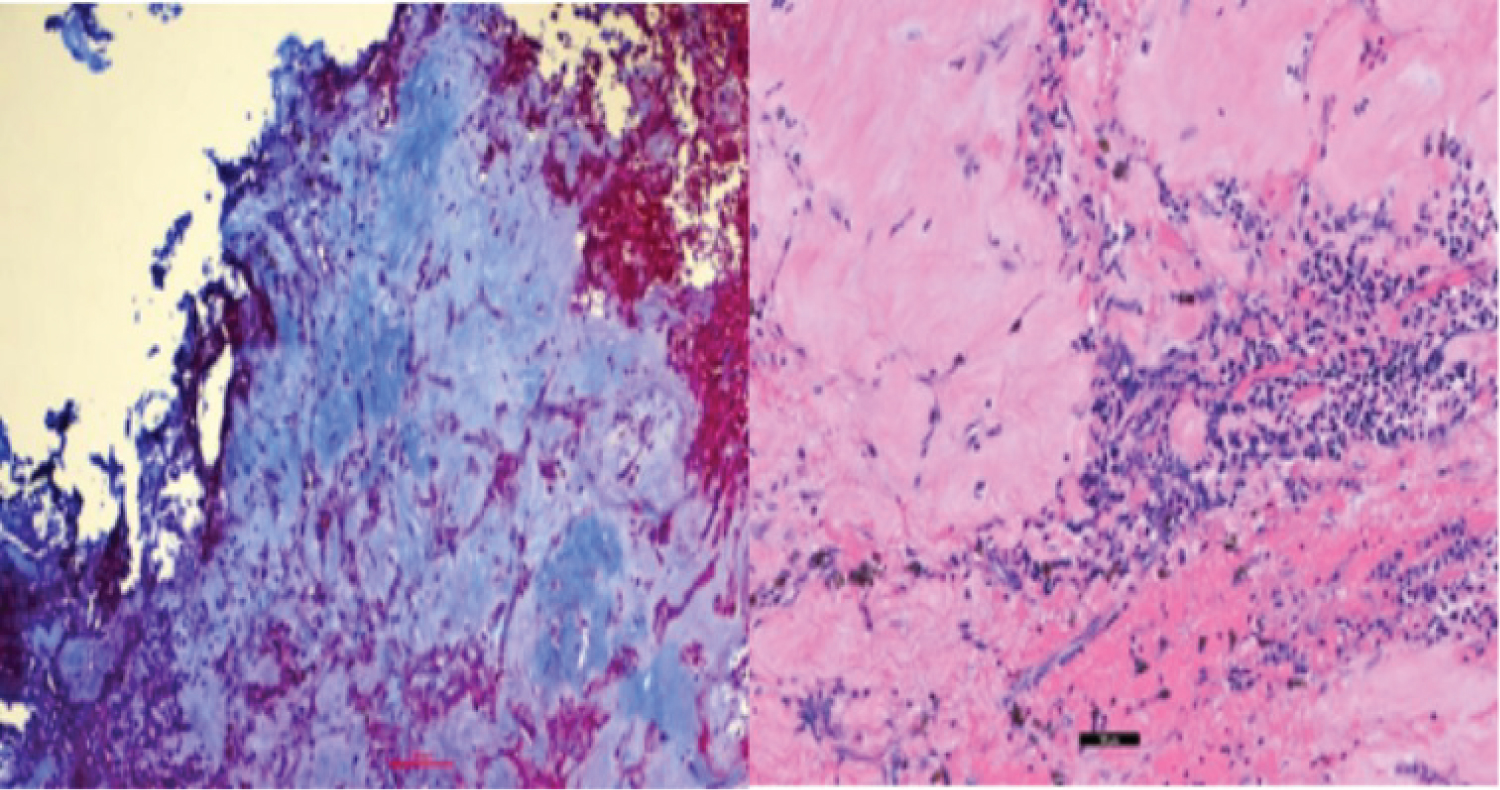
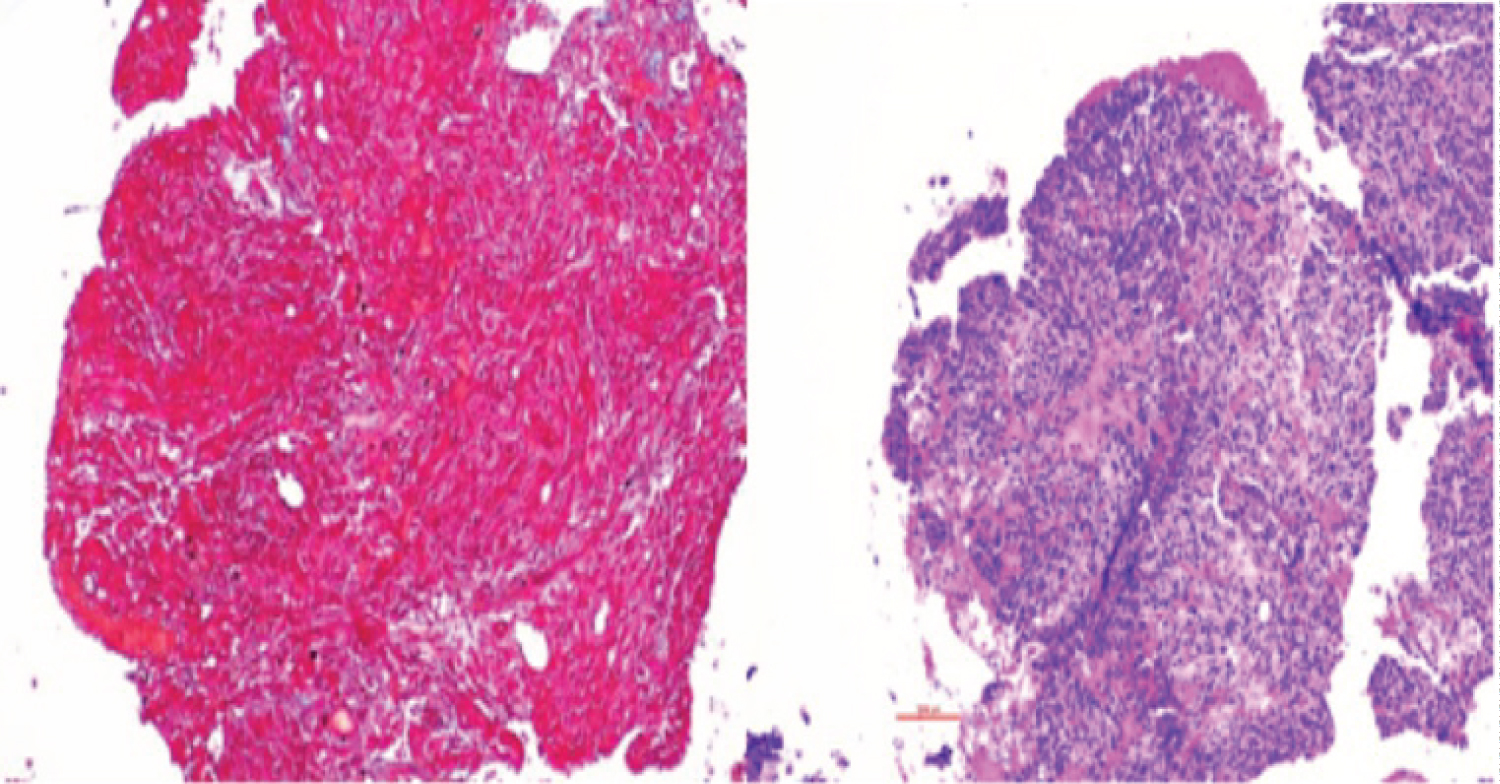
The patient was initially scheduled for surgical resection; however, surgery was delayed due to a nasal septal perforation in the setting of cocaine use. In the interim, he was started on cabergoline 0.5 mg, twice weekly. After one month of treatment, prolactin levels decreased to 13.1 ng/mL. After two months of treatment, the patient had significant improvement of his temporal visual field in the right eye, however, OCT report demonstrated diminished RNFL thickness (78µ right eye, 73µ left eye). At this time, he developed severe headaches. MRI showed heterogeneous internal signal enhancement of the lesion, suggestive of hemorrhage, with persistent invasion into the cavernous sinus, sphenoid sinus, and central skull base (Figure 5). As a result, surgical resection of the tumor was completed without any complications. The pathology report revealed a sparsely granulated lactotroph tumor, with neoplastic cells arranged in clusters within fibrotic tissue. Overall specimen fibrosis on Masson’s trichrome staining was 50-60% (Figure 6).
The patient had an uncomplicated post-operative course and was discharged without cabergoline. Follow-up endocrine labs two weeks after surgery revealed a prolactin level of 14.3 ng/mL and a persistently low total testosterone level of 35 ng/dL. Humphrey visual field testing demonstrated grossly full vision in both eyes and the patient reported only occasional headaches. Repeat prolactin levels 2-months after surgery showed an increase to 589.7 ng/mL. Cabergoline treatment was recommended at this time. However, the patient was lost to follow-up. As a result, post-operative imaging was not obtained.
Case 4
A 26-year-old male with no significant past medical history presented to the emergency department with 1.5 months of progressive vision loss in the left eye and daily headaches. Aside from fatigue and decreased libido, further review of systems was negative. Physical exam was notable for decreased visual acuity in the left eye, diffuse left peripheral vision loss, and right superotemporal field loss. OCT values were within normal limits. Initial MRI of the brain showed a large sella and suprasellar mass measuring 3.4 cm (CC) × 2.2 cm (TV) × 2 cm (AP). The lesion elevated and compressed the optic chiasm with areas of hypointensity and fluid levels, likely secondary to underlying hemorrhage. Laboratory work-up revealed a prolactin level of 957.8 ng/mL, TSH 1.02 uU/mL, FT4 0.2 ng/dL, LH 0.6 mIU/mL, FSH 1.5 mIU/mL, total testosterone 2 ng/dL, IGF-1 74 ng/mL, and cortisol 2.1 ug/dL.
Due to imaging evidence of hemorrhage and concern for apoplexy, transnasal transsphenoidal removal of the adenoma was performed (Figure 7). Surgical resection was uncomplicated and pathology report revealed a sparsely granulated lactotroph adenoma. The neoplastic cells were arranged in solid clusters and exhibited irregularly shaped nuclei with speckled chromatin and prominent nucleoli. Cellular cytoplasm showed a juxtanuclear eosinophilic region and a basophilic peripheral zone. Ki67 immunohistochemical staining was focally elevated up to 11%. Overall specimen fibrosis was 15% on Masson’s trichrome staining (Figure 8).
The patient was discharged home on hydrocortisone (20 mg, twice daily) and levothyroxine (50 µg daily). Three weeks after surgery, prolactin levels declined to 77.3 ng/mL, cortisol improved to 10.1 ug/dL, and testosterone continued to be low at a total level of 15 ng/dL. TSH was 0.52 uU/mL and FT4 was 0.7 ng/dL. He reported his vision was fully restored, headaches were resolved, and there was an improvement in his libido with increased morning erections. Two months after surgery, prolactin remained elevated at 80.1 ng/mL and the patient was initiated on cabergoline 0.25 mg, twice weekly. MRI at 3 months revealed gross total resection, with persistently elevated prolactin to 225.3 ng/mL. Treatment with cabergoline was increased to 0.5 mg, twice weekly, While use of hydrocortisone and levothyroxine was tapered off due to normalization of hormone levels. Follow-up imaging 6 months later demonstrated tumor along the posterior and left aspects of the pituitary. After this time, the patient was lost to follow-up with no additional endocrine labs obtained (Table 1).
Discussion
In this report, we describe three cases of prolactinomas treated with cabergoline therapy, resulting in significantly increased tumor fibrosis prior to surgical resection. Pre-operative medical treatment with dopamine agonists has remained the standard of care for prolactin-secreting pituitary adenomas, demonstrating efficacy in reducing prolactin levels and markedly decreasing tumor size [6]. However, the use of medical therapy has been shown to result in significant side-effects within the gastrointestinal, neurological, and cardiovascular systems [7,8]. Bromocriptine use, in particular, has been found to result in substantial tumor, cardiac valve, and retroperitoneal fibrosis, yet patients treated with cabergoline have been found to be less likely to experience such effects [9,10]. Additionally, the fibrotic effect of dopamine agonist therapy has been most commonly observed after several years of treatment [11]. Surprisingly, in this report, cabergoline use was associated with increased tumor fibrosis after 6 months of treatment or less.
Despite understanding the potential for dopamine agonist therapy to increase collagen deposition within tumors, there has been debate regarding the effects of fibrosis on clinical outcomes. While some may argue that preoperative medical therapy results in improved patient outcomes by allowing for greater tumor resection, other reports have documented that increased adenoma fibrosis is associated with more difficult tumor removal and decreased ability to achieve gross total resection [12,13]. Not only can fibrosis create a more challenging surgical case, it may also elevate the risk of intra- or post-operative complications. Prior studies have demonstrated that pre-operative radiation therapy leads to a higher rate of surgical morbidity, such as cerebrospinal fluid leaks, postulated to be due to radiation-induced tissue fibrosis [14,15]. The effect of cabergoline therapy on fibrosis has thus been thought to result in similar operative complications.
Aside from the surgical risks that may result from dopamine agonist use, biochemical dysregulation may be more likely to persist among those treated with pre-operative medical therapy. Menucci, et al., demonstrated that pituitary adenomas with increased fibrosis were less likely to exhibit hormone normalization after surgery compared to those that were deemed non-fibrous [9]. Further studies have corroborated these results, finding that even a short duration of pre-operative dopamine agonist treatment decreased the chances of hormonal homeostasis [16]. As a result, those treated with upfront cabergoline treatment may be at higher risk of requiring a significantly prolonged duration of medication use. As a result of the potential consequences of medical treatment prior to surgery, some physicians argue that surgical intervention should be prioritized as a first-line treatment as doing so may optimize long-term patient outcomes and mitigate post-operative complications.
At this time, current indications for the surgical resection of pituitary adenomas include resistance to DA therapy, intolerance to medication side effects, pituitary apoplexy, and patient preference [16]. Resistance to medical therapy has been estimated to occur in 20-30% of prolactinomas treated with bromocriptine and 10-20% of those treated with cabergoline, with tumor size and invasiveness being predictive factors for resistance [17]. In fact, one surgical series found DA resistance to be one of the most common indications for surgical intervention, accounting for over 64% of cases [16]. DA intolerance, on the other hand, is a less likely cause of surgical intervention. While high rates of cabergoline side effects have been reported, the severity of such effects has not proven to be a common cause of drug discontinuation. One cohort study, for example, documented side effects in 68% of women with hyperprolactinemic amenorrhea treated with cabergoline, with only 3% requiring medication cessation [18]. Given the moderate rates of tumor resistance, the prevalence of undesirable side effects, and the increased chances of long-term post-operative medication use after pre-operative cabergoline use, it can be argued that surgical intervention should be considered prior to medical management for many patients, particularly those who present with visual compromise.
In consideration of the negative outcomes that pre-operative cabergoline use may impose on patients, it is necessary to determine indications for initial management of prolactinomas with surgical intervention. One retrospective study of patients treated with upfront surgical resection found that micro- or macroprolactinomas with Knosp grade 0 were most likely to not require post-operative DA therapy [19]. However, given the increase in tumor fibrosis associated with cabergoline use, initial surgical management may prove advantageous for patients with large tumors, as well. Therefore, in contrast to current recommendations, patients with both small and large tumors may benefit from surgery prior to medical therapy, especially those experiencing visual deficits.
Conclusion
Prolactinomas treated with pre-operative cabergoline for at least one month prior to surgical resection exhibit increased tumor collagen deposition. Given that tumor fibrosis can decrease the ability to achieve gross total resection, increase the risk of operative complications, and result in the need for prolonged DA treatment after surgery, surgery may be the optimal first-line treatment for patients with prolactinomas presenting with visual compromise.
Declarations of Interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Petersenn S, Fleseriu M, Casanueva FF, et al. (2023) Diagnosis and management of prolactin-secreting pituitary adenomas: A pituitary society international consensus statement. Nat Rev Endocrinol 19: 722-740.
- Chanson P, Maiter D (2019) The epidemiology, diagnosis and treatment of Prolactinomas: The old and the new. Best Pract Res Clin Endocrinol Metab 33: 101290.
- Molitch ME (2017) Diagnosis and treatment of pituitary adenomas: A review. JAMA 317: 516-524.
- Majumdar A, Mangal NS (2013) Hyperprolactinemia. J Hum Reprod Sci 6: 168-175.
- Moraes AB, dos Santos Silva CM, Neto LV, et al. (2013) Giant prolactinomas: The therapeutic approach. Clin Endocrinol 79: 447-456.
- Alsubaie S, Almalki MH (2014) Cabergoline treatment in invasive giant prolactinoma. Clin Med Insights Case Rep 7: 49-51.
- Verhelst J, Abs R, Maiter D, et al. (1999) Cabergoline in the treatment of hyperprolactinemia: A sstudy in 455 patients. J Clin Endocrinol Metab 84: 2518-2522.
- Su J, Simonsen ULF, Carlsen J, et al. (2017) Pulmonary artery occlusion and mediastinal fibrosis in a patient on dopamine agonist treatment for hyperprolactinemia. Front Pharmacol 8: 492.
- Menucci M, Quiñones-Hinojosa A, Burger P, et al. (2011) Effect of dopaminergic drug treatment on surgical findings in prolactinomas. Pituitary 14: 68-74.
- Herzog A, Minne H, Ziegler R (1989) Retroperitoneal fibrosis in a patient with macroprolactinoma treated with bromocriptine. BMJ 298: 1315.
- Esiri MM, Bevan JS, Burke CW, et al. (1986) Effect of bromocriptine treatment on the fibrous tissue content of prolactin-secreting and nonfunctioning macroadenomas of the pituitary gland. J Clin Endocrinol Metab 63: 383-388.
- Mohan N, Chia YY, Goh GH, et al. (2017) Cabergoline-induced fibrosis of prolactinomas: A neurosurgical perspective. BMJ Case Rep 2017: bcr2017220971.
- Sughrue ME, Chang EF, Tyrell JB, et al. (2009) Pre-operative dopamine agonist therapy improves post-operative tumor control following prolactinoma resection. Pituitary 12: 158-164.
- Boling CC, Karnezis TT, Baker AB, et al. (2016) Multi-institutional study of risk factors for perioperative morbidity following transnasal endoscopic pituitary adenoma surgery. Int Forum Allergy Rhinol 6: 101-107.
- Maiter D (2019) Management of dopamine agonist-resistant prolactinoma. Neuroendocrinology 109: 42-50.
- Barnett GC, West CML, Dunning AM, et al. (2009) Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat Rev Cancer 9: 134-142.
- Kim EH, Kim J, Ku CR, et al. (2023) Surgical treatment of prolactinomas: Potential role as a first-line treatment modality. Yonsei Med J 64: 489-496.
- Webster J, Piscitelli G, Polli A, et al. (1994) A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med 331: 904-909.
- Andereggen L, Frey J, Andres RH, et al. (2021) First-line surgery in prolactinomas: Lessons from a long-term follow-up study in a tertiary referral center. J Endocrinol Invest 44: 2621-2633.
Corresponding Author
Isabella L Pecorari, MS, Montefiore Medical Center, The University Hospital for the Albert Einstein College of Medicine, 3316 Rochambeau Avenue, Bronx, New York, 10467, USA, Tel: 718-920-4216, Fax: 718-547-4591.
Copyright
© 2024 Pecorari IL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.