Literature Review of Percutaneous Needle Fasciotomy + Extracorporeal Shockwave (ECSW) Therapy in Comparison to Fasciectomy
Abstract
Dupuytren's Contracture (DC) is a progressive fibrotic thickening of the palmar aponeurosis of the hand. Often first presenting with nodules in the palm but leading to a fixed flexion of the digits as further collagen deposition leads to rope like cords being formed that can have a severe impact on one's functionality. Historically, a variety of interventions have been used, but those that are used most common in current practice include limited fasciectomy (LF), percutaneous needle fasciotomy (PNF), and collagenase clostridium histolyticum injections (CCH). Of these, PNF and CCH have the advantage of being less invasive than a LF. Fasciectomy, on the other hand, has the disadvantage of being a more invasive treatment option, yet yields superior results in treating severe cases and has lower recurrence rates than the less invasive treatments. A different avenue which will be explored, is the possible role of Extra-Corporeal Shockwave (ECSW) Therapy in the treatment of DC. Such therapy has received notoriety in the treatment of conditions such as plantar fasciitis and tendinitis calcarea, and while it has been used for DC the authors would like to explore other possibilities for this modality to find out the extent of its application. The authors hypothesized that treating DC with a combination of percutaneous needle fasciotomy and ECSW Therapy would be able to establish a non-inferiority when compared to limited fasciectomy. To test this hypothesis a literature review was performed, and after application of inclusion and exclusion criteria there were 52 scholarly articles utilized in this paper. Results indicated that while PNF seems to be the preferrable non-invasive treatment option, it has major issues with contracture recurrence when compared to LF. PNF does have the advantage of being cost-effective when compared to LF. ECSW Therapy has been shown to be efficacious in the treatment of DC from both a pain reduction standpoint along with a functional one, yet no studies to date have combined ECSW Therapy with PNF to gauge long term efficacy. Based upon these results, the authors conclude that the combination of ECSW Therapy and PNF in the treatment of DC warrants further investigation, as it may bolster the long-term efficacy of PNF while remaining minimally invasive along with cost effective.
Keywords
Dupuytren's Contracture, Fasciectomy, Fasciotomy, Shockwave, Recurrence
Introduction
Dupuytren’s Contracture, or Dupuytren’s Disease, is a common condition seen by hand surgeons which follows a predictable and progressive course resulting in loss of function. DC is a fibro-proliferative disorder characterized by the formation of hard nodules at onset, followed by longitudinal fibrous bands forming in the palmar aponeurosis [1]. The nodules themselves represent contraction of the tissues, while the cords bind nodules to surrounding tissues and cause the flexion contracture associated with the condition which can be seen in (Figure 1) [2,3].
This progression has been described as having three phases: The proliferative, involution, and residual stages [2,4]. In the proliferative stage, an increase in disorganized myofibroblasts is seen in the palmar aponeurosis and this is mainly under the control of local mediators such as TGF-B1 and periostin [2,4-8]. This is followed by the involution stage, where these cells rearrange along tension lines [2,8]. And finally, the residual stage, where collagen is deposited to form cords and myofibroblasts along with nodules regress [2,5]. The result of this process is contracture of the metatarsophalangeal (MCP) and proximal interphalangeal (PIP) joints, causing loss of extension in one or more digits [1,9]. This contracture is most likely to affect the 4 th or 5 th digits of the dominant hand [2,9]. This progression is most commonly assessed clinically by use of the Tubiana staging system (Table 1), which was developed by Tubiana, et al. in 1968 but still widely used today [5].
It has been estimated that the worldwide prevalence of DC is 8.2%, with the continent of Africa having the highest prevalence of 17.2% and the American continent having a prevalence of 2.3% [10]. DC was previously correlated with those of Nordic ancestry, but more recent studies have called this correlation into question [11,12]. Regardless of ancestry, genetic factors have been found to be a main factor that contributes to the development of the disease [11]. DC is more common in men than women, and most often appears during or after the fifth decade of life [2].
There are a number of conditions that have been associated with the development of DC, including: diabetes mellitus, dyslipidemia, liver disease, epilepsy, and HIV infection [2,5,11]. The most closely associated condition is diabetes mellitus, as both Type 1 and Type 2 are associated with increased prevalence of DC [2,10]. DC is also more common in smokers and those who consume alcohol and has been linked to the use of antiretrovirals along with anticonvulsants [2]. While a definitive link has not been determined, it has been postulated that the mechanism behind these conditions’ relation to DC lies in an alteration of local circulation which affects cytokine and growth factor production leading to collagen deposition [2].
Another linkage to the development of DC comes from trauma, as this is an association that has been hypothesized since the 17 th century and studies have found evidence of such a link [1]. Although the pathophysiology may be poorly understood, it has been estimated that up to 1/5 of patients with DC who seek medical intervention had an injury that led to the contracture [1]. Along with trauma, some occupational associated risks have been determined. Those who work with vibrating tools have been found to have increased incidence of DC, especially if doing so for multiple years [11,13].
A small portion of patients with DC also have fibrotic lesions of the plantar fascia or penis, known as Ledderhose’s disease and Peyronie’s disease, respectfully [2]. Lesions over the knuckles may also be present, and these are referred to as Garrod’s pads [14]. Patients with these widespread manifestations are said to have Dupuytren’s diathesis, which indicates a systemic and progressive course that is associated with earlier onset and more frequent recurrence [14].
Recurrence following treatment is one of the primary issues faced when providing care to a patient with DC. Therefore, a consensus definition of recurrence is necessary to establish. Kan, et al. accomplished just that, and their definition of recurrence is "more than 20 degrees of contracture recurrence in any treated joint at one-year post-treatment compared to six weeks post-treatment" which has been widely accepted [15].
Extracorporeal Shockwave Therapy
Extracorporeal shockwave therapy is a form of treatment that has been rising in popularity over more recent years, as new applications for its use are being discovered. While its popularity has increased as of late, the modality has been around for decades and was born from extracorporeal shockwave lithotripsy (ESWL), which provided a non-invasive option for the treatment of kidney stones [16]. While EWSL is aimed at destruction of the kidney stones, ECSW Therapy is predominately used to catalyze a regenerative process at the cellular level which has greatly expanded the possible applications for the modality [16].
In ECSW Therapy, the high energy acoustic waves generated are able to propagate through the tissue and produce changes at the cellular level to induce the healing process [16]. Contrary to their effects in ESWL, the effects of the waves when used in ECSW Therapy can induce changes such as anti-inflammation, neovascularization, tissue regeneration, anti-apoptosis, and chondroprotection [17]. Triggering of osteogenesis has even been demonstrated [18]. Though the exact mechanism of this mechano-transduction still requires further elucidation, there are a few processes which have been observed [16]. There is an initial release of mRNA from the nucleus of cells, which activates organelles to release proteins with healing properties [16]. It has also been observed that the process induces production of growth factors by use of both free and oxygen radicals [18]. The net effect of this mechano-transduction process is activation of a regenerative cascade, and the applications of such are still being discovered as further research is conducted [16]. Along with these actions, ECSW therapy has been found to have more direct anti-fibrotic effects. Fischer, et al. found that ECSW Therapy administered after insertion of silicone implants was able to both decelerate and degrade the capsular fibrosis that ensued [19]. With application of multiple rounds of ECSW Therapy, the degradation was accompanied by alterations in pro-fibrotic proteins such as TGF-B1 [19].
Of note is the fact that there are two different forms of ECSW Therapy which are commonly used, focused and radial ECSW Therapy. The different manners in which they exert their effects leads to different applications in the clinical setting. Focused ECSW Therapy is characterized by a rapid rise in pressure, and the waves generated can be directed to and converge on a selected depth in the tissue [17]. Radial ECSW Therapy, on the other hand, has a lower pressure exertion that is maximal at the body surface and diminishes as the waves propagate deeper into the tissue in a diverging fashion [17]. These distinct differences necessitate the different clinical applications of the two forms.
ECSW Therapy has shown therapeutic efficacy for the treatment of several different orthopaedic conditions. These include fracture non-unions [20,21], plantar fasciitis [16], and tendinitis calcarea [22]. It is also commonly used for lateral epicondylitis, though the efficacy of such use is not as successful as the other conditions mentioned above [16].
ECSW Therapy has also been used to treat conditions closely associated with DC, including Ledderhose’s Disease and Peyronie’s Disease. In terms of treating Peyronie’s Disease, ECSW Therapy has produced results that are widely conflicting across different studies. Varying results have been produced in regard to pain reduction, reduction in penile plaques and curvature, and even erectile dysfunction related to Peyronie’s Disease [23]. Therefore, although ECSW Therapy has been used for Peyronie’s Disease, the applications and efficacy remain to be elucidated. Results have been more promising, however, in the treatment of Ledderhose’s Disease. ECSW Therapy has been found to be effective in reducing pain levels associated with the disease and has even been found to provide functional improvement in those affected [24,25].
Percutaneous Needle Fasciotomy (PNF)
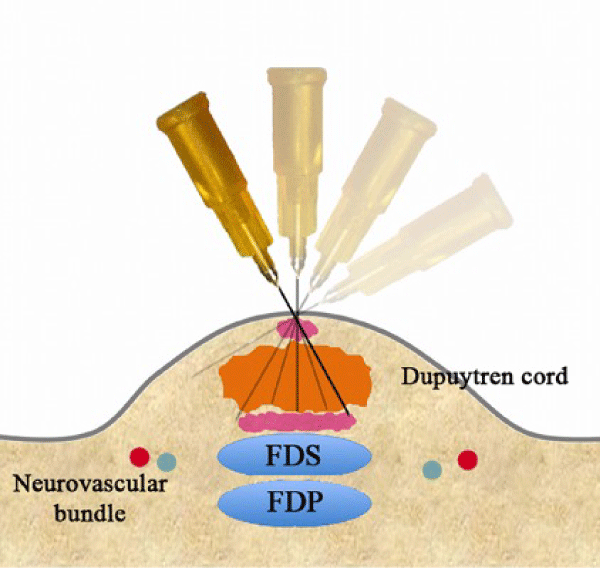
Given the fact that PNF exists as a minimally invasive treatment option that is also relatively inexpensive, it should come as no surprise that the procedure itself is quite simple. First, a local anesthetic is administered to the dorsal and volar aspect of the cord [26]. Once the area has been anesthetized, tension is induced on the cord by extending the digit, and a needle is repetitively passed through the cord in a fan like distribution (Figure 2) until rupture of the cord is either felt or heard [26]. Rupture of the cord is achieved via these repeated perforations under tension, a fairly straightforward mechanism compared to other treatment modalities. Following treatment, physical therapy is rarely necessary, rather, at home stretching and early use of the affected extremity are the recommendations for the patient [26].
Limited Fasciectomy (LF)
Fasciectomy in any form has been a viable option in the treatment of DC for decades, all with a common goal of surgical excision of diseased fascia in order to allow contracture correction [27]. While procedures such as open fasciotomy, segmental fasciectomy, and dermofasciectomy are certainly performed, limited fasciectomy is the most commonly used and therefore will be the procedure which the authors will center our discussion around [27]. The procedure itself is performed with the patient under general, regional, or local anesthesia, a decision which varies by case and by surgeon [27]. The incision is then made, and a variety of techniques exist for the surgeon to choose from, including transverse, Z-plasty, Bruner, and midlateral approach [27]. Following the incision is a dissection of diseased fascia, investigation for possible PIP joint contracture, assessment after correction is completed, and then closure [27]. The patient should expect splinting and occupational therapy following the procedure to ensure the most ideal outcome [27].
Methods
In this literature review, one author performed a search of the PubMed online database for all scientific articles included in the paper. Search terms used to find articles can be found in Table 2, along with the number of articles generated by the search term.
There existed much overlap in the search terms, therefore many of the articles reviewed were present in multiple searches. Inclusion of articles was based upon the results yielded with the search terms, along with a decision being made based upon their relevance to the topic. Priority was given to randomized control trials, systematic reviews, and meta-analyses as our primary article types to include, especially for search terms which yielded numerous results. For such terms a meta-analysis or systematic review was often utilized to better encompass the scope of the term while also narrowing down search results. Exclusion criteria included non-English articles, and beyond that articles were excluded if they were deemed not relevant to the topic being reviewed. The majority of articles excluded were done so after a review of the title or abstract showed that the article did not provide relevance to the topic being discussed in this paper. No time constraints were used in the selection process, as some of the information found in older articles is still accepted today. However, if two articles addressed the same information, the priority was given to the more current article in order to reflect the more up to date understanding of the given topic. Along similar lines, if numerous articles were generated by a search term, then those with the more current understanding were selected for.
The PubMed search failed to yield scholarly articles with information related to the cost of ECSW. As such, a Google search was performed. Search terms used to identify these sources were “shockwave therapy cost for plantar fasciitis” and “high energy shockwave therapy price range”. These searches yielded three of the references used in the section labeled “Extracorporeal Shockwave Therapy”.
Results
Of the articles generated by the literature search performed on PubMed, 52 met the inclusion criteria and were utilized in this paper. Another three references were generated by the Google search that was performed.
Extracorporeal Shockwave Therapy
Based upon its applications in similar disease states, it should come as no surprise that ECSW Therapy has been used in the treatment of DC. Outcomes have been promising in varying regards, and it appears that the benefits of ECSW Therapy in terms of treating DC are still being discovered. One aspect where it has been very promising is in reduction of pain levels. In a smaller study performed by Abdulsalam, et al. pain levels were reduced from 8.7+/-0.5 to 2.0+/-0.9, and tenderness was reduced from 8.5+/-0.5 to 2.5+/-0.9 following weekly treatments over the span of 8 weeks [28]. In a larger study performed by Knobloch, et al. patients received one session of ECSW Therapy per week for three weeks, and the researchers observed a reduction in pain on the visual analog scale from 3.6 +/- 1.8 to 1.9 +/- 0.8 (-47%) after 18 months [29].
While pain reduction has been demonstrated in studies, another exciting prospect is the possibility of functional improvement by way of ECSW Therapy. Noternicola, et al. not only demonstrated pain reduction, but also found statistically significant functional recovery along with extension deficit recovery [30]. Likewise, Aykut, et al. found a statistically significant improvement in function after intervention with ECSW Therapy by way of improvement in MAYO wrist score, grip strength, and 16 of the 23 patients having a table-top test that turned negative [31]. A case report showed similar outcomes in a patient who was treated with radial ECSW Therapy, which led to significant improvement in ability to perform activities of daily living along with correction of the hand deformity that was present [32]. The mechanism by which these functional changes occur is unclear, but as mentioned earlier ECSW Therapy was found to have antifibrotic effects following the insertion of silicone implants, and ECSW Therapy has been found to inhibit the expression of TGF-B1 and alpha-smooth muscle actin when being applied to hypertrophic scars [19,33]. These actions, along with the anti-inflammatory effects mentioned prior, are likely the explanation of improved clinical outcomes in patients treated with ECSW Therapy.
While prices of ECSW Therapy vary based upon a multitude of factors, the cost per session for treatment of plantar fasciitis and other musculoskeletal conditions has been estimated by one source to be around $250 dollars per session [34]. Another source differed in their cost estimation and found it to be $300-$500 per session [35] Notably, this cost estimate is pertaining to low energy ECSW Therapy sessions. Costs of sessions for Dupuytren’s Contracture could be estimated to be similar, given the fact that it is an orthopaedic condition. However, given the range of prices found when trying to determine the cost of a session of ECSW Therapy to treat DC, the authors will err on the side of caution and estimate the price to be $500 per session for low energy ECSW Therapy the purpose of this paper. High energy ECSW Therapy sessions are more expensive, ranging from $1,000-$3,000, yet have the advantage of requiring fewer treatment sessions when compared to low energy ECSW Therapy [35,36]. For the purpose of this paper, the authors will estimate the cost of high energy ECSW Therapy to be $2,000 per session. While these costs can certainly add up if considering multiple sessions, it would still have to be compared to the cost of more invasive treatment options such as surgery to perform a fasciectomy.
Percutaneous Needle Fasciotomy (PNF)
There are two commonly used treatment options for DC that are less invasive and have been shown to be efficacious. These being percutaneous needle fasciotomy (PNF) and collagenase clostridium histolyticum (CCH) injections. With two options available, a choice must be made as to which treatment method to proceed with. There exists much variation in treatment practices, both due to preferences of the physician along with giving respect to the individual case of the patient. With that said, PNF has become a more preferred treatment method when compared to CCH injections and reasoning for that is two-fold. While there are studies which have observed similar efficacy between the two treatment options, especially in treating mild contractures (Tubiana I or II) [46-48][37-39], this is not the case in all studies. Several studies found PNF to be a superior treatment option, and this was determined primarily by a lower rate of recurrence and need for additional intervention, along with a lower rate of adverse effects [40-43]. Given the conflicting evidence, the authors will hypothetically assume that the two treatment methods are similar in terms of efficacy. Yet even with this assumption, still lies the second reason why PNF has become preferred over CCH, the cost of the treatment. Cost of treatment is considerably lower for PNF when compared to CCH, therefore even if efficacy is relatively similar, PNF would be the treatment of choice based on price [26,44,45]. Leafblad, et al. performed a study examining this very topic and found that the standardized direct cost per digit was $624 for PNF and $4,189 for CCH. These values increased to $1,540 for PNF and $5,952 for CCH at 5 years when re-interventions were taken into account [46]. Therefore, even if the two treatment modalities are similar in terms of efficacy, which the literature for that is inconsistent at best, there remains a significant cost difference that should be considered when choosing a form of treatment for a given patient.
The efficacy of PNF, though not without flaws, is promising. Strömberg, et al. demonstrated that 79% of patients retained a straight MCP joint at their 2-year follow-up [39]. Mehdiyev, et al. found that at 3 years, the mean passive extension deficit following PNF was 9 degrees [44]. Similarly, Scherman, et al. found that extension deficits were reduced by 70% at their 12-month follow-up after treatment with PNF [47]. There is a common theme throughout the literature, however, and that is that intervention efficacy with the minimally invasive modalities wanes over time and recurrence of DC is a major issue. Scherman, et al. found the DC recurrence rate to be 42.5% at 36 months following intervention with PNF. Leafblad, et al. found the need for re-intervention following PNF to be 23% at 2 years, but then 61% at 5 years [46]. The rate discovered by van Rijssen, et al. 84.9%, was even higher [48]. Issues with recurrence are not isolated to treatment with PNF, rather it is a common theme for both primary minimally invasive treatment options. Multiple studies have found that there are no significant differences with recurrence rates between patients treated with PNF and those treated with CCH at 3-year follow-ups [38,47]. While recurrence rates pose a significant issue, PNF has the benefit of being a minimally invasive and cost-effective treatment option that can be performed again and again, and many patients opt to do so [26]. With that said, recurrence remains an issue needing to be addressed moving forward. Along similar lines, there is the issue with contracture that involves the PIP joint rather than just the MCP joint. Such contractures are notoriously more problematic in terms of effective treatment, as Mehdiyev, et al. found lower rates of clinical success in treating PIP joints when compared to MCP joints, and Abe found that successful corrections were present in 67% of patients with moderate PIP joint contractures at only 30 days post-intervention [38,44]. Further issues arise in terms of recurrence at the PIP joint, which van Rijssen found to be 70% at 5-year follow-up, and Skov et al found to be 68% at 2-year follow up [48,49]. Therefore, even though PNF is a fantastic option for treatment of DC, issues are still present, especially in terms of contracture recurrence.
Limited Fasciectomy (LF)
The benefit of the more invasive treatment techniques is a higher efficacy, and there are many studies which corroborate with this general principle. In a study performed by van Rijssen, et al. patients who underwent LF had a 79% improvement in total passive extension deficit at 6 weeks, and this was in comparison to a 63% improvement seen in patients following PNF [50]. The difference in these outcomes was primarily seen among patients with a Tubiana stage III or IV contracture [50]. Another follow up was performed at 5 years following intervention by van Rijssen, et al. which found that the disease had recurred in 20.9% of LF patients compared to 84.9% of PNF patients, and that recurrence occurred significantly sooner in the PNF patients [48]. In a study by Leafblad, et al. rates of re-intervention were 24% for PNF and 4% for fasciectomy after 2 years [9][46]. At 5 years, the rate of re-intervention was 61% for PNF and 4% for Fasciectomy [46]. Similarly, Toppi, et al. found that at 2 years, 12% patients who underwent a fasciectomy were told they would require another operation whereas 30% of patients who were treated with PNF were given the same prognosis [51]. In a review performed by Crean, et al. the recurrence rates were 39% for fasciectomy compared to 62% for Fasciotomy [52]. As can be seen, the common theme across the literature is that though the exact results may have a wide range, intervention with the more invasive fasciectomy does provide superior results from an aspect of long-lasting relief when compared to PNF.
Given the fact that it is more invasive, it comes as no surprise that recovery times are longer and more severe adverse effects are liable to occur. In a meta-analysis performed by Cooper, et al. they found no statistical difference in the rates of mild complications, yet fasciectomy was associated with 74 severe complications compared to the 2 associated with the less invasive CCH injections [53]. Although some studies describe the overall rate of adverse events as being higher with minimally invasive treatment options or comparable between the two, this is primarily claimed while considering minor complications rather than selecting for major ones [40,52]. Such minor complications are predominately associated with CCH rather than PNF [40,52]. Other studies, however, have found more distinct differences. Toppi, et al. found that patients undergoing fasciectomy were 7.57 times more likely to have a postoperative infection when compared to those being treated with PNF [51]. Likewise, Soreide, et al. found a higher rate of adverse events associated with LF compared to PNF [54]. Severe adverse events associated with fasciectomy include infection, nerve injury, neurapraxia, CRPS, and arterial injury [53]. The invasive nature of LF also helps to explain the cost associated with the procedure, which Leafblad, et al. found to be $5,291 per digit for LF compared to $624 per digit for PNF [46]. Another aspect which must be taken into account is the recovery time associated with the treatment modality. As mentioned earlier, patients who undergo PNF are rarely subjected to extensive post-operative interventions. LF patients, on the other hand, can expect a post-surgery compressive dressing followed by occupational therapy and night splinting for varying amounts of time [46,53,55]. Such extended measures must be considered not only from a physical standpoint, but from a financial one as well.
Therefore, although LF poses a fantastic option for the treatment of DC, it is not without the flaws associated with its invasive nature. The success it has with deformity correction and recurrence rates set the standard. If such success were able to be obtained with minimally invasive measures, then a new standard of care may be in the works. Such an alternative will be addressed in the coming section.
Discussion
The goal of this paper was to conduct a review of the literature to determine if a new methodology for the treatment of DC should be explored. LF has established itself as the superior option in terms of results, especially when taking into account recurrence rates, yet has a major disadvantage in terms of invasiveness. PNF, on the other hand, is both minimally invasive and cost effective but is lacking in terms of long-term efficacy. Therefore, the authors desired to find a method in which PNF may be bolstered in order to produce more desirable outcomes, and ECSW Therapy may provide such an alternative. Throughout the literature review performed by the authors, no papers were found which have combined PNF and ECSW Therapy in the treatment of DC.
PNF has established itself as a mainstay in the treatment of DC due to its previously discussed advantages, especially when considering less severe contractures. Yet issues arise when reviewing the data, especially years following intervention. Both van Rijssen and Leafblad highlighted the discrepancies between PNF and LF in their studies and have shown that if a patient is looking for long term relief, they typically become restricted to the invasive techniques [46,48]. This issue is more apparent when considering the age of the patient. Whereas an elderly patient may be looking for a few years of relief and therefore be satisfied with PNF, a younger patient affected by DC would more likely desire be treated with a method that resembles more of a cure than a short-term solution. Such patients are very limited in terms of options, and if they are unable to undergo surgery for any reason then they are even further out of luck. Presently, no cure exists for DC. However, what the authors are proposing is a method which may be able to provide a lasting efficacy which resembles LF while also remaining minimally invasive. To be more specific, if a treatment regimen could be established that combines PNF with ECSW Therapy then it may pose a minimally invasive option which could rival LF in terms of efficacy.
ECSW Therapy has been gaining notoriety in the treatment of several conditions, and while its application in terms of pain reduction in DC has been shown, the authors are more concerned with its success from a functional standpoint. The studies by Noternicola, et al. and Aykut, et al. have opened the door for such a discussion, as they were able to demonstrate extension deficit recovery and functional improvement following ECSW Therapy [30,31]. These results have been obtained without a clear understanding as to why this modality is beneficial, though multiple plausible mechanisms are laid out earlier in the paper. Nevertheless, with efficacy being demonstrated it seems apparent that this non-invasive modality should be incorporated into the arsenal of treatment options against DC. It is our estimation that combining it with PNF could prove to be quite beneficial. PNF has shown to be efficacious on its own, with Strömberg et al finding 79% of MCP joints remaining straight at 2-year follow up [39]. However, studies focusing on recurrence rates highlight the modality’s need of some support if it is to rival LF in the long term. Why then, could it not be established to perform a PNF and then over time supplement that treatment with sessions of ECSW Therapy? Both have shown to be efficacious in treating DC, and both have the benefit of being non-invasive, so it stands to reason that a combination of the two may be able to provide a non-invasive option in the treatment of DC with long term efficacy. Furthermore, such a combination may be able to prove more effective in treating problematic cases such as severe contractures or ones involving the PIP joints that have proven quite difficult for PNF to be beneficial against over the years. While still merely speculative, given the data present on each as a viable treatment option along with the potential upside of discovering a non-invasive long-term option, it seems to be a more than worthy avenue to explore in the future.
Another primary goal of this proposed treatment combination would be to remain cost-effective while also being minimally invasive. Therefore, a comparison of costs between PNF and LF must be drawn, and Leafblad, et al. did just that. Standardized direct costs per digit were $624 for PNF and $5,291 for fasciectomy, and these costs increased to $1,540 for PNF and $5,507 for fasciectomy at 5 years when accounting for continued treatment and further interventions [46]. Given the fact that the increase in cost seen at 5 years in the PNF group was due to the need for further interventions, and that what the authors are proposing is an alternative intervention, the authors will focus on the initial cost for PNF which was $624. With this number in mind, a patient would be able to undergo multiple sessions of either high or low energy ECSW Therapy in the years following initial intervention with PNF yet remain below the 5-year cumulative cost of fasciectomy, $5,507. The authors have estimated the cost to be $500 per session for low energy ECSW Therapy and $2,000 for high energy ECSW Therapy. Therefore, multiple sessions of either intensity would be able to be performed and the cost of treatment, including the initial intervention with PNF of $624, would still be lower than the 5-year cost of intervention with fasciectomy. This, of course, depends on the total number of ECSW Therapy sessions performed, but it remains an exciting prospect that this combination may prove to be worthwhile from a standpoint of long-term efficacy while also being cost effective and minimally invasive. The cost effectiveness of this treatment combination may become even more pronounced in the coming years, as ECSW Therapy is currently a cash-pay treatment option and if it were to become covered by insurance it would certainly benefit our proposed treatment combination from the aspect of the patient.
Conclusion
Based upon the review of the literature performed, the authors conclude that a new methodology in the treatment of DC may need further examination. Due to the shown efficacy of both PNF and ECSW Therapy as viable treatment options, the authors propose that a combination of the two may be able to provide long term relief from DC that rivals the efficacy of LF in terms of recurrence rates and treating severe contractures yet has the unquestionable benefit of being a minimally invasive combination. This exciting proposition warrants further investigation, if a non-inferiority were able to be determined then it may alter the landscape of treating DC. Furthermore, a deeper comparison between the treatment methods from a financial standpoint should be performed, as it appears that our proposed combination may also be cost-effective in addition to being efficacious and minimally invasive.
References
- Samulenas G, Rimdeika R, Braziulis K, et al. (2020) Dupuytren’s contracture: Incidence of injury-induced cases and specific clinical expression. Medicina (B Aires) 56: 323.
- Mansur HG, Oliveira ER de, Gonçalves CB (2018) Epidemiological analysis of patients with Dupuytren’s disease. Rev Bras Ortop (Sao Paulo) 53: 10.
- Dutta A, Jayasinghe G, Deore S, et al. (2020) Dupuytren’s contracture-current concepts. J Clin Orthop Trauma 11: 590-596.
- Luck JV (1959) Dupuytren’s contracture; a new concept of the pathogenesis correlated with surgical management. J Bone Joint Surg Am 41: 635-664.
- Khashan M, Smitham PJ, Khan WS, et al. (2011) Dupuytren’s disease: Review of the current literature. Open Orthop J 5: 283-288.
- Black EM, Blazar PE (2011) Dupuytren disease: An evolving understanding of an age-old disease. J Am Acad Orthop Surg 19: 746-757.
- Tomasek JJ, Vaughan MB, Haaksma CJ (1999) Cellular structure and biology of Dupuytren’s disease. Hand Clin 15: 21-34.
- Picardo NE, Khan WS (2012) Advances in the understanding of the aetiology of Dupuytren’s disease. The Surgeon 10: 151-158.
- DiBenedetti DB, Nguyen D, Zografos L, et al. (2011) Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: Results from a population-based study. Hand (NY) 6: 149-158.
- Salari N, Heydari M, Hassanabadi M, et al. (2020) The worldwide prevalence of the Dupuytren disease: A comprehensive systematic review and meta-analysis. J Orthop Surg Res 15: 495.
- Ruettermann M, Hermann RM, Khatib Chahidi K, et al. (2021) Dupuytren’s Disease-Etiology and Treatment. Dtsch Arztebl Int 118: 781-788.
- Ng M, Thakkar D, Southam L, et al. (2017) A genome-wide association study of dupuytren disease reveals 17 additional variants implicated in fibrosis. Am J Hum Genet 101: 417-427.
- Descatha A, Jauffret P, Chastang JF, et al. (2011) Should we consider Dupuytren’s contracture as work-related? A review and meta-analysis of an old debate. BMC Musculoskelet Disord 12: 96.
- Hindocha S, Stanley JK, Watson S, Bayat A (2006) Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am 31: 1626-1634.
- Kan HJ, Verrijp FW, Hovius SER, et al. (2017) Recurrence of Dupuytren’s contracture: A consensus-based definition. PLoS One 12: e0164849.
- Auersperg V, Trieb K (2020) Extracorporeal shock wave therapy: An update. EFORT Open Rev 5: 584-592.
- Ko NY, Chang CN, Cheng CH, et al. (2022) Comparative effectiveness of focused extracorporeal versus radial extracorporeal shockwave therapy for knee osteoarthritis-randomized controlled study. Int J Environ Res Public Health 19: 9001.
- Wang FS, Wang CJ, Chen YJ, et al. (2004) Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1α and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem 279: 10331-10337.
- Fischer S, Mueller W, Schulte M, et al. (2015) Multiple extracorporeal shock wave therapy degrades capsular fibrosis after insertion of silicone implants. Ultrasound Med Biol 41: 781-789.
- Cacchio A, Giordano L, Colafarina O, et al. (2009) Extracorporeal shock-wave therapy compared with surgery for hypertrophic long-bone nonunions. J Bone Joint Surg Am 91: 2589-2597.
- Furia JP, Juliano PJ, Wade AM, et al. (2010) Shock wave therapy compared with intramedullary screw fixation for nonunion of proximal fifth metatarsal metaphyseal-diaphyseal fractures. J Bone Joint Surg Am 92: 846-854.
- Gerdesmeyer L, Wagenpfeil S, Haake M, et al. (2003) Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA 290: 2573-2580.
- Bakr AM, El Sakka AI (2021) Extracorporeal shockwave therapy in peyronie’s disease: Systematic review and meta-analysis. J Sex Med 18:1705-1714.
- Knobloch K, Vogt PM (2012) High-energy focussed extracorporeal shockwave therapy reduces pain in plantar fibromatosis (Ledderhose’s disease). BMC Res Notes 5: 542.
- Hwang JT, Yoon KJ, Park CH, et al. (2020) Follow-up of clinical and sonographic features after extracorporeal shock wave therapy in painful plantar fibromatosis. PLoS One 15: e0237447.
- Strömberg J (2019) Percutaneous needle fasciotomy for dupuytren contracture. JBJS Essent Surg Tech 9: e6.
- Dias JJ, Aziz S (2018) Fasciectomy for dupuytren contracture. Hand Clin 34: 351-366.
- Abdulsalam AJ, Shehab D, Elhady AA, et al. (2019) High-energy focused extracorporeal shockwave therapy relieved pain in Dupuytren’s disease: A series of seven hands. Eur J Phys Rehabil Med 55: 862-864.
- Knobloch K, Hellweg M, Sorg H, et al. (2022) Focused electromagnetic high-energetic extracorporeal shockwave (ESWT) reduces pain levels in the nodular state of Dupuytren’s disease-a randomized controlled trial (DupuyShock). Lasers Med Sci 37: 323-333.
- Notarnicola A, Maccagnano G, Rifino F, et al. (2017) Short-term effect of shockwave therapy, temperature controlled high energy adjustable multi-mode emission laser or stretching in Dupuytren’s disease: A prospective randomized clinical trial. J Biol Regul Homeost Agents 31: 775-784.
- Aykut S, Aydin C, Öztürk K, et al. (2018) Extracorporeal shock wave therapy in Dupuytren’s disease. Sisli Etfal Hastan Tip Bul 52:124-128.
- Brunelli S, Bonanni C, Traballesi M, et al. (2020) Radial extracorporeal shock wave therapy: A novel approach for the treatment of Dupuytren’s contractures: A case report. Medicine 99: e20587.
- Cui HS, Hong A, Kim JB, et al. (2018) Extracorporeal shock wave therapy alters the expression of fibrosis-related molecules in fibroblast derived from human hypertrophic scar. Int J Mol Sci 19: 124.
- (2023) How shockwave therapy helps heal sports and overuse injuries. Orthopaedics and Rehab, UT Southwestern Medical Center.
- Lowell Scott Weil (2011) ESWT for plantar fasciitis: What do the long-term results reveal? Podiatry Today 38.
- (2023) High energy shockwave-ESWT.
- Zhou C, Hovius SER, Pieters AJ, et al. (2017) Comparative effectiveness of needle aponeurotomy and collagenase injection for dupuytren’s contracture: A multicenter study. Plast Reconstr Surg Glob Open 5: e1425.
- Abe Y (2020) Comparison of treatment outcomes after collagenase injection and percutaneous needle fasciotomy for dupuytren’s contracture: Objective and subjective comparisons with a 3-Year Follow-Up. Plast Reconstr Surg 145: 1464-1474.
- Strömberg J, Sörensen AI, Fridén J (2018) Percutaneous needle fasciotomy versus collagenase treatment for dupuytren contracture: a randomized controlled trial with a Two-Year Follow-up. J Bone Joint Surg Am 100: 1079.
- Obed D, Salim M, Schlottmann F, et al. (2022) Short-term efficacy and adverse effects of collagenase clostridium histolyticum injections, percutaneous needle fasciotomy and limited fasciectomy in the treatment of Dupuytren’s contracture: A network meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 23: 939.
- Hirase T, Suresh R, Cotton MO, et al. (2021) Percutaneous needle fasciotomy versus collagenase injection for dupuytren’s contracture: A systematic review of comparative studies. J Hand Microsurg 13: 150.
- Arnold DMJ, Lans J, Westenberg R, et al. (2022) Additional treatment after collagenase injections and needle fasciotomy for dupuytren’s disease: A retrospective cohort study. J Hand Microsurg 14:138-146.
- Sanjuan Cerveró R, Carrera Hueso FJ, Vazquez Ferreiro P, et al. (2018) Efficacy and adverse effects of collagenase use in the treatment of Dupuytren’s disease: A meta-analysis. Bone Joint J 100:73-80.
- Mehdiyev T, Maffei D, Müller V, et al. (2022) Dupuytren disease: A retrospective cohort study comparing collagenase injection and percutaneous needle fasciotomy. Plast Reconstr Surg Glob Open 10: e4604.
- Brazzelli M, Cruickshank M, Tassie E, et al. (2015) Collagenase clostridium histolyticum for the treatment of Dupuytren’s contracture: Systematic review and economic evaluation. Health Technol Assess 19: 1-202.
- Leafblad ND, Wagner E, Wanderman NR, et al. (2019) Outcomes and direct costs of needle aponeurotomy, collagenase injection, and fasciectomy in the treatment of Dupuytren contracture. J Hand Surg Am 44: 919-927.
- Scherman P, Jenmalm P, Dahlin LB (2015) One-year results of needle fasciotomy and collagenase injection in treatment of Dupuytren’s contracture: A two-centre prospective randomized clinical trial. J Hand Surg Eur 41: 577-582.
- Van Rijssen AL, Ter Linden H, Werker PMN (2012) Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: Percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg 129: 469-477.
- Skov ST, Bisgaard T, Søndergaard P, et al. (2017) Injectable collagenase versus percutaneous needle fasciotomy for dupuytren contracture in proximal interphalangeal joints: A randomized controlled trial. J Hand Surg Am 42: 321.e3-328.e3.
- Van Rijssen AL, Gerbrandy FSJ, Linden H Ter, et al. (2006) A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: A 6-Week Follow-Up Study. J Hand Surg Am 31: 717-725.
- Toppi JT, Trompf L, Smoll NR, et al. (2015) Dupuytren’s contracture: An analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg 85: 639-643.
- Crean SM, Gerber RA, Le Graverand MPH, et al. (2011) The efficacy and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: A structured review of published studies. J Hand Surg Eur 36: 396-407.
- Cooper TB, Poonit K, Yao C, et al. (2020) The efficacies and limitations of fasciectomy and collagenase clostridium histolyticum in Dupuytren’s contracture management: A meta-analysis. J Orthop Surg 28: 2309499020921747.
- Soreide E, Murad MH, Denbeigh JM, et al. (2018) Treatment of Dupuytren’s contracture: A systematic review. Bone Joint J 100: 1138-1145.
- Almadani YH, Vorstenbosch J, Efanov JI, et al. (2021) Healing, inflammation, and fibrosis: Dupuytren’s disease: an outcomes-focused update. Semin Plast Surg 35: 216-222.
Corresponding Author
Jonathan Fincher, Medical Student, Louisiana State University Health Shreveport School of Medicine, 10856 Woodpine Trail, Bethany, La 71007, USA.
Copyright
© 2025 Stevens J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.