Congenital Talipes Equinovarus: A Review
Abstract
An Abnormal anatomical variation of any part of the body is a predictor of functional aberration of that part. Congenital talipes equinovarus imposes some functional compromise on the foot, resulting in structural adaptation or modification of the local anatomy in conformity with the direction of the structural stress that subserves the abnormal function. The foot deformities of congenital talipes equinovarus interfere with the locomotive ability of the individual by alteration of gait biomechanical parameters, leading to an imbalance in the gait. Evidence suggests that 80% of infants with congenital talipes equinovarus deformity live in developing countries. The aim of this review article is to promote the awareness of congenital talipes equinovarus deformity among the health care providers' population within the study locality. A review of the literature on the subject of congenital talipes equinovarus deformity was done. Anatomical perspectives, epidemiological information, aetiological factors, patho-anatomy and treatment of congenital talipes equinovarus were extracted and presented.
Keywords
Congenital clubfoot, Deformities, Structural anatomy, Functional compromise
Introduction
The study of human gross anatomy often focuses on the normal structural disposition of anatomical structures as a basis for a normal functional performance of these structures. Abnormal anatomical variation of any part is a predictor of abnormal function of that part. This is an important concept in clinical anatomy, where application of normal anatomy underlies the understanding of functional aberrations that may occur in the event of abnormal structural outlay of body parts or organs. Normal structural anatomy is a function of an uninterrupted intrauterine development by intrinsic or extrinsic factors [1]. The effects of such interruptions may extend beyond the gross anatomical and functional deficiencies of the involved organ systems to also affect their molecular and microscopic integrity. All these effects are diverse outcomes of impaired histogenes is arising from intrinsic or extrinsic factors [2].
The functional integrity of the foot depends on normal gross anatomical structure, such that foot deformities are always associated with some functional compromise. The resultant abnormal function results in structural adaptation or modification of the local anatomy in conformity with the direction of the structural stress that subserves the abnormal function [2]. This is the picture of the relationship between anatomical structure and function in such foot deformity as the congenital talipes equinovarus (CTEV), otherwise known as clubfoot. When left unattended, this relationship between abnormal anatomy and function in the deformed foot enters a vicious cycle, which paves the way for some far-reaching complications in the local foot anatomy and function in succeeding early childhood years and beyond. Such complications include rigid and painful foot, as well as gait anomaly. However, children with clubfoot have different gait patterns. Usually, they have different anatomical features and develop their own adaptation to those constraints. Therefore, individual adaptations result in diverse movement patterns, and this is the reason children with clubfoot have large differences in gait patterns [2].
Congenital talipes equinovarus (congenital clubfoot) is one of the most common structural congenital abnormalities affecting the lower limb, with a generally accepted incidence of one to two per 1000 live births [3,4]. However, the incidence of CTEV has been reported to vary across the regions of the world from 0.6/1,000 individuals in Asia, 0.9/1,000 individuals in Australia to 6.9/1,000 individuals in Hawaii, Polynesia and Maori [5,6]. The incidence in males is reportedly higher than in females [5], with a male to female ratio of 4:1. From a global perspective, it is reported that approximately 100,000 children are born world-wide each year with clubfoot. About 80% of these children are believed to live in developing countries where many of them may never receive the expected optimal treatment. When neglected, clubfoot becomes a serious reason for physical, social, and psychological disability among patients with congenital musculoskeletal defects [7].
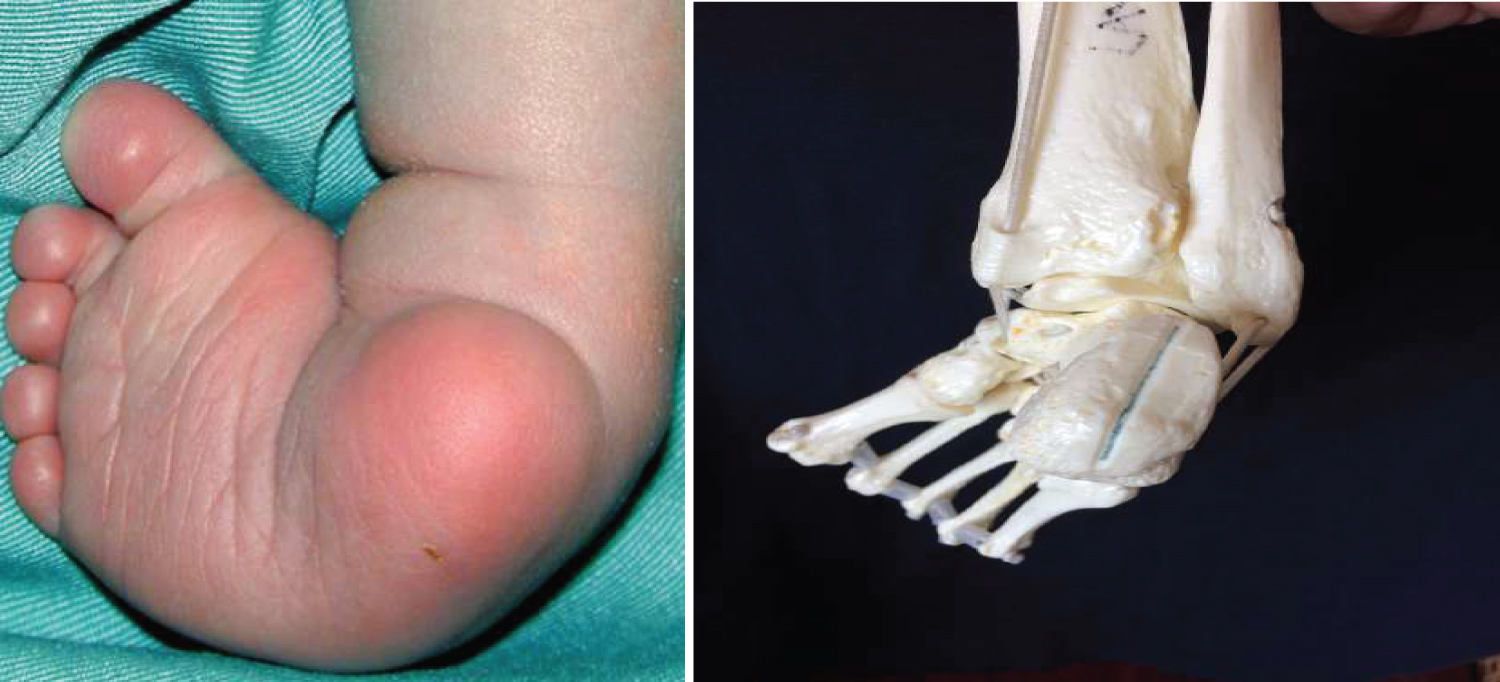
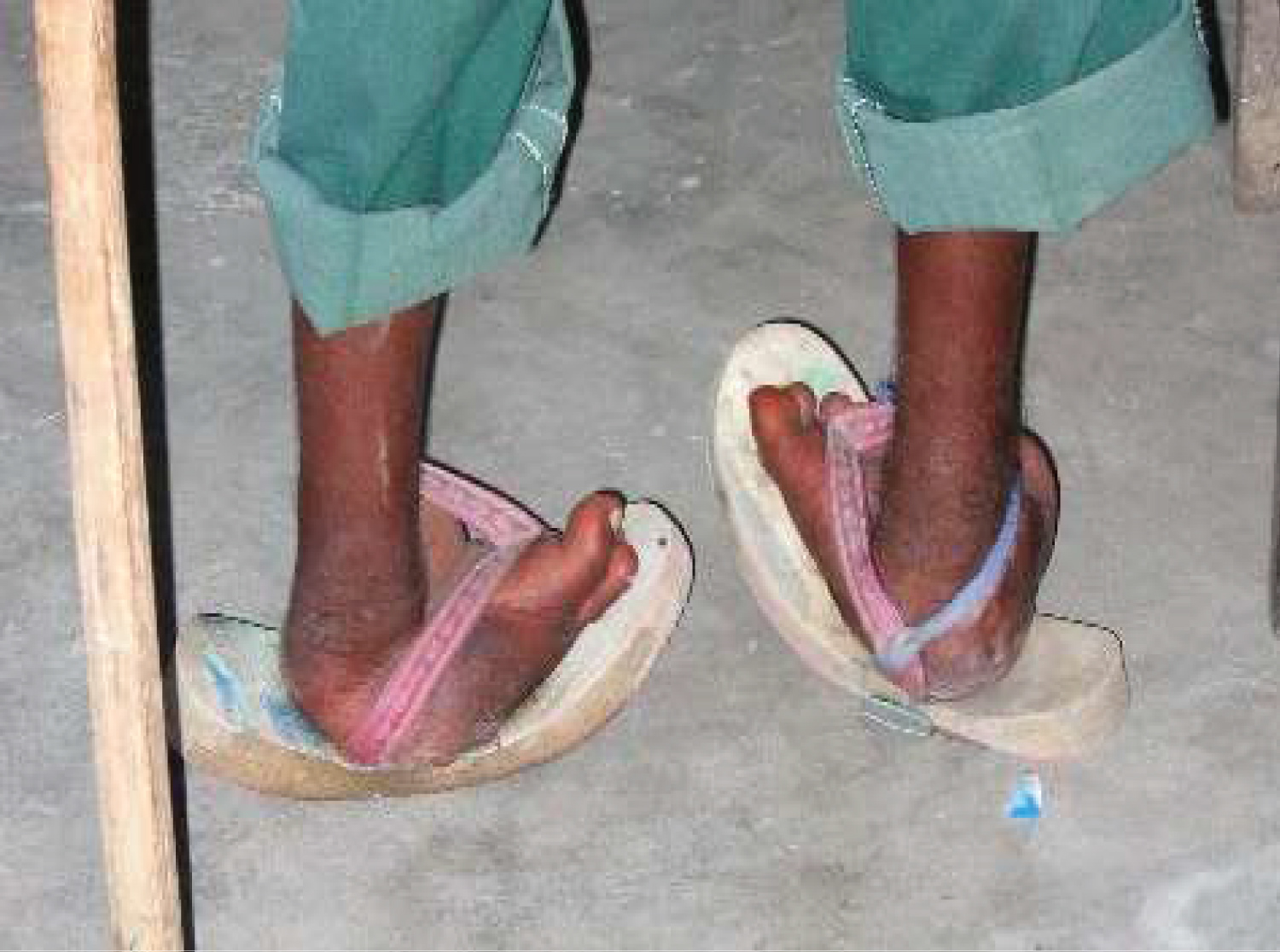
The anatomic deformity of clubfoot is easily recognised. For descriptive purposes, clubfoot deformity has four constant features, namely, equinus, mid-foot cavus, fore-foot adduction and hind-foot varus. The deformity in clubfoot is both cosmetic and functional with associated hypoplasia of skin, muscles, bones, tendons, ligaments and neurovascular bundle on the medial side. The affected foot is smaller than the normal foot [4,8]. Functional adaptation occasioned by these deformities in an untreated clubfoot results in additional local anatomical changes such as callosity of the lateral border of the foot due to weight bearing on this part, increasing deformation of the tarsal bones of the foot, skin and bone infections, stiffness of the foot, limitation in mobility, and inability to wear standard shoes [4].
Although clubfoot may be associated with many other congenital abnormalities, it is more commonly an isolated idiopathic birth defect, which may affect either one foot or both feet. When it is associated with other congenital anomalies, it is referred to as syndromic clubfoot. When it is an isolated defect, it is referred to as idiopathic clubfoot [9]. About half of the infants with clubfoot have bilateral involvements, and unilateral deformity occurs more often on the right side [5,9]. There is an associated posteromedial ankle and foot soft tissue contractures which deform and displace tarsal bones, giving rise to characteristic deformities of equinus, heel varus, mid-foot adductus and cavus [9,10]. These deformities are responsible for the plantar flexed, inverted, and adducted position of the foot. The deformation of the normal anatomy of the affected foot is conspicuously obvious at birth. The aetiology of CTEV is unknown but several theories have been advocated to explain it. However, most infants who have congenital clubfoot have no identifiable genetic, syndromal, or extrinsic cause [11].
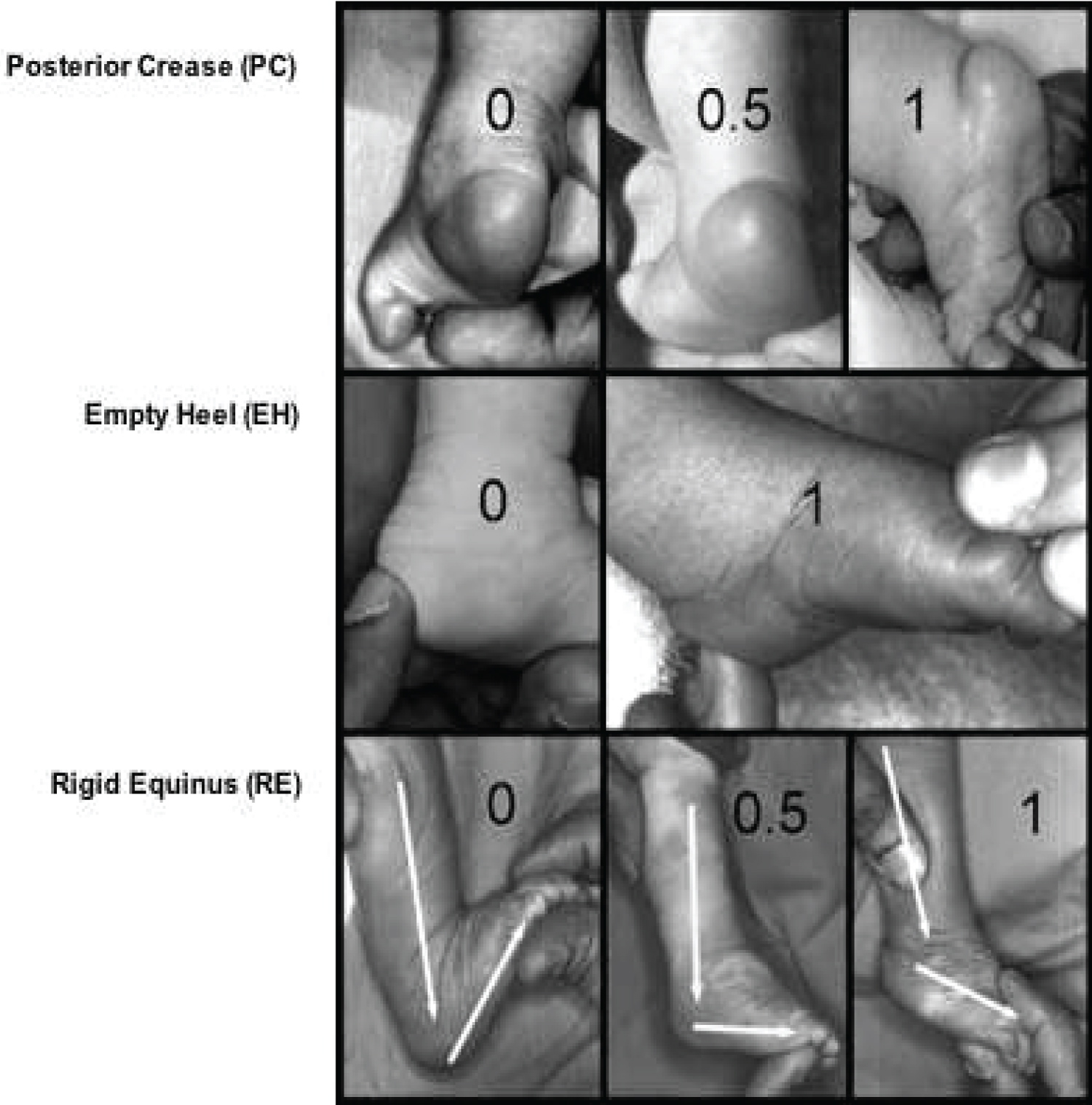
The severity of clubfoot and its response to treatment can be objectively assessed to a large extent using the instrument of the Pirani score [12]. This is a valid and reliable method that clinically assesses the amount of deformity present in an unoperated congenital clubfoot less than two years [6]. This scoring system is based on six clinical signs of contracture in clubfoot. Each clinical sign is given a value of 0, if no abnormalities are identified; 0.5, if moderate abnormalities are identified; and 1.0, if severe abnormalities are identified. This assessment is divided into the mid-foot contracture score and the hind-foot contracture score. The six clinical signs are divided into three signs related to abnormalities in the hind-foot and mid-foot respectively. The signs to be assessed in the hind-foot are the severity of the posterior crease, the emptiness of the heel, and the rigidity of the equinus. Three signs related to abnormalities in the mid-foot include medial crease, curved lateral border, and the lateral head of the talus [6,12].
Evidence suggests that 80% of infants with congenital clubfoot live in developing countries [7,13-15], and the condition is reportedly the commonest congenital musculoskeletal deformity in Nigeria [16-20], accounting for 52.8% of all malformations with live birth incidence of 3.4/1000. It is difficult to cover up functional deficiencies resulting from structural anomalies in the lower limb. The foot is very vital to the maintenance of a normal ambulatory gait, being involved in every component of gait, namely, heel strike, mid-stance, swing and toe off. These elements of gait become a challenge in a foot afflicted with CTEV. CTEV interferes with locomotive ability of the individual by alteration of gait biomechanical parameters, with unilateral clubfoot causing more imbalances in gait compared with bilateral clubfoot [2]. Therefore, the most striking functional challenge occasioned by congenital clubfoot is the impaired quality of locomotive effort by the individual.
The restoration of a functional, painless, plantigrade foot with good mobility is the aim and hallmark of successful treatment of congenital clubfoot. It is a pointer to the restoration of normal or near-normal structural anatomy of the foot in the subjects. The integrity of functional anatomy of the foot is directly related to that of its structural anatomy. This work is a review article aimed at promoting awareness of congenital talipes equinovarus deformity among the health care providers' population, outside of orthopaedic specialty, within and outside the study locality.
Normal Functional Anatomy of the Paediatric Foot
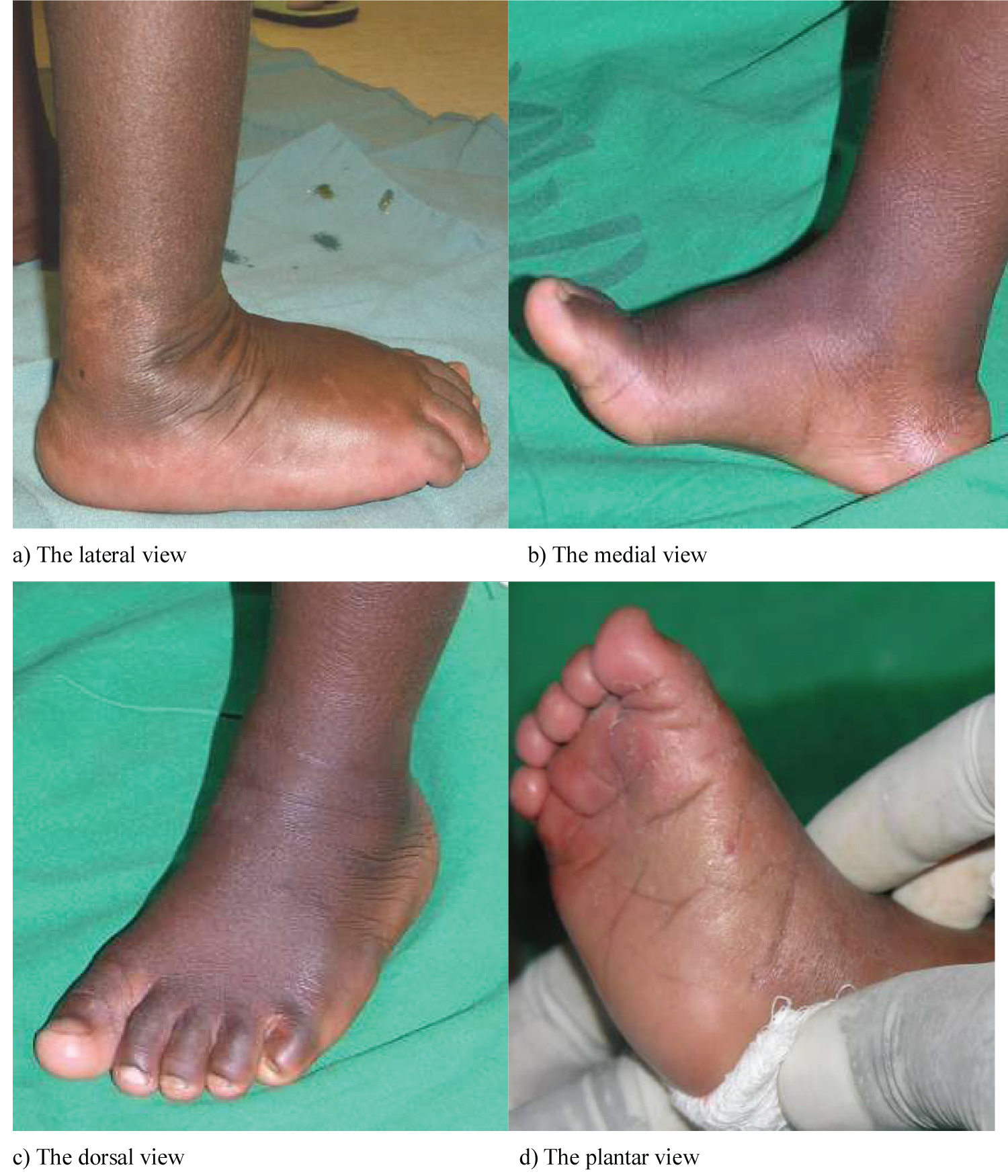
The healthy foot at birth is generally flexible and well aligned. However, foot contractures that may occur because of some deformities are usually known to resolve with exercises, observation, or both. The longitudinal arch is generally absent at birth but slowly and spontaneously develops. Also, there is medial deviation of the forefoot that resolves spontaneously in over 90% of cases [21]. For descriptive purposes, there are four views of the normal foot as depicted in Figure 1. They include the medial, lateral, dorsal and plantar views [22]. Three regions exist in the foot, namely, the hind-foot, the mid-foot and the forefoot. The calcaneum and the talus are together known as the hind-foot. The tarsal bones (the cuboid, navicular, and cuneiforms) are known as the mid-foot. The metatarsals and toes are known as the forefoot. In practice the forefoot is firmly connected to the mid-foot as the metatarsals are interlinked with the cuneiform bones [22].
The foot grows continuously with the growth of the child, but this growth accelerates ahead of the adolescent growth spurt. Metatarsal growth plates are located proximally on the first metatarsal and distally on the second to fifth metatarsals [21]. The tuberosity of the calcaneus grows through the calcaneal apophysis. According to Andersen and co-workers [23], foot growth decelerates at 12-13 years of age in girls and 14-15 years of age in boys. Distal epiphyses tend to close first, followed by diminished growth through the mid-foot and cessation of growth 1-2 years before the growth in height ends [21,23].
The bony structure of the paediatric foot consists of seven tarsal bones, five metatarsals, and five digits. Each tarsal bone has a defined shape with complex articular surfaces, variable ossification pattern, centripetal growth, and links to adjacent bones through thick ligaments [21]. The calcaneus and the talus usually are ossified at birth, and the cuboid ossification centre usually appears at birth or shortly after. The navicular tarsal bone is the last of the large tarsal bones to develop primary ossification, usually around three years of age [24]. The longitudinal arch of the foot is formed by the combination of all tarsal and metatarsal bones. The toes are composed of phalanges stabilized by ligaments, allowing only flexion and extension across joints. The bones of the foot are held in position by ligaments, as the joints of the foot have no intrinsic stability. In addition to the bony and ligamentous structure of the foot, there are intrinsic and extrinsic muscles affecting this complex. The extrinsic muscles have their origin above the ankle and include the tibialis anterior, the tibialis posterior, the extensor hallucis longus, the extensor digitorum communis, the flexor hallucis longus, the flexor digitorum communis, two peroneal muscles, and the gastrocsoleus complex. The intrinsic muscles of the foot are primarily in the plantar aspect of the foot, and are arranged in four layers. The most superficial layer includes the abductor hallucis, the flexor digitorum brevis, and the abductor digiti minimi. The second layer is the quadratus plante and the lumbricals. The third layer is the flexor hallucis brevis, the adductor hallucis, and the flexor digiti minimi brevis. The fourth layer includes the interossei. The intrinsic dorsal musculature is the extensor digitorum brevis muscle that arises from the distal lateral calcaneus and ligaments of adjacent joints. The balance among all of these muscles is critical for proper function as well as avoidance of cavus deformity [21]. The foot is also endowed with a rich neurovascular structure.
This intricate arrangement of the bony and soft tissue structures of the normal foot is responsible for the functional integrity of the foot. The congenital talipes equinovarus deformity causes a fundamental disorganisation of the structural and functional anatomy of the foot as outlined, thus changing the foot from a supple and mobile to a rigid and immobile structure, with diverse functional challenge.
Embryogenesis of the Foot
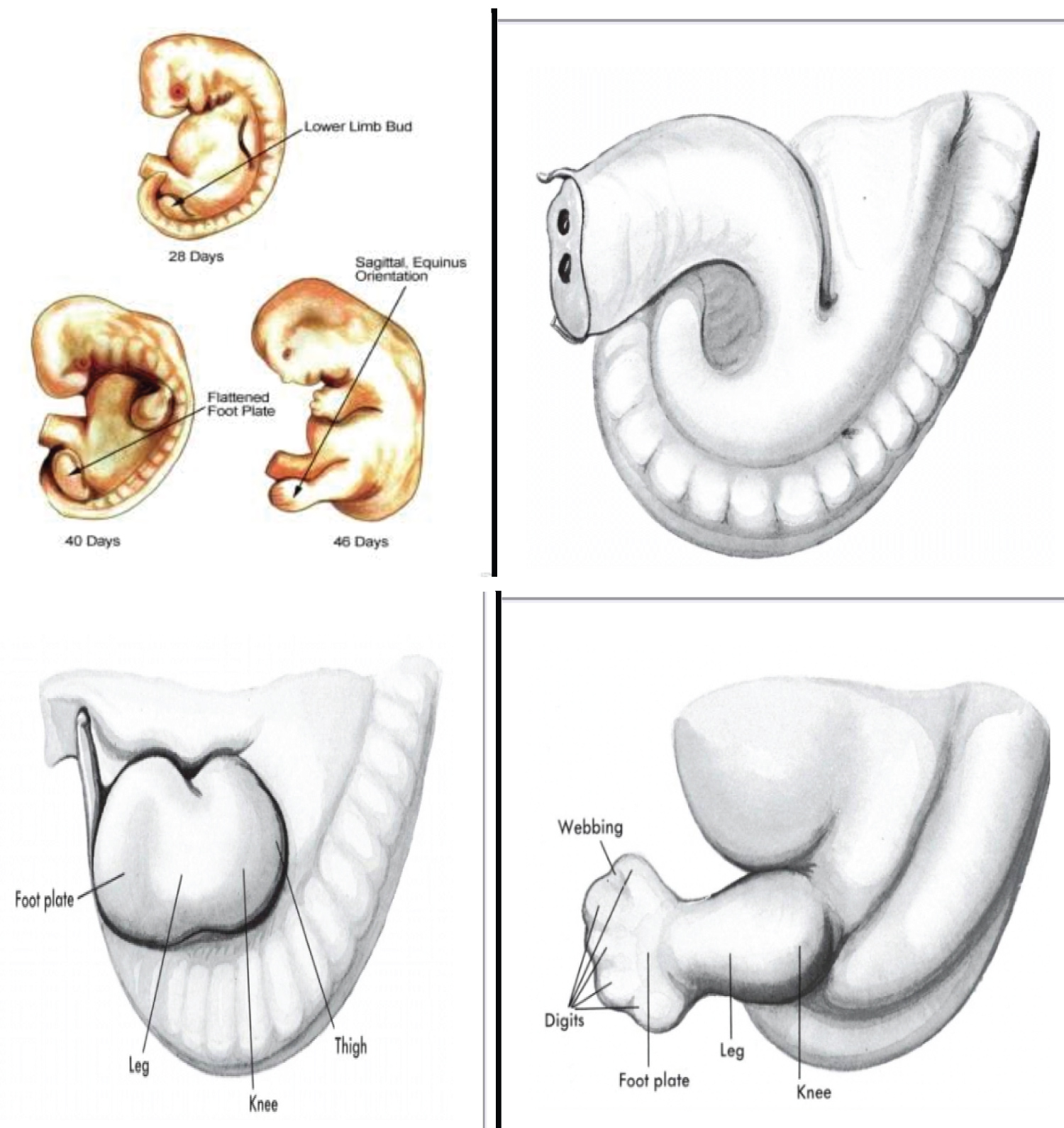
The embryo of two weeks post fertilization is curved irregularly in a semicircle and presents no external evidence of a lower limb bud in the caudal area. At three weeks, a slightly longitudinal swelling is discernible opposite the five lumber and first sacral myotomes. Once initiated, the ontogeny of the lower limb progresses in a rapid sequential fashion, and definite morphologic changes are recognizable at two days interval. The surface of the foot plate is located in the transverse plane, and the ventral surface, the future plantar surface, faces the head.
An inward rotation occurs, and the future flexor surface obliquely faces the median saggital plane of the trunk. When viewed from the ventral aspect of the embryo, the rotation of the foot plate -a fundamental change- is counter clockwise on the left and clockwise on the right. Morphologically, no toe rays are present in the toe plate. However, older embryos of this group have an indication of the great toe on the tibial or preaxial border.
During fetal development, important rotational changes take place that alter the leg-foot relationship. Initially the feet, their soles facing each other, are in equinus relative to the leg. A progressive internal rotation of the thigh and leg occurs, and the foot is then in equinus, supination and external rotation relative to the leg (Figure 2) [1]. Subsequently the foot dorsiflexes and pronates, bringing the foot close to the adult neutral position [1,3,25].
Patho-Anatomy of the Congenital Talipes Equino Varus (CTEV)
The basic pathological definition of the congenital talipes equinovarus describes it as a congenital dysplasia of the musculoskeletal tissue distal to the knee joint, manifesting in the form of deformity of the foot and ankle. Biologically speaking, clubfoot is not an embryonic malformation, but rather a developmental deformation which is rarely detected with ultrasonography before the 16th week of gestation [26]. Further biological aberration in the affected foot suggests an excessive pull of the tibialis posterior, aided by the gastrosoleus and the long toe flexors. The ligaments of the posteromedial aspects of the ankle and foot are very thick and taut. There is evidence that excessive collagen synthesis occurs in the ligaments, tendons and muscles around the foot and ankle, and this may persist until the child is three or four years, and is thought to be the reason for relapses in the affected children [3,26]. This phenomenon is also responsible for the pathological contractures and rigidity of the soft tissue structures of the foot and ankle in clubfoot [3,26,27].
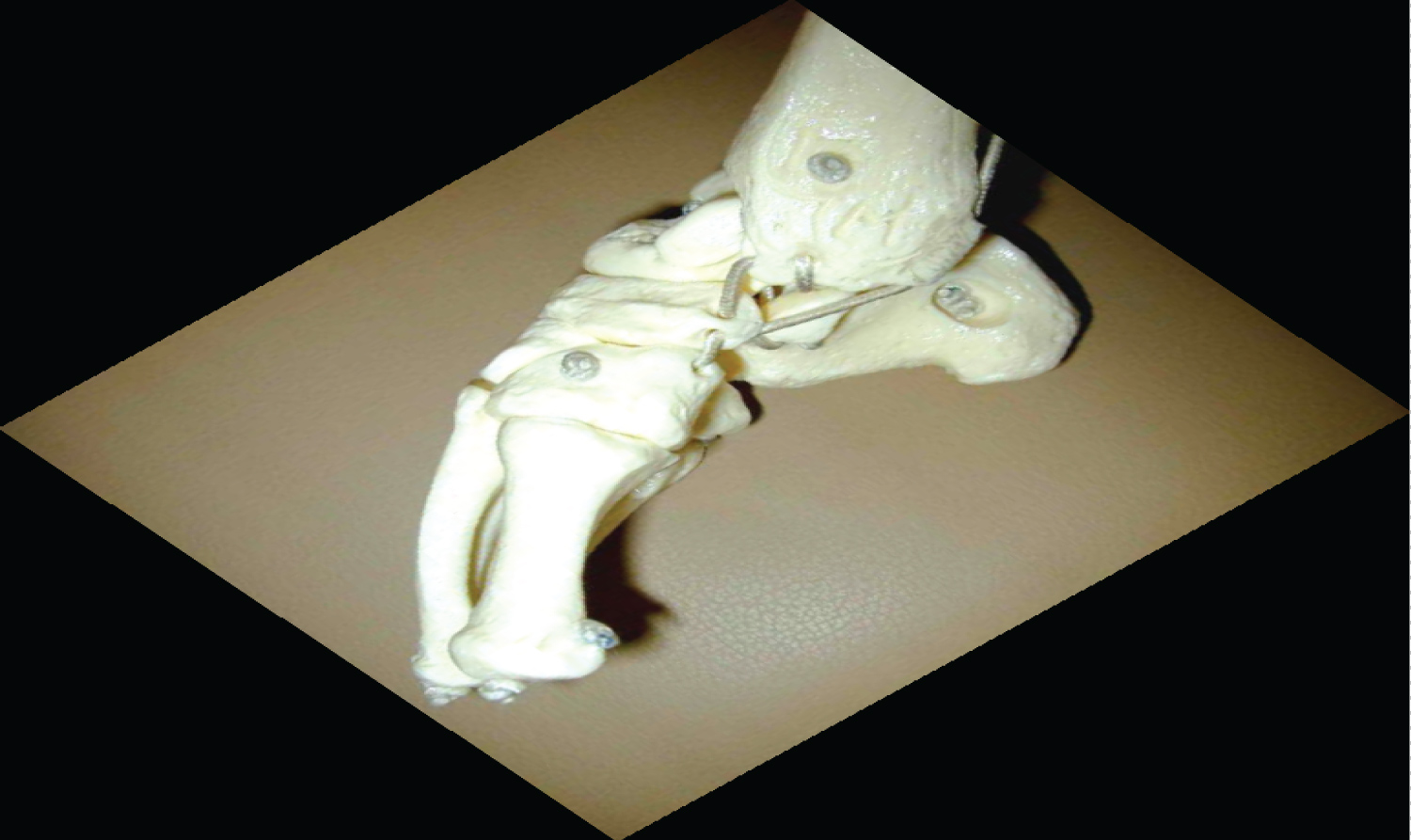
Numerous anatomical studies of clubfoot have confirmed the gross changes in the shape and position of the talus, navicular, calcaneum and cuboid. The most severe deformities are located in the hind-foot, where the talus and the calcaneus are in accentuated equinus, the calcaneus is positioned medially and angulated in varus, and the navicular exhibits accentuated medial deviation [3]. The tendons, tendon sheaths, ligaments and fascia of the foot have undergone adaptive changes and became fibrotic or contractured [27]. Moreover, the posterior ligaments of the ankle, such as those from the medial and plantar region, are shortened and thickened. The triceps surae, tibialis posterior and flexor muscles of the toes are shortened [3].
The specific details of the patho-anatomic and kinematic changes in the bony framework of the hind-foot have been well documented as follows: [3,7,26]
i. Talus
This is the most deformed and the least displaced of the bony framework of the hind-foot. The head and neck of talus are deviated medially and plantar-ward. The body is rotated externally in the ankle mortise, as its superior articular surface escapes from the mortise and remains uncovered by its tibial articular counterpart. The talar neck is short and medially deviated. In some cases the talar neck may be absent [3]. The entire talus is smaller than normal, suffers from disturbance of vascular supply and has an eccentrically placed ossification centre. There is a decreased subtalar motion due to the severe shortening of the medial and posterior tarsal ligaments and the tightness of the tibialis posterior and gastrosoleus muscles [7].
ii. Navicular
The navicular is in extreme inversion, medially displaced over the talar head [28], so as to articulate with the medial surface of the talar head, in close proximity to the medial malleolus [7,26]. This exposes the lateral head of the talus, which is easily palpable upon clinical examination.
iii. Calcaneus
The calcaneus is adducted and directed medially on the horizontal plane. It is in equinus and inverted under the talus, with the anterior tuberosity facing the lateral malleolus, thus leaving the calcaneus in a varus disposition [3,26,28]. Both the calcaneus and talus are in plantar flexion, producing varus deformity of the hindfoot [7].
iv. Cuboid
The cuboid is displaced medially in relation to the calcaneus [28]. The tarsometatarsal joints and the metatarsal diaphyses are medially displaced and cause adduction of the forefoot, which is pronated in relation to the hind-foot. The first and the second metatarsals are angulated plantar-ward in relation to the fifth, which is in alignment with the hind-foot. This arrangement is responsible for the cavus seen in clubfoot [3].
As already pointed out, considerable soft tissue changes involving the ligamentous and fascial tissues of the ankle and foot occur in congenital clubfoot. Also, the calf muscles, the foot and its bones are smaller. There is a contracture and shortening of the triceps surae muscle [3,29].
Vascular malformations have been described in congenital clubfoot, and the origin is thought to be either congenital or an adaptive response to severe and prolonged deformity. The most frequent anomaly is absence or hypoplasia of the anterior tibial artery, which is present proximally in the leg, but ends abruptly in the ankle or in the calf, with deficient or absent anastomotic network. In rarer situations, there can be more profound vascular insufficiencies, which also compromise the perfusion provided by the posterior tibial artery. In these cases, the blood supply occurs separately through the fibular artery [3,30,31].
General Considerations in the Aetiogenesis of Congenital Anomalies
A general look at the causes and the risk factors in congenital defects as a whole is thought to provide a platform for understanding the aetiogenesis of such conditions as congenital clubfoot. This is without prejudice to the belief by scholars that the aetiogenesis of congenital clubfoot is not well understood.
Approximately 50% of all congenital anomalies cannot be linked to a specific cause [32]. A United States research examined the frequency and causes of birth defects by looking at medical records for over 270,000 births between 2005 and 2009. The researchers found 5,504 cases of birth defects, or about 2% of total births. However, they found the cause behind only one in five of these birth defects, with the rest (79.8%) remaining a mystery [33]. This implies that the cause of congenital malformations is known only in about 20% of cases, according to Farlie [33]. These known causes are majorly environmental (4.1%), twin pregnancies (1.4%) and genetic (94.4%) [33]. Therefore, although majority of the congenital malformations cannot be linked to any specific aetiologic factor, there are some known genetic, environmental and other causes or risk factors.
The aetiological factors proposed have diverse history, based on prevailing understanding, culture, and religion. Worldwide historically, the role of the supernatural had been in the forefront of aetiological considerations but has changed with advances in embryology and teratology. However, the role of the supernatural in the aetiology of congenital malformations still has a stronghold in many African settings and this poses an enormous psychosocial challenge for the affected [34].
i. Genetic Factors
Genes play an important role in many congenital anomalies, and this might be through inherited genes that code for an anomaly, or resulting from sudden changes in genes known as mutations. Consanguinity (when parents are related by blood) also increases the prevalence of rare genetic congenital anomalies and nearly doubles the risk for neonatal and childhood death, intellectual disability and other anomalies. Some ethnic communities (such as Ashkenazi Jews or Finns) have a comparatively high prevalence of rare genetic mutations such as Cystic Fibrosis and Haemophilia C [32]. Genetic factors include chromosomal abnormalities and single gene defects.
ii. Socioeconomic Factors
Low-income may be an indirect determinant of congenital anomalies, with a higher frequency among resource-constrained families and countries. It is estimated that about 94% of severe congenital anomalies occur in low- and middle-income countries [32]. An indirect determinant, this higher risk relates to a possible lack of access to sufficient, nutritious foods by pregnant women, an increased exposure to agents or factors such as infection and alcohol, or poorer access to healthcare and screening. Factors often associated with lower-income may induce or increase the incidence of abnormal prenatal development [32].
iii. Demographic Factors
Maternal age is also a risk factor for abnormal intrauterine fetal development. Advanced maternal age increases the risk of chromosomal abnormalities, including Down syndrome [32]. Young maternal age increases the risk of malformations such as gastroschisis [34].
iv. Environmental Factors
These include agents that pregnant women are exposed to, which are capable of inducing structural anomalies in a developing fetus. These agents are known as teratogens, derived from the Greek words teratos (monster) and gen (producing). Examples of teratogens include alcohol, tobacco, certain pesticides, radiation, and drugs such as antiepileptics and anticoagulants [34]. Maternal exposure to these teratogens during pregnancy may increase the risk of having a fetus or neonate affected by congenital anomalies. Working or living near, or in, waste sites, smelters or mines may also be a risk factor, particularly if the mother is exposed to other environmental risk factors or nutritional deficiencies [32]. The precise mechanisms by which these agents cause malformations are not clearly understood but include alteration in gene expression, cell migration and differentiation, and histogenes is [34,35].
v. Infections
Maternal infections such as syphilis, rubella and influenza virus are a significant cause of congenital anomalies in low- and middle-income countries. Maternal rubella infection is associated with such anomalies as congenital cataracts and congenital heart defects. More recently, the effect of in-utero exposure to Zika virus on the developing fetus has been reported [32]. In 2015, Brazil detected cases of Zika virus and a spatio-temporally associated increase in microcephaly. By 2016, Brazil reported that of 4180 suspected cases of microcephaly, 270 were confirmed, 462 were discarded and 3448 are still under investigation. This is compared to an average of 163 microcephaly cases recorded nationwide per year. With 6 of the 270 confirmed cases of microcephaly showing evidence of Zika infection, health authorities and agencies began investigating and conducting comprehensive research to confirm a causal link.
According to the WHO [32] following the Zika outbreak in French Polynesia, health authorities reported an unusual increase in the number of congenital malformations in babies born between March 2014 and May 2015. Cleft lip and palate, neural tube defects, and cardiac malformations are examples of congenital malformations arising from viral infections [32].
vi. Maternal Nutritional Status
Maternal folate insufficiency increases the risk of having a baby with a neural tube defect (spina bifida and anencephaly), while excessive vitamin A intake may affect the normal development of an embryo or fetus. Deficiencies of other vitamins and minerals such as zinc have been implicated in the aetiology of some congenital malformations [34].
Theories of Aetiogenesis of Congenital Talipes Equinovarus
Notwithstanding that the specific aetiogenesis of congenital clubfoot is not well understood, certain theories have been propounded to explain the origin of this condition [3,4,7]. These theories are grouped into extrinsic and intrinsic factors, comprising intrauterine position of the fetus, mechanical compression or increase of intrauterine hydraulic pressure, interruption in fetal development, viral infections, maternal smoking, vascular deficiencies, muscular alterations, neurological alterations, defect in the development of bony structures and genetic defects [3,4,7].
Although clubfoot may be associated with many other congenital abnormalities, it is more commonly an isolated idiopathic birth defect [4]. Previous studies have proposed several risk factors that are associated with clubfoot, including male gender, maternal smoking, maternal age, maternal marital status, parity, maternal education, and maternal diabetes [11,36]. Palma, et al. [36] found that males were twice as likely to have clubfoot as females. Also, infant birth in the winter months correlated with an increased risk of clubfoot. Maternal characteristics found to be significantly associated with increased risk of clubfoot were young maternal age at conception and low maternal education. Young paternal age also had a correlation with increased risk of clubfoot in the child. Paternal smoking and the presence of any household smoking were strongly associated with an increased risk of clubfoot [36].
Specific Theories of Aetiology of Clubfoot
i. Mechanical Factors In-utero
This is the oldest theory and was first proposed by Hippocrates. He believed that the foot was held in a position of equinovarus by external uterine compression. However, Parker in 1824 and Browne in 1939 believed that diminution of amniotic fluid, as in oligohydramnios, prevents foetal movement and renders the foetus vulnerable to extrinsic pressure [4,27].
ii. Neuromuscular Defect
This theory holds that equinovarus foot is always the result of neuromuscular defect. However, histological and electromyographic studies by some investigators have not demonstrated any abnormalities of the muscles in clubfoot [4,27].
iii. Primary Plasma Defect
In 1963, Irani and Sherman dissected 11 equinovarus feet and 14 normal feet and found no primary abnormalities of the nerves, vessels, tendon and muscles insertions. Constantly, they found abnormalities in the anterior part of the talus. The talus was undersized and its anterior portion was medially rotated. They suggested that the deformity probably resulted from a primary germ plasma defect [4,27,37].
iv. Arrested Foetal Development
This theory has to do with the impact of the intrauterine environment and environmental influences. The effect of the intrauterine environment was reported by Heuter and Von Volkman. In 1963, they were the first to suggest the arrest of foetal development early in embryonic life as a cause of congenital clubfoot. Although this theory was corroborated by Bohm in 1929 [4,27], investigators such as Mau and Bessel-Hagen opposed it [4,27].
Environmental influences refer to the effects of teratogenic agents on foetal environment and development as are well exemplified by the effect of rubella and thalidomide in pregnancy. Many authors believe that there are various environmental factors responsible for the appearance of a clubfoot, as there are various substances capable of producing a temporary growth arrest [4,27].
v. Polygenic Theory of Hereditary Pattern
Clubfoot tends to be familial in a significant number of cases. Wynne Davis supported the polygenic theory and showed a rapid decrease in incidence of clubfoot from first to second to third degree relatives. About 2.9% of siblings in the first degree relatives had this deformity as compared to 0.6% in second degree relatives or 1-2 per thousand in general population. The chances of getting affected in siblings are more than 25 times [4,27].
vi. Cultural Beliefs
In some countries and cultures there are different beliefs about what causes a child to be born with clubfoot. Such beliefs include spiritual influences, spells, or curses. Sometimes the mothers might be blamed for it. These ideas can cause the child with clubfoot to be excluded from such societies and stigmatized. Therefore, it is important to explain to families that children with clubfoot are a valuable part of the community [22].
Epidemiology of Congenital Talipes Equinovarus
The incidence of clubfoot is approximately one case per 1,000 live births in the United States. Also, an analysis using data from European studies reported that the total prevalence of congenital clubfoot in Europe was 1.13/1,000 births [38]. The incidence differs among ethnicities to the tune of 75 cases per 1000 live births in the Polynesian islands, particularly in Tonga. It is believed that 80% of infants with congenital clubfoot live in developing countries [7,13-15], and the condition is reportedly the commonest congenital musculoskeletal deformity in Nigeria [16-20], accounting for 52.8% of all malformations with live birth incidence of 3.4/1000.
The male-to-female ratio has been reported to be 2:1. Bilateral involvement is found in 30-50% of cases, and patients with bilateral clubfoot are said to have a wider range of severity. There is a 10% chance of a subsequent child being affected if the parents already have a child with a clubfoot. The overall prevalence of clubfoot was 1.29/1,000 live births, with figures of 1.38 among non-Hispanic whites, 1.30 among Hispanics, and 1.14 among non-Hispanic blacks or African Americans. Maternal age, parity, education, and marital status were significantly associated with clubfoot, along with maternal smoking and diabetes [11,36].
Components of Clubfoot Deformity
i. Cavus- The arch of the foot is higher than normal. This is because the first metatarsal is plantar flexed in relation to the calcaneum and hind foot (Figure 3). There is forefoot plantar flexion.
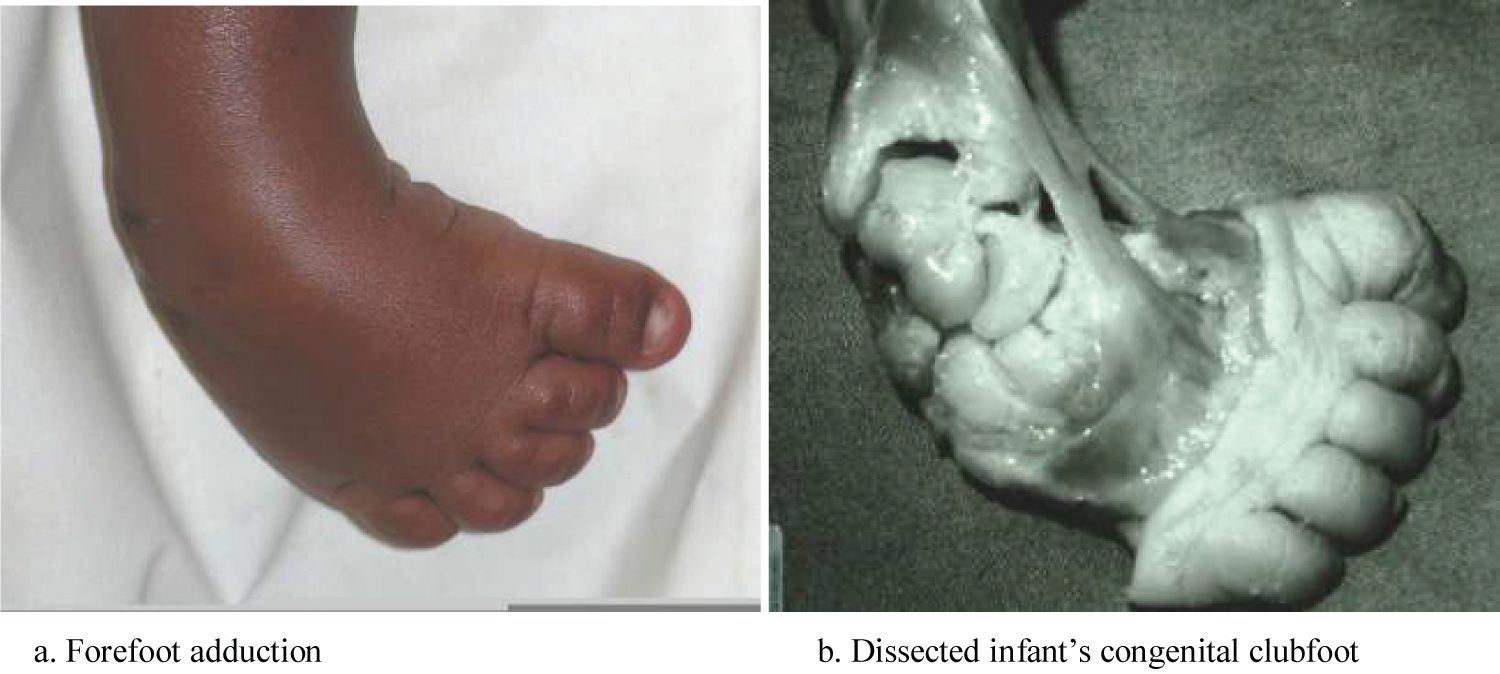
ii. Adduction-the forefoot is moved towards the midline at the talonavicular and tarso metatarsal joints (Figure 4).
iii. Varus- This deformity is described in relation to the heel. The heel in the clubfoot is in varus and angled towards the midline at the subtalar joint (Figure 5).
iv. Equinus- This is plantarflexion of the foot at the ankle joint. The calcaneum is plantar flexed in relation to the tibia (Figure 6).
Soft tissue contractures associated with these clubfoot deformities include the posterior contracture, medial contracture, subtalar and plantar contractures. The posterior contracture involves the Achilles tendon, tibiotalar capsule, talocalcaneal capsule, posterior talofibular ligament and calcaneofibular ligament. These structures perpetuate and resist equinus correction. The medial contracture involves the tibialis posterior, deltoid ligament, talonavicular capsule and spring ligament. The subtalar contracture is from the talocalcaneal ligament. The plantar contracture is produced by the abductor hallucis, intrinsic flexors, quadratus plantae and plantar aponeurosis [39].
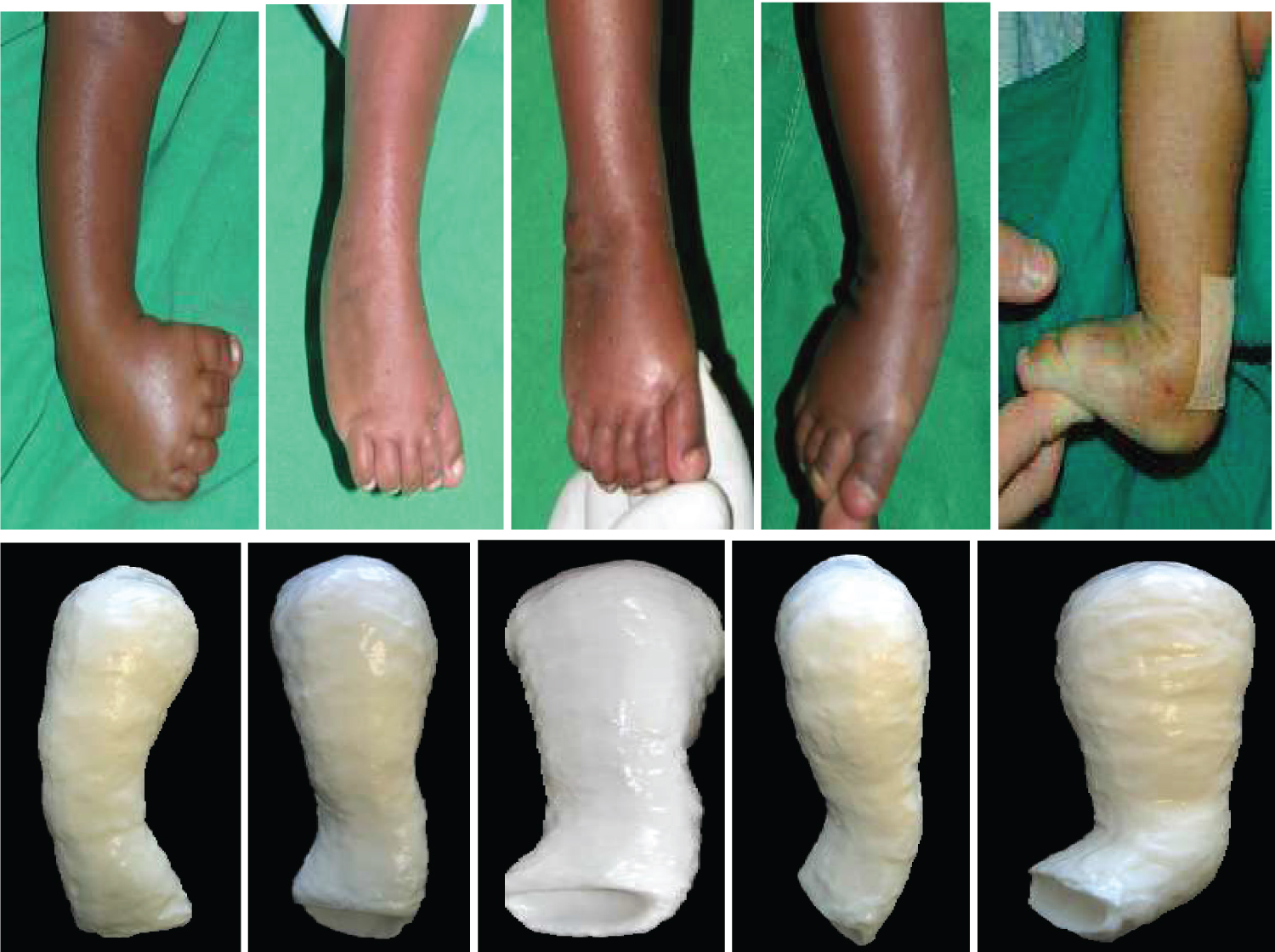
The persistence of these soft tissue contractures coupled with secondary bony changes are responsible for worsening the above deformities and for the final appearance of the foot in an untreated clubfoot, as shown in the clinical photograph below (Figure 7).
Assessment of the Severity of CTEV using the Pirani Score
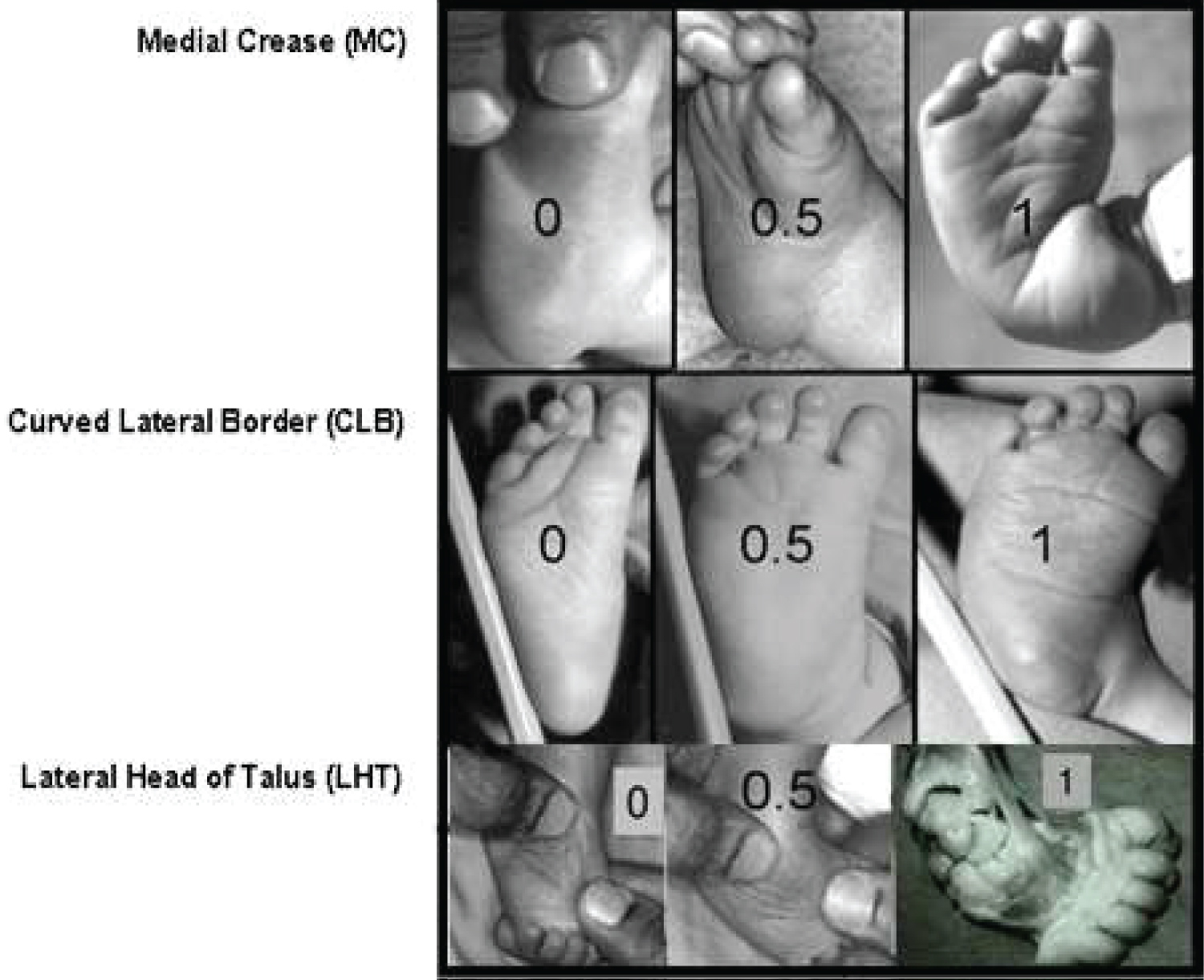
The Pirani scoring system is a tool for measuring the severity of clubfoot deformity and for monitoring progress in the course of treatment. The scoring system is named after Shafique Pirani, the inventor of the Pirani scoring system [22]. The Pirani scoring system is based on six well-described clinical signs of contracture characterizing a severe clubfoot [22]. If the sign is severely abnormal it scores 1; If it is partially abnormal it scores 0.5; and if it is normal it scores 0. Three of the signs are in the mid-foot and another three in the hind-foot. Total Score (TS) varies from 0 to 6 and is the sum of mid-foot and hind-foot contracture scores (Figure 8 and Figure 9) [22].
Mid-foot Contracture Score (MFCS)
This varies between 0 and 3. The signs scored in mid-foot contracture score are Medial Crease (MC), Curved Lateral Border (CLB), and Lateral Head of Talus (LHT). These 3 signs are each scored 0, 0.5, or 1, depending on the severity (Figure 8).
Hind-foot Contracture Score (HFCS)
This varies between 0 and 3. The signs scored in the hind-foot contracture score are Posterior Crease (PC), Empty Heel (EH), and Rigid Equinus (RE). These 3 signs are each scored 0, 0.5, or 1, depending on the severity (Figure 9).
Total Score (TS)
This varies from 0 to 6 and is the sum of mid-foot and hind-foot contracture scores (TS = MFCS + HFCS) (Lavy, 2017). Higher Pirani scores correlate positively with the severity of the clubfoot deformity at first assessment, and the scores at subsequent assessments indicate the level of correction or otherwise, at the point in time.
Treatment of Clubfoot
Historically speaking, the treatment of clubfoot has been characterized by controversies, has evolved through many forms from antiquity, and has continued to present a challenge [15,40]. The goals of management of clubfoot are to obtain an obvious plantigrade, stable and straight foot with no need to modify shoes and a good range of foot motion without pain [15,41]. From the beginning of the 20th century till now, the management of clubfoot has fluctuated between conservative and surgical procedures, depending on the personality of the clubfoot. It is recommended that treatment should start soon after birth, within the first week of life, when the joints and bones of the infant's foot are more supple and flexible to allow successful repair. The first line of treatment is usually non-operative or conservative, and surgical treatment is reserved for cases that fail to respond to the first line of treatment, as well as for severe rigid clubfoot [15,42].
The history of conservative treatment of clubfoot dates back to Hippocrates in 400 BC. The first researched and written description of clubfoot was performed by Hippocrates of Kos in 400 BC, when he propounded that clubfoot was curable for the majority of cases, by manipulative correction remarkably similar to current non-operative methods [15,43]. Conservative approach to clubfoot treatment is divided into two phases, namely, the pre- and post-Ponseti eras. The classic examples of the pre-Ponseti techniques are the Kite's and the French techniques [44].
The Ponseti technique, developed by Dr. Ignatio Ponseti, is currently the gold standard in the overall management of congenital clubfoot. The Ponseti method of treatment was developed based on extensive anatomical study of the foot. It is a simple, cost-effective treatment method that has become widely used around the world. Numerous research studies in countries across the globe in the last 20 years have shown that more than 90% of cases of newborn children with idiopathic clubfoot can be treated effectively with the Ponseti technique [3,15,22]. The goals of Ponseti treatment are to have functional, pain-free feet, capable of wearing normal shoes and devoid of permanent disability [22]. Ponseti treatment for congenital clubfoot has completely replaced the previous methods (pre-Ponseti methods) of treating clubfoot, which were a mix of surgical and conservative techniques. The Ponseti method consists of the corrective phase and the maintenance phase. During the corrective phase, on weekly basis, the position of the foot is gradually corrected using a series of manipulations and plaster of Paris casts. Then, finally, a small outpatient procedure is performed to cut the Achilles tendon (percutaneous tenotomy). The corrective phase usually takes 4-8 weeks (Figure 10).
During the maintenance phase, after the corrective phase has achieved a good position for the foot, it must be kept in the corrected position for the next 4-5 years, to avoid a relapse of the condition (Figure 11). This is achieved by putting the child's feet into a foot abduction brace for 23 hours a day for the first 12 weeks. Thereafter, night-time bracing is continued until child is 4-5 years-old. For older children presenting late with clubfoot, appropriate adjustment is necessary to accommodate the peculiarities of the older child, without breaching the established management principles [3,15,22].
Long-term follow up of treated cases is important to detect recurrences or relapses. Recurrence (or relapse) is defined as the development of one or more of the original deformities (equinus, varus, adductus and cavus) after initial full correction of all deformities of both the hind-foot and the fore-foot [7,45,46]. The cause of recurrence is unknown, but it is logically the same mechanism that initially caused the deformities to develop and it is related to rapid growth of the foot. Recurrence is rare after the age of 4-5 years and almost never occurs after 7 years of age [7]. A key essence of treatment is to improve the quality of life of the affected children, but the concept of quality of life in this instance should be measured from the patient's perspective [7]. In a study on 170 subjects (252 clubfeet) with an average follow-up of 16 years, female patients were found to be less satisfied and more concerned about the shape of their feet and legs compared with their male counterparts, even without demonstrable difference in physical function [7]. One possible explanation could be that women are more cosmetically conscious than men. In the long run, more than 80% of the feet yield good functional long-term results [7].
Conclusion
Congenital talipes equinovarus imposes some functional compromise on the foot, resulting in structural adaptation or modification of the local anatomy. The foot deformities of congenital talipes equinovarus interfere with the locomotive ability of the individual, leading to an imbalance in the gait. Evidence suggests that 80% of infants with congenital talipes equinovarus deformity live in developing countries. The goals of management of clubfoot are to obtain an obvious plantigrade, stable and straight foot with no need to modify shoes and a good range of foot motion without pain. Recurrence after treatment is common, and requires long-term follow up of treated cases to detect.
Disclosure
This manuscript was adapted from a dissertation submitted by the first author to the postgraduate school of the University of Uyo, Nigeria, in December 2021, in partial fulfillment of the requirements for the award of the Master of Science degree (MSc) in Anatomy.
Conflict of Interests
The authors have no conflict of interests to declare.
References
- Sarrafian SK, Kelikian AS (2016) Development of the foot and ankle. Musculoskeletal key.
- Soares RJ, Cerqueira ASO, Mochizuki L, et al. (2016) Revista Brasileira de Educacao Fisica e Esporte/Brazilian. J Phys Educ Sport 30: 279-285.
- Maranho DA, Volpon JB (2011) Congenital clubfoot. Acta Ortop Bras 19: 163-169.
- Manisha R, Priyanka K (2017) Congenital clubfoot: A comprehensive review. Orthopaedics & Rheumatology Open Access 8: 555728.
- Ricco A, Richards B, Herring J (2014) Tachdjian’s paediatric orthopaedics. In: Herring J, (5th edn), Philadelpia, Elsevier 785-818.
- Martanto TW, Dominica H, Irianto KA, et al. (2020) The Pirani score evaluation on patients with clubfoot treated with the Ponseti method in public hospital. EurAsian J Bio Sci 14: 3419-3422.
- Wallander HM (2010) Congenital clubfoot. Aspects on epidemiology, residual deformity and patient reported outcome. Acta Orthop Suppl 81: 1-25.
- Strach EH (1986) Clubfoot through the Centuries. Prog Pediatr Surg 20: 215-237.
- Ansar A, Rahman AE, Romero L, et al. (2018) Systematic review and meta-analysis of global birth prevalence of clubfoot: A study protocol. BMJ Open 8: e019246.
- Janicki JA, Narayanan UG, Harvey B, et al. (2009) Treatment of neuromuscular and syndrome-associated (non-idiopathic) clubfeet using the Ponseti method. J Pediatr Orthop 29: 393-397.
- Patel M (2019) Clubfoot (Talipes) treatment & management. Medscape.
- Pirani S, Hodges D, Sekeramayi F (2008) A reliable and valid method of assessing the amount of deformity in the congenital clubfoot deformity. In: Orthopaedic Proceedings. The British Editorial Society of Bone & Joint Surgery 90: 53-53.
- Saltzman HM (2009) Foot focus: International initiative to eradicate clubfeet using the Ponseti method. Foot Ankle Int 30: 468-471.
- Smythe TH (2018) Evidence to improve clubfoot services in Africa with Zimbabwe as a case study. PhD (research paper style) thesis, London School of Hygiene and Tropical Medicine.
- Hegazy MA, Khairy HM, Hegazy AA, et al. (2021) Clubfoot in children: An overview. The Foot and Ankle Online Journal 13: 10.
- Omololu B, Ogunlade SO, Alonge TO (2005) Pattern of congenital orthopaedic malformations in an African teaching hospital. West Afr J Med 24: 92-95.
- Adewole OA, Giwa SO, Kayode MO, et al. (2009) Congenital clubfoot in a teaching hospital in Lagos, Nigeria. AJMMS 38: 203-206.
- Ukoha U, Okafor A, Ogugua I, et al. (2011) Incidence of congenital talipes equinovarus among children in SouthEast Nigeria. Int J Biol Sci INT J BIOL SCI 2: 712-715.
- Mejabi JO, Esan IO, Adegbehingbe OO, et al. (2016) The Pirani scoring system is effective in assessing severity and monitoring treatment of clubfeet in children. British Journal of Medicine and Medical Research 17: 1-9.
- Ugorji TN, Nwakamma JI, Anoliefo KT, et al. (2020) Epidemiology and pattern of clubfoot in Enugu, South-East Nigeria. Am J Biomed Sci 11: 190-193.
- Kasser JR (2006) The foot. In: Morrissy RT, Weinstein SL, Lovell and Winter’s Paediatric Orthopaedics, (6th Edn), Lippincott Williams & Wilkins 1258.
- Lavy C (2017) Africa clubfoot training: Basic & Advanced clubfoot treatment provider courses participant manual, University of Oxford 15-27.
- Anderson M, Blais MM, Green WT (1956) Lengths of the growing foot. J Bone Joint Surg Am 38: 998-1000.
- Ogden J (2000) The foot. In: Ogden, Journal of Skeletal Injury in the Child 2000, New York, Springer Verlag 38-68.
- Miedzybrodzka Z (2003) Congenital talipes equinovarus (clubfoot): A disorder of the foot but not the hand. J Anat 202: 37-42.
- Praveen KR (2017) https://www.slideshare.net/mobile/PraveenKumarReddyGorantla/ctev-82394819
- Nordin S, Aidura M, Razak S, et al. (2002) Controversies in congenital clubfoot: Literature review. Malays J Med Sci 9: 34-40.
- Pirani S, Zeznik L, Hodges D (2001) Magnetic resonance imaging study of the congenital clubfoot treated with the Ponseti method. J Pediatr Orthop 21: 719-726.
- Ponseti IV (1996) Congenital clubfoot: Fundamentals of treatment. Oxford: Oxford University Press 1-140.
- Hootnick DR, Levinsohn EM, Crider RJ, et al. (1982) Congenital arterial malformations associated with clubfoot: A report of two cases. Clin Orthop Relat Res 167: 160-163.
- Kruse L, Gurnett CA, Hootnick D, et al. (2009) Magnetic resonance angiography in clubfoot and vertical talus: A feasibility study. Clin Orthop Relat Res 467: 1250-1255.
- WHO (2016) Document on congenital anomalies. World Health Organization. Geneva, Switzerland.
- Farlie P (2017) Why we don’t know what causes most birth defects.
- Emordi MV, Osifo DO (2018) Challenges of congenital malformations: An African perspective. Annals of Paediatric Surgery 14: 1-7.
- Kumar P, Burton B (2007) Congenital malformations: Evidence-based evaluation and management. USA: McGraw Hill Professional 283-299.
- Palma M, Cook T, Segura J, et al. (2013) Descriptive epidemiology of clubfoot in Peru: A clinic-based study. Iowa Orthop J 33: 167-171.
- Irani RN, Sherman MS (1963) The pathological anatomy of clubfoot. Journal of Bone Joint Surgery 45: 45-52.
- Wang H, Barisic I, Loane M, et al. (2019) Congenital clubfoot in Europe: A population-based study. Am J Med Genet A 179: 595-601.
- Sahoo J (2014) Clubfoot/CTEV pdf. Svnirtar.nic.in 1-9.
- Mittal RL (2019) Epidemiology of clubfoot. In: Clubfoot: A Comprehensive Approach (Past, Present, and Future). Boca Raton, FL: CRC Press/Taylor & Francis Group 5.
- Diepstraten AF (1996) Congenital clubfoot. Acta Orthopaedica Scandinavia 67: 305-312.
- Ippolito E, Farsetti P, Valentini MB (2014) Management of clubfoot 84-92. In: Bentley G, European surgical orthopaedics and traumatology 2014. Springer Berlin Heidelberg 57.
- Philippe H, Maxime H, Jacques P, et al. (2017) History of clubfoot treatment part 1: From manipulation in antiquity to splint and plaster in Renaissance before tenotomy. Int Orthop 41: 1693-1704.
- Anand A, Sala DA (2008) Clubfoot: Etiology and treatment. Indian J Orthop 42: 22-28.
- Ponseti IV, Smoley EN (1963) Congenital club foot: The results of treatment. J Bone Jt Surg 45: 261-344.
- Ponseti IV (2000) Clubfoot management. J Pediatr Orthop 20: 699-700.
Corresponding Author
Dr. Dim EM, Department of Orthopaedics and Traumatology, Faculty of Clinical Sciences, University of Uyo, Nigeria, Tel: +234-703-753-4952
Copyright
© 2022 Dim EM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.