Safety of Intraoperative Neurophysiological Monitoring during Indirect Decompression in Lumbar Spinal Surgery - Descriptive Report
Summary
Objective: Degenerative disease of the lumbar spine often compromises the life quality of patients. In recent decades, surgical procedures on the lumbar spine have been oriented to reduce risks, costs, and perioperative complications by performing minimally invasive surgeries, specifically anterior lumbar interbody fusion (ALIF) and/or lateral lumbar interbody fusion (LLIF). A large number of studies have evaluated these procedures, but those studies almost exclusively evaluated clinical and radiological outcomes. This study aims to evaluate the safety of intraoperative neurophysiological monitoring (IONM) during indirect decompression of the lumbar canal.
Methods: This descriptive case series study included 18 patients undergoing a surgical procedure for indirect decompression of the lumbar spinal canal (ALIF/LLIF only or multilevel) from 2018-2019 in the Hospital Universitario San Ignacio (HUSI). The authors measured neurophysiological parameters intraoperatively, with somatosensory-evoked potentials (SSEPs) and motor-evoked potentials (MEPs). Changes in amplitude and latencies were evaluated.
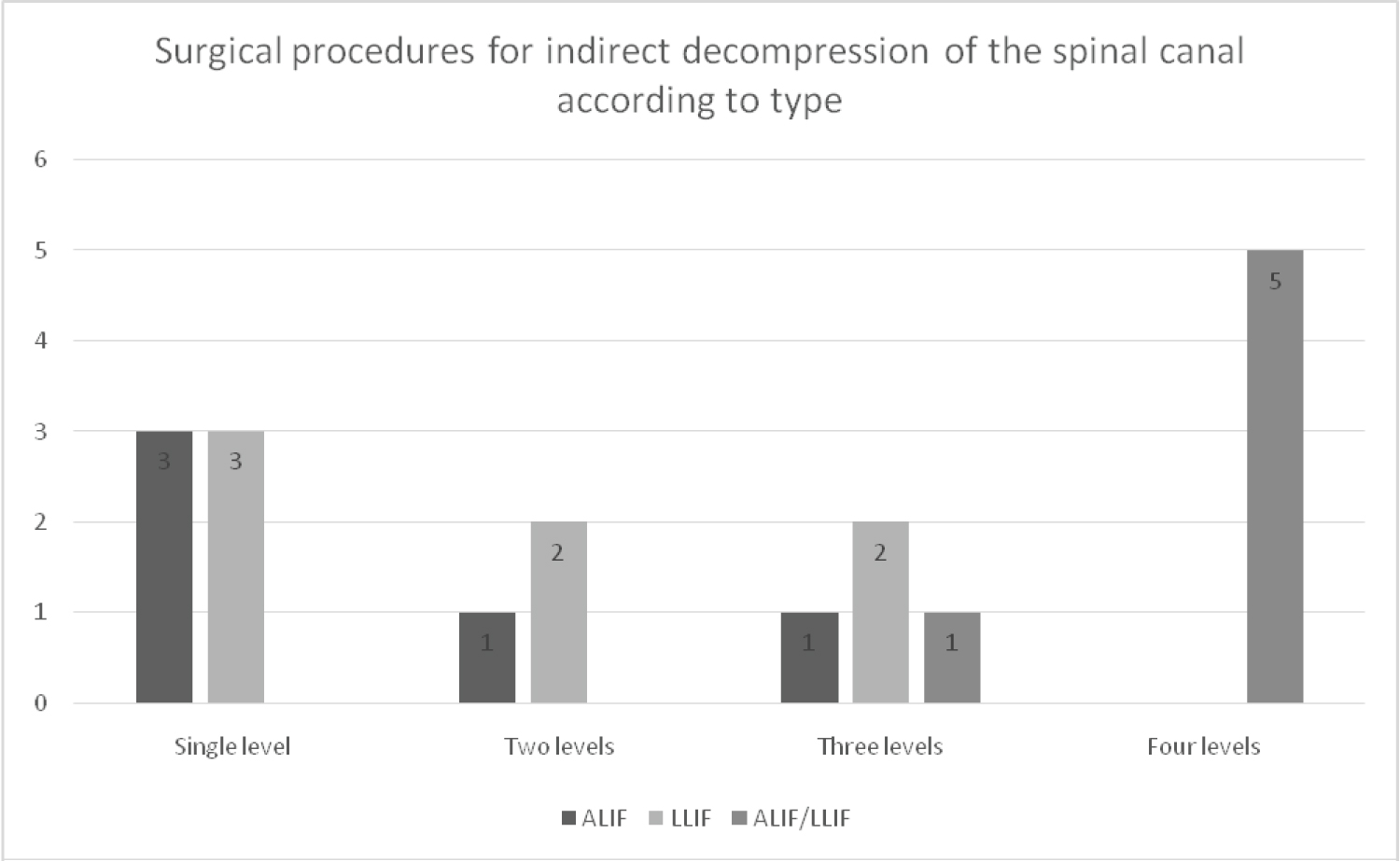
Results: Eight subtypes of surgery were performed: 3 single-level ALIF (16.6%), 1 two-level ALIF (5.5%), 1 three-level ALIF (5.5%), 3 single-level LLIF (16.6%), 2 two-level LLIF (11.1%), 2 three-level LLIF (11.1%), 1 three-level LLIF/ALIF (5.5%), and 5 four-level LLIF/ALIF (27.7%). For all cases, SSEPs and MEPs basal registries were compared with the registries after performing the interbody fusion. No changes were evidenced in the neurophysiologic parameters. There was no postoperative neurologic deficit in any of the patients.
Discussion: Multimodal Intraoperative Neurophysiological Monitoring (MIONM) gives surgeons the certainty that no neurological damage occurs during a surgical procedure. Interbody fusion is a safe procedure when it is performed with an adequate surgical technique and multimodal monitoring. Multimodal monitoring allows the measurement of several responses, with higher sensitivity and specificity than monitoring single responses. Future research is needed to develop and validate protocols regarding the most appropriate monitoring technique (SSEPs, MEPs, EMGs, EMGt, or multimodal).
Keywords
Axial lumbar pain, Radicular lumbar pain, Anterior lumbar interbody fusion, Lateral lumbar interbody fusion, Somatosensory-evoked potentials, Motor-evoked potentials
Introduction
In the last decade, surgical procedures on the lumbar spine have been oriented to reduce risks, costs, and perioperative complications by performing minimally invasive surgeries, specifically anterior lumbar interbody fusion (ALIF) and/or lateral lumbar interbody fusion (LLIF). The anterior and the lateral approaches are associated with higher rates of fusion compared to posterolateral techniques alone [1]. The anterior and lateral techniques have the advantages of reducing morbidity rates associated with the surgical approach. These techniques also create a larger area for fusion that increases disc height and lumbar lordosis and contributes to indirect decompression of the lumbar canal and neural foramina [2]. Lumbar stenosis can lead to neurogenic claudication or radicular symptoms when present within the foramen, when nonoperative pain management fails to alleviate those symptoms, surgical interventions may be necessary and as previously stated, minimally techniques have been developed to avoid the morbidity of traditional open surgery [3].
Spine surgeons always face choosing the surgical approach, among anterior, lateral, or a combination of the two. It is known that ALIF has a higher rate of successful interbody fusions due to wider exposure of the surgical field. This exposure reduces complications, such as vertebral body fracture, but increases other complications, such as injuries of intra-abdominal organs, sympathetic plexus, or large blood vessels. The ALIF also lengthens surgical time and patient exposure [4,5]. The LLIF, on the other hand, avoids exposure of intra-abdominal viscera with a reduction in the possibility of injuries to those organs or large blood vessels. It also shortens the recovery period and hospital stay [5]. These techniques, however, carry a slightly higher index of neurological complications of different sorts. The LLIF employs the creation of a corridor through the psoas muscle, with the risk of iatrogenic injuries of the lumbar plexus located within the muscle, and complications such as transient motor weakness, hypoesthesia, and femoral nerve injury, among others. There are also certain mechanisms of injury difficult to identify, such as the secondary ischemia due to constant traction of nerve roots [6]. This mechanism of injury has a low frequency of occurrence, but it has the potential to produce an important neurological deficit [7]. The IONM has recently been used as a tool to avoid such a complication, as it allows a real-time assessment of the spinal medulla function during the surgical procedure [8-11].
The role of IONM during pedicle fixation was not appreciated until recent times. It is now considered a highly valuable tool, in addition to surgical technique and intraoperative fluoroscopy [12]. However, to the best of our knowledge, the use of IONM during minimally invasive procedures for indirect decompression of the lumbar canal, such as ALIF and LLIF, has not been reported. That is the reason for this paper.
Methods
This descriptive case series study evaluated intraoperative neurophysiological parameters in 18 patients of all ages who underwent a surgical procedure for indirect decompression of the lumbar canal (ALIF/LLIF only, or multilevel), from 2018-2019 in the Hospital Universitario San Ignacio (HUSI). Patients with tumors, infections (confirmed with MRI and blood panel test), or non-adequate images were excluded from the study. Data were obtained from Sistema de Administración Hospitalaria Integrado (SAHI), the integrated database of the HUSI. The study was approved by the institutional review board. Each patient underwent imaging studies with magnetic resonance imaging of the lumbar spine, panoramic X-ray (for measurement of sagittal and spine-pelvic parameters), and dynamic X-rays. All measurements were performed by two attending spine neurosurgeons. The best surgical approach for each patient (in this case, anterior, lateral, or combined) was defined according to these measurements. All patients underwent pre-anesthetic assessment before the surgery and obtained anesthesiology approval.
During each surgery, a physiatrist performed IONM with a Medtronic NIM-Eclipse™ E4 system, that measured basal and final amplitudes and latencies of SSEPs and MEPs. Registries were made at the beginning of the procedure (basal registry) and immediately as the interbody fusion cages were positioned (post-fusion registry), and these were compared. Box-and-whisker plots were made to explore the variation in SSEPs and MEPs measurements before and after the intervention.
The spontaneous electromyographic activity was also measured, though this parameter was not taken into account due to the high sensitivity of the electrode to nerve stimulation (by retraction or irrigation). Also, the electrode picks up interference (from cauterization devices, electrocardiography electrodes, or drilling devices) that may be misinterpreted as electromyographic activity.
The alarm signs were a prolongation of latencies of 10% or more or a reduction of amplitudes of 50% or more. In such cases, a careful evaluation of the surgical field was required, to prevent any neurological complication. All patients were given a liquid diet and allowed assisted ambulation 4 hours after surgery. A follow-up X-ray was taken on the first postoperative day. Patients were released after 24-48 hours of postoperative surveillance. All patients had a follow-up appointment at two weeks, one month and every six months thereafter and the mean follow-up in time was 3.8-years.
Results
During the selection process, 61 patients underwent interbody fusion procedures. All patients were referred to neurosurgery consultation due to axial or radicular lumbar pain, and all had undergone percutaneous procedures for pain management (facet or epidural block) without improvement, which was the reason to perform surgery. In 18 (30% of the patients), IONM was performed; 10 (56%) were women and 8 (44%) were men. The majority of patients were older than 60 years (83%) and only 3 patients were younger than age 60 (Table 1). Regarding the type of intervention, 8 subtypes of surgery were performed: 3 single-level ALIF (16.6%), 1 two-level ALIF (5.5%), 1 three-level ALIF (5.5%), 3 single-level LLIF (16.6%), 2 two-level LLIF (11.1%), 2 three-level LLIF (11.1%), 1 three-level LLIF/ALIF (5.5%) and 5 four-level LLIF/ALIF (27.7%) (Figure 1).
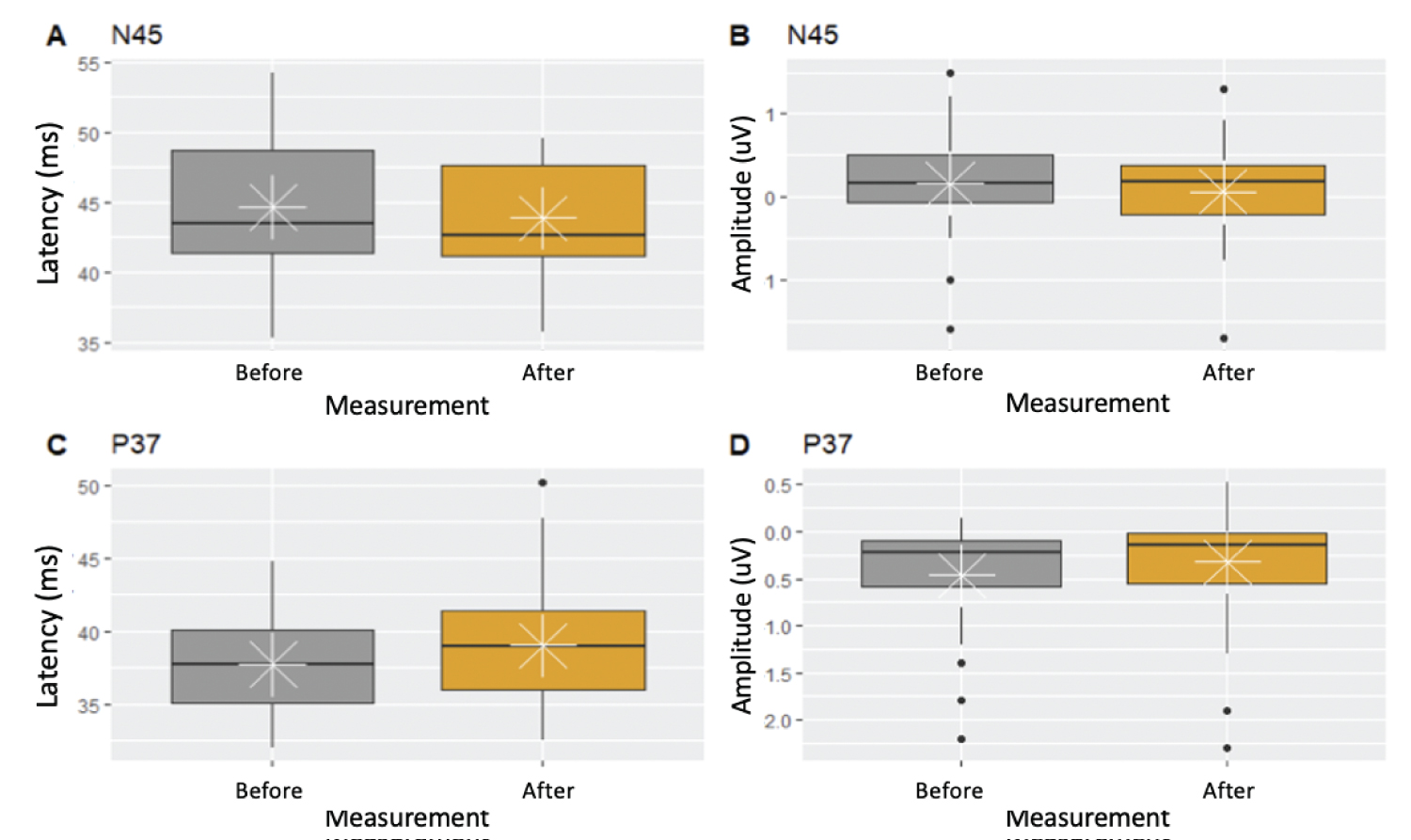
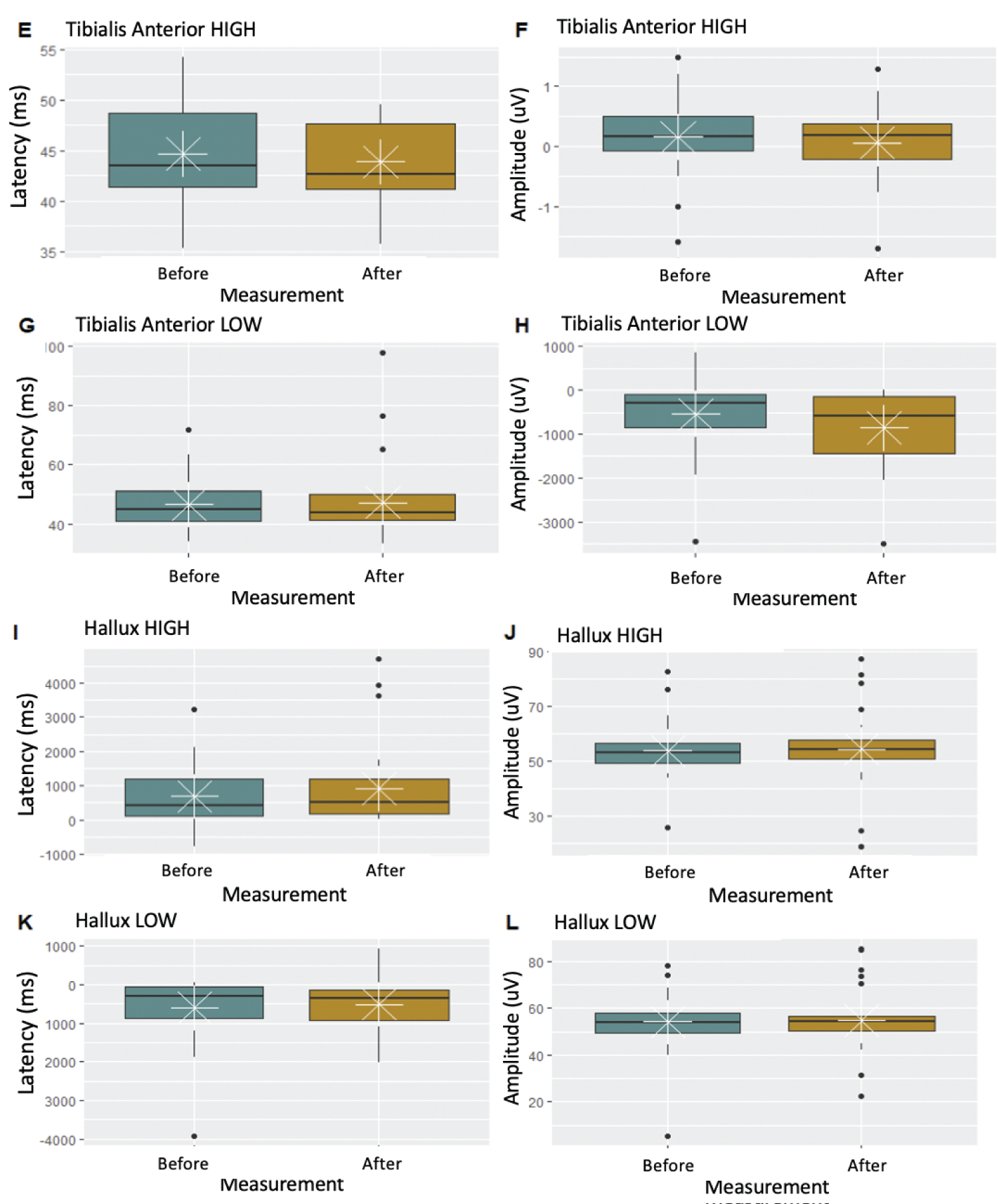
Box-and-whisker plots evidenced no changes in neurophysiological parameters during the performance of surgical procedures (Figures 2 and Figures 3).
No neurological deficit or complication of another type (organ or vascular injury) was reported in any patient after interbody fusion procedures. There was no fatal outcome during procedures or patient follow-ups.
Discussion
The IONM emerges from an important limitation for clinical evaluation of nervous system function during surgical procedures. Registries obtained through IONM provide a real-time assessment of changes in the patient, which guide the surgeon in a procedure and ease intraoperative decision-making.
Despite advances in neuromonitoring technologies, there are no prospective studies with a high level of evidence that validate the efficacy of IONM. Generally, the use of IONM is prompted by the surgeon's preference or by medicolegal issues [10].
Latency is the time it takes for an electrical stimulus to elicit a response; amplitude is the size of that response. Surgical goal is to avoid reduction in amplitudes or prolongation of latencies after performing decompression when the interbody cage is positioned. Evaluation of SSEPs and MEPs in each patient undergoing indirect lumbar canal decompression by minimally invasive procedures in this study showed no variability in the potentials. This assessment verified the integrity of the nervous system during surgical procedures, which correlated with the evidence of no neurological deficit in the postoperative period.
It is important to take into account factors, such as the anesthetic regimen, unrelated to the procedure that might affect the neurophysiological results. Especially, MEPs can be significantly altered by the type and level of anesthesia [13]. Hemodynamic parameters can also alter evoked potentials. A reduction in cerebral blood flow alters the cortical evoked responses, as well as the subcortical responses in SSEPs. Hematocrit reduction of 10-15% has been associated with prolonged latency, which normalizes when hematocrit increases. Most studies have been performed with SSEPs, and results are extrapolated to MEPs [10]. For these reasons, it is crucial to control all variables that might prompt changes in neurophysiological parameters before reaching conclusions and making decisions based on potentially biased results.
The use of IONM is increasing in spine surgical procedures. There are, however, doubts about immediate changes in neurophysiological parameters and their diagnostic values. A study by Garcés, et al. failed to demonstrate a reduction in the incidence of misplaced pedicle screws. With increased costs and surgical time, the authors did not find a benefit for IONM during TLIF-MIS [14]. Ibrahim, et al. studied 121 patients and concluded that neuromonitoring has a poor positive predictive value. They concluded it is an inappropriate test for preventing neurological injury [15].
Conversely, Biscevic, et al. Consider that an adequate selection of patients, surgical planning, surgical technique, and a diligent IONM are key points in preventing a neurological deficit in spine surgery. The authors propose a combination of MEP and SSEPs (the same recommendation of the institution in the present study) as the preferred method of monitoring [16].
Sutter, et al. Consider that IONM is an effective and precise tool to evaluate medullar and radicular function during spine surgery, helping reduce neurological complications. The authors emphasize multi modal monitoring as fundamental, given that measuring different responses results in sensitivity and specificity of 93.0% and 99.1%, respectively. In their cohort, the comparison with the use of single modalities showed variability from 13-81% for detection of neurological complications [17].
Cofano, et al. found IONM as a reliable indicator of functional injury, especially during lateral transpsoas access for interbody fusion and the divergent trajectory for cortico-pedicular screws. In these particular techniques, because of reduced visual exposure, neuromonitoring is indeed essential to exploit the full potential of minimally invasive surgery, while avoiding damage to nervous structures [18].
Performing IONM during interbody-fusion procedures for degenerative disease of the lumbar spine provides certainty that no neurological damage occurs during a surgical procedure, just as this study finds. These procedures are safe when an adequate technique is used, depending on the surgeon's expertise. These minimally invasive interbody fusions result in shorter surgical time, reduced hospital stay, and lower rates of complication. They are also associated with unharmed intraoperative neurophysiological parameters. This proves the integrity of the nervous system and confirms the safety of these procedures.
The strengths of our paper are its originality and novelty, to the best of our knowledge there has not been published any other paper evaluating the safety of intraoperative neurophysiological monitoring during indirect decompression in lumbar spinal surgery. This study also has an important limitation, the small sample size, which is due to the fact that during ALIF procedures the IONM is not performed as a routine, so in a close future this could be considered as a routine parameter during these types of procedures for investigation purposes.
Conclusions
There is clear controversy regarding the use of IONM. There is, however, an increasing tendency to prefer its use. Along with this preference, there is a supporting body of evidence such as the experience of the institution in this study. The lack of evidence of immediate positive changes in measurements performed brings us to the understanding that IONM is not a predictive factor of prognosis of clinical improvement, but it is a factor for safety and for clinical correlation with perioperative neurological complications. Future investigation should develop and validate protocols with the most adequate monitoring (SSEPs, MEPs, EMGt, or multimodal).
Conflict of Interest
None.
Funding
This study did not receive any specific support from public or commercial agencies, or non-profit sectors.
References
- Mummaneni PV, Dhall SS, Eck JC, et al. (2014) Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: Interbody techniques for lumbar fusion. J Neurosurg Spine 21: 67-74.
- Heary RF, Kumar S, Bono CM (2008) Decision making in adult deformity. Neurosurgery 63: 69-77.
- Castellvi AE, Nienke Thomas W, Marulanda German A, et al. (2014) Indirect decompression of lumbar stenosis with transpsoas interbody cages and percutaneous posterior instrumentation. Clin Orthop Relat Res 472: 1784-1791.
- Bassani R, Gregori F, Peretti G (2019) Evolution of the anterior approach in lumbar spine fusion. Rev World Neurosurgery 131: 391-398.
- Härtl R, Joeris A, McGuire R (2016) Comparison of the safety outcomes between two surgical approaches for anterior lumbar fusión surgery: Anterior lumbar interbody fusion (ALIF) and extreme lateral interbody fusion (ELIF). Springer Berlin Heidelberg 25: 1484-1521.
- Riley M, Doan A, Vogel R, et al. (2018) Use of motor evoked potentials during lateral lumbar interbody fusión reduces postoperative déficits. The Spine Journal 18: 1763-1778.
- Ahn H, Fehlings MG (2008) Prevention, identification, and treatment of perioperative spinal cord injury. Neurosurg Focus 25: E15.
- Ajiboye RM, Zoller SD, D Oro A, et al. (2017) Utility of intraoperative neuromonitoring for lumbar pedicle screw placement is questionable: A review of 9957 cases. Spine (Phila Pa 1976) 42: 1006-1010.
- Imirizaldu L, Urriza J, Olaziregui O, et al. (2009) Intraoperative neurophysiological monitoring in spine surgery. An Sist Sanit Navar 32: 125-133.
- Lall RR, Lall RR, Hauptman JS, et al. (2012) Intraoperative neurophysiological monitoring in spine surgery: Indications, efficacy and role of preoperative checklist. Neurosurg focus 33: E10.
- Daniel JW, Botelho RV, Milano JB, et al. (2018) Intraoperative neurophysiological monitoring in spine surgery: A systematic review and meta-analysis. Spine (Phila Pa 1976) 43: 1154-1160.
- Alemo S, Sayadipour A (2010) Role of intraoperative neurophysiologic monitoring in lumbosacral spine fusion and instrumentation: a retrospective study. World Neurosurgery 73: 72-76.
- Bithal PK (2014) Anaesthetic considerations for evoked potentials monitoring. J Neuroanaesthesiol Crit Care 1: 2-12.
- Garces J, Berry JS, Valle Giler E, et al. (2014) Intraoperative neurophysiological monitoring for minimally invasive 1-and 2-level transforaminal lumbar interbody fusion: Does it improve patient outcome? Ochsner J Spring 14: 57-61.
- Ibrahim T, Mrowczynski O, Zalatimo O (2017) The impact of neurophyisiological intraoperative monitoring during spinal cord and spine surgery: A critical analysis of 121 cases. Cureus 9: e1861.
- Biscevic M, Sehic A, Krupic F (2020) Intraoperative neuromonitoring in spine deformity surgery: Modalities, advantages, limitations, medicolegal issues - surgeons' views. EFORT Open Rev 5: 9-16.
- Sutter M, Eggspuehler A, Jeszenszky D, et al. (2019) The impact and value of uni- and multimodal intraoperative neurophysiological monitoring (IONM) on neurological complications during spine surgery: A prospective study of 2728 patients. Eur Spine J 28: 599-610.
- Cofano F, Zenga F, Mammi M, et al. (2019) Intraoperative neurophysiological monitoring during spinal surgery: Technical review in open and minimally invasive approaches. Neurosurg Rev 42: 297-307.
Corresponding Author
Dr. Diego Armando Devia Manosalva, Department of Neurosurgery, Hospital Universitario San Ignacio, Carrera 7 # 40-62, Bogotá DC, Colombia
Copyright
© 2021 Devia DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.