Negative Pressure Wound Therapy: An Asset When Treating Pediatric Osteoarticular Infection
Abstract
Background
Pediatric osteoarticular infection can lead to serious sequelae and permanent disability. The purpose of this study was to describe use of Negative Pressure Wound Therapy (NPWT) as an advantageous treatment option for pediatric patients with osteoarticular infection. NPWT has not been used in pediatric articular infections and has only been published in animal models.
Methods
Retrospective review of 18 patients (20 acute osteoarticular infections) with a mean age of 92.83 months (range: 4-187 months) and a mean follow-up of 31.61 months (range: 10-94 months). The presence of osteoarticular infection was confirmed via clinical symptoms, lab results, and magnetic resonance imaging (MRI); patients underwent debridement, cultures and the placement of NPWT. Seven patients had more than one NPWT. Laboratory values, pain scores, and complications were assessed.
Results
There were 20 acute osteoarticular infections, presenting with a mean temperature of 37.75 ℃; length of stay (LOS) was a mean of 7.38 days (range, 3 to 23 days). There was an improvement in pain from presentation to post-operative care as measured by the Visual Analog Scale (VAS). 50% percent of patients with infections required two or fewer doses of opiates post-operatively. Inflammatory markers dropped precipitously after surgery and normalized within one month after surgery. There were no intra-operative complications or recurrences of infection at follow up (31.61 months).
Conclusion
NPWT is a reliable treatment option for pediatric osteoarticular infection. In the future, a comparative study may investigate how NPWT hastens recovery, leads to less pain medication, and improves infection containment.
Keywords
Pediatric, Osteomyelitis, Septic arthritis, NPWT
Abbreviations
NPWT: Negative Pressure Wound Therapy; MRI: Magnetic Resonance Imaging; LOS: Length of Stay; VAS: Visual Analog Scale; MRSA: Methicillin-Resistant Staphylococcus Aureus; IDSA: Infectious Diseases Society of America; MSSA: Methicillin-Susceptible Staphylococcus Aureus; AHO: Acute Hematogenous Osteomyelitis; SIRS: Systemic Inflammatory Response Syndrome
Introduction
Osteoarticular infection in children may be difficult to diagnose early in its course, a period characterized by increasing bone pain [1]. This condition can lead to serious sequelae and permanent disability including sepsis, growth arrest, avascular necrosis, joint destruction or subluxation, and pathologic fractures [2-6]. Treatment is often complicated by Methicillin-Resistant Staphylococcus Aureus (MRSA) infection, which has a more severe clinical course with increased immediate and late morbidity [4,7].
Since 2001, the Infectious Diseases Society of America (IDSA) has been concerned with the explosion of drug-resistant infections and with the relative standstill in the development of new antimicrobial agents [8]. From 2001 to 2010 the percentage of patients with MRSA osteomyelitis and Methicillin-Susceptible Staphylococcus Aureus (MSSA) has increased from 11.8% to 34.8% at a high-volume academic pediatric hospital [9]. Another large pediatric academic institution has found 51% Acute Hematogenous Osteomyelitis (AHO) MRSA rates [10]. These numbers reveal a pressing necessity for early and aggressive treatment which should include debulking of the bacterial load and the associated toxins. Wound care with local bactericidal treatment is essential and should be used with antibiotics for the treatment and cure of these infections [8]. Immediate intravenous antibiotics are helpful with sepsis but cannot penetrate the relative avascular zone of the osteomyelitis, regardless of pathogen, until the levels are therapeutic [11]. Regardless, there is a 40% failure rate of antibiotics such as Vancomycin when treating osteomyelitis [10]. This is critical since these children can easily progress to Systemic Inflammatory Response Syndrome (SIRS) [12].
Negative Pressure Wound Therapy (NPWT) may have utility in treating osteomyelitis. However, there are a paucity of studies detailing its safety and efficacy with osteoarticular infection. Intra-articular use of NPWT has only been published in animals and has not been used in intra-articular infections clinically [13]. The purpose of this study was to describe the use of NPWT as an advantageous treatment option with osteoarticular infection.
Methods
Institutional Review Board approval was obtained prior to the initiation of this retrospective study performed at a single-institution. The study included 18 pediatric patients diagnosed with 20 acute osteoarticular infections who underwent treatment with NPWT between January 2010 and January 2016. The mean age was 92.83 months (range: 4-187 months); the mean follow-up was 31.61 months (range: 10-94 months). Patients were admitted through the emergency department (ED) with pain or change in weight bearing status and treated by the same pediatric orthopedic surgeon. Magnetic Resonance Imaging (MRI) was used to identify the location and extent of bone involvement (abscess or bone edema). The clinical and outcome parameters evaluated included: 1) Temperature; 2) Inflammatory markers, including a) C-reactive protein (CRP), b) erythrocyte sedimentation rate (ESR), and white blood cell count (WBC); 3) LOS; 4) Pain (using the Visual Analog Scale (VAS) in the ER at presentation and 24 hours after the first debridement and NPWT) and opiate use; and 5) Intra- and post-operative complications.
Once the presumptive diagnosis of acute osteoarticular infection was made, all patients were brought to the operating room for emergent treatment. The diagnosis was further verified with a bone culture and a synovial fluid culture. Appropriate debridement was accomplished along with antibiotic pulse lavage or bulb syringe irrigation. Depending on the extent of bone involvement revealed on MRI, drilling or slotting of the bone was performed.
Following the initial debridement, NPWT was used to remove exudate and assist with healing [KCI Wound V.A.C.® (Kinetic Concepts, Inc., San Antonio, Texas)] [14-16]. If a septic joint was encountered, silver impregnated open-cell reticulated polyurethane foam was placed to the depth of the wound or up to and crossing the open joint capsule. For superficial wounds, an ellipse-shaped piece of foam was cut and placed in the area. A second piece of full-thickness foam was cut in a circular fashion with a diameter large enough to allow the NPWT suction device to sit fully on the foam without touching the patient's skin. The circle of foam was placed over the one centimeter of foam extruding from the wound. Self-adherent plastic or Duoderm (ConvaTec Inc; Greensboro, North Carolina), was placed over the foam on the skin to protect it from excoriation. A small hole was made in the middle of this plastic film, and the suction device, which had its own self-adherent surface, was placed down over the hole (the vacuum may fail if the device contacts the skin). Care must be taken to make sure the suction device is well-positioned and held securely in place on the foam. Fine mesh Vaseline gauze can be used to protect all neurovascular or tendon structures.
Once the foam is in place, the operating room suction apparatus was applied to the NPWT tube, which was attached to the mechanical suction device at 125-mmHg continuous suction. The exudate was delivered to a disposable canister attached to the NPWT machine.
To aid with the closure of larger wounds, 3-0 nylon stitches were placed in a horizontal mattress configuration approximately one-third of the way from the end of the wound so that part of the wound itself was closed while still allowing drainage. This was to decrease the area and volume of the wound, thus allowing for faster healing and for the maintenance of pressure on the skin (to make it easier to close at the next visit to the operating room).
If patients were diagnosed with a virulent organism or if a significant amount (> 3-cc) of purulent exudate was present in the wound, then another NPWT device was applied when patients returned to the operating room for the second washout procedure. The device was removed, and the wound was closed if there was: 1) Negligible output from the device; 2) Minimal (< 3-cc) exudate in the wound with no purulence in the operating room; and 3) Stable vital signs. All decisions regarding antibiotic choices were made by a fellowship-trained pediatric infectious disease specialist.
When the NPWT device was applied to a joint, the limb was internally rotated and adducted to assist drainage. Post-operatively, nurses periodically checked patient limb position and rearranged the limb if necessary. Moving the limb to nondependent positions at regular intervals aids the pumping action of the NPWT device. Septic knees were flexed and extended to aid drainage (continuous passive motion is used in larger patients). Septic ankles were dorsiflexed and plantar flexed to aid NPWT. In general, there was exudate draining towards the canister with joint motion to decrease joint space. This is expected as with Boyle's Law: PV = k where pressure x volume is equal to a constant. Therefore, decreased volume in the joint due to position will increase pressure, thus aiding the action of the NPWT. This was assumed since pressures in the joint were not measured.
The patients were followed bi-weekly by infectious disease doctors. Antibiotics and inflammatory markers were ordered and followed. Initial empiric antibiotic treatment protocol consisted of cefazolin and vancomycin unless patients were allergic to cephalosporins in which case they were started on clindamycin and vancomycin pending culture sensitivities. Vancomycin was discontinued for OSSA (oxacillin-sensitive Staphylococcus aureus) pathogens. Patients were changed to orals if sensitivities were known, CRP normalized, and clinical improvement was seen. Patients were followed orthopedically with a wound check at 2 weeks post-operatively and x-ray of the involved area at 4-6 weeks, and then at 3, 6, and12 months post-operatively. Patients where there is concern of growth disturbance are reimaged every 6 to 12 months.
All de-identified data was put into a Microsoft Excel spreadsheet (Excel, Microsoft Corporation, Redmond, Washington) for statistical analysis. Statistical comparisons were performed using a Student's t-test for means. A P value of less than 0.05 was considered statistically significant. The statistical software Graph Pad Prism version 5.01 (GraphPad Software Inc., La Jolla, California), was used for all statistical calculations.
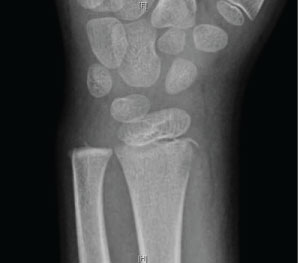
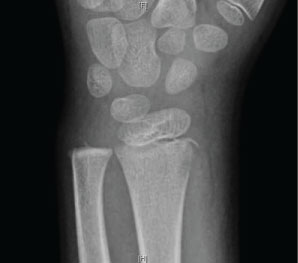
To exemplify this treatment, we reported on using NPWT to care for a seven-year-old with osteomyelitis who had atraumatic wrist pain. A plain radiograph was read as an avulsion fracture of the radial styloid (Figure 1). The patient was diagnosed with wrist overuse due to video game use, splinted, and discharged home without an orthopedic consult requested.
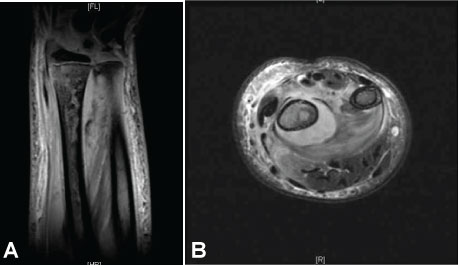
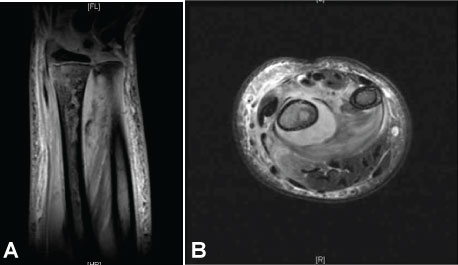
The patient returned to the ED two days later and was febrile to 38.4 ℃. The wrist and forearm were severely swollen, warm, and erythematous. The previously obtained radiograph of the forearm was reviewed and reread as a lucent lesion of the distal radius. Laboratory testing revealed a CRP of 218, ESR 66, and WBC 12.7. An emergency MRI revealed changes consistent with osteomyelitis of the radius involving 7-cm of the canal, including an intra-osseous abscess starting at the growth plate, continuing proximally, and a subperiosteal abscess along the shaft circumferentially measuring 9-cm, dissecting towards the ulna (Figure 2A and Figure 2B).
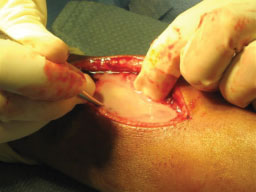
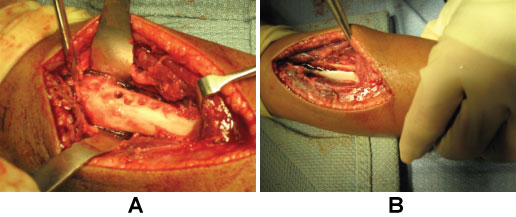
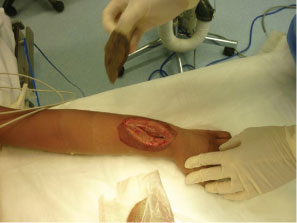
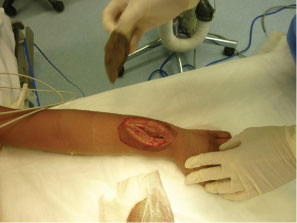
After MRI, the patient was taken directly to the operating room. The patient's vital signs were 40 ℃, BP 100/56, pulse 144. He was given one dose of Vancomycin prior to starting the procedure. A dorsal incision was made, revealing an abscess containing 40-cc of purulent material (Figure 3). A 3-mm drill was used to make a series of holes in the radius 7-cm long starting at the growth plate while pus exuded from the drill holes. The holes were connected to form a slot, and the canal was cleared using a curette (Figure 4A and Figure 4B). The purulent material was sent for culture, and pathology.
The wound was irrigated with three liters of saline/bacitracin by pulse lavage, then a NPWT was applied with silver coated polyurethane foam. The patient was taken to the Pediatric Intensive Care Unit for overnight monitoring due to a temperature of 40 ℃, tachycardia, and hypotension (meeting SIRS criteria). By post-operative day 1, the patient was afebrile and stable, and his pain level was scored as VAS 0 post-operatively (versus VAS 10 upon ED presentation). While the patient was comfortable and mobile with NPWT, his initial Gram stain showed gram-positive cocci in clusters, which later grew MRSA.
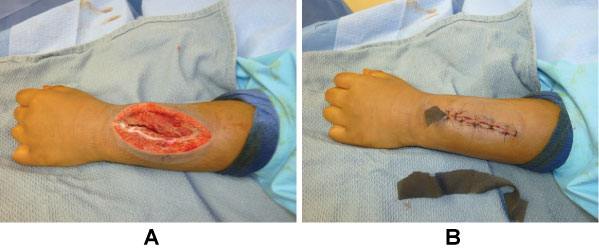
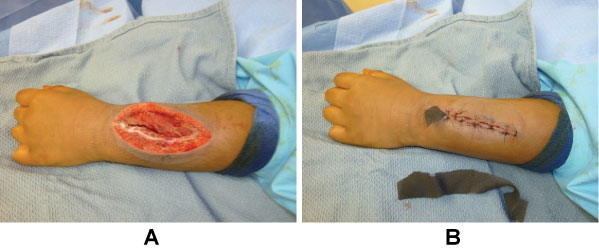
The patient returned to the operating room two days later to remove the NPWT and for reevaluation of the wound. The wound had > 3-cc purulent fluid present with severe soft tissue swelling (Figure 5). It was irrigated once again with pulse lavage and another NPWT was applied. The patient's CRP improved to 104; Vancomycin levels remained subtherapeutic.
The patient continued to do well and returned to the operating room three days later. After removal of the NPWT, the wound was clean with minimal swelling (Figure 6A). The wound was irrigated again and closed (Figure 6B). Labs were improved (CRP 44) and Vancomycin levels were still subtherapeutic. The next day, the patient was switched to Clindamycin due to pathogen sensitivities, a percutaneous intravenous catheter (PICC) was placed, and the patient was discharged home.
The patient's CRP level was normal within one week of discharge. Although oral antibiotics were considered, the family felt that there would be better compliance with the PICC line, which was discontinued after four weeks of antibiotics.
Radiographs taken one and three months after the procedure showed the bone remodeling at the slotted site with no other findings. At the 3-year follow-up appointment, the physical exam and plain radiographs revealed no signs of past infection or growth plate disturbance.
Results
This group consisted of 20 acute osteoarticular infections (18 patients), presenting with a mean temperature of 37.75 ℃. There was a mean 7.38-day LOS (range, 3 to 23 days), and an improvement in pain from presentation to post-operative care as measured by the VAS. 50% percent of patients with infections required two or fewer doses of opiates post-operatively (Table 1). Inflammatory markers dropped precipitously after surgery and normalized within one month of surgery. There were no intra-operative complications or recurrences of infection at follow-up (31.61 months). Acute complications were seen in three patients who were admitted with septic shock. Patient follow-up confirmed resolution of all residual effects from bone slotting. All patients with a septic hip were prescribed an abduction brace for one month after discharge to protect against late subluxation. One patient had early signs of hip avascular necrosis without clinical symptoms at the last follow-up.
Discussion
IDSA guidelines for osteoarticular infection emphasize that the "adequate surgical debridement" of intra/extraosseous abscesses is critical for effective therapy [7]. Those patients with AHO caused by MRSA have more severe infections with more abscesses. Classic orthopedic principles that support aggressive wound care for infected bone are typically applied, the rationale being that antibiotics cannot penetrate the avascular zone of necrotic bone or an abscess [11,17]. This entails early aggressive surgical debridement (with the goal of debulking bacteria, avascular bone, and associated toxins), non-closure of the wound, routine use of NPWT with a second washout and reevaluation of the wound several days later. It is also possible to use topical silver in the wound as a bactericidal agent (part of the NPWT) to further decrease the bacterial load and facilitate treatment. Up until the advent of antibiotics in the 1940's, silver was used extensively as an antibacterial in wounds. Now that antibiotics have lost efficacy in this new era of pathogens we must again employ silver ions as bactericidal agents [18].
This case series demonstrates that early aggressive surgical debridement involving the placement of NPWT with silver nanoparticles to optimize the healing environment is both safe and efficacious. In this population, after discussion with the parents, an aggressive treatment protocol, including NPWT was used in all patients. Rapid diagnosis and immediate surgical intervention are essential for MRSA and other resistant MSSA/AHO [7]. IDSA guidelines recommend the use of intravenous Vancomycin that can be started empirically [7]. This drug is currently broadly effective against circulating strains of MRSA but is much less potent than beta-lactam drugs against S. aureus. It also has notoriously poor bone penetrance in healthy bone, and even worse penetrance in compromised bone [7,19]. The course of the disease may be monitored by CRP [19]. CRP levels have been noted to drop expeditiously with NPWT [20].
NPWT has been the contemporary answer to wound care as it has been used for open fractures and bone infections in adults and for a variety of pediatric problems [21-25]. Although initially not advocated for the treatment of infections, it has been used successfully for this purpose [23]. Various mechanisms of continuous irrigation-drains and suction devices-have been used in wound therapy but have had inconsistent results [26,27]. NPWT can positively affect treatment outcomes as it has been shown to: 1) Promote angiogenesis; 2) Increase vascular flow with associated increases in oxygen, nutrients and antibiotic delivery; 3) Increase granulation tissue; 4) Decease bacterial count; 5) Induce tissue stress which promotes proliferation; and 6) Maintain the balance of wound moisture [21,28].
NPWT is well-tolerated by these patients and the literature shows that pain scores drop precipitously with this technique as it allows for less opiate use [29]. Although antibiotics were necessary to ensure patient survival, wound management was essential to preserving the viability of the bone. Although a PICC line may be required, many patients can be switched to oral therapy, thus avoiding the complications associated with PICC line usage [30].
In conclusion, we illustrate the safe and efficacious use of NPWT as a treatment option for pediatric patients with osteoarticular infections. This demonstrates how essential wound care along with contemporary technology and the judicious use of antibiotics are an effective way of addressing this condition in the pediatric population. Future studies should focus on the long-term safety and efficacy of treatment protocols that utilize NPWT and identify which patients would benefit from this treatment upon initial presentation. Absolute indications for irrigation and debridement without the use of NPWT versus treatment solely with NPWT should be further explored.
Acknowledgement for Editorial Assistance
Zachary Cherna, Atlantic Health Morristown Medical Center, Department of Orthopaedics.
Conflicts of Interest and Source of Funding
None declared.
References
- Conrad DA (2010) Acute hematogenous osteomyelitis. Pediatr Rev 31: 464-471.
- Belthur MV, Birchansky SB, Verdugo AA, et al. (2012) Pathologic fractures in children with acute Staphylococcus aureus osteomyelitis. J Bone Joint Surg Am 94: 34-42.
- Harik NS, Smeltzer MS (2010) Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther 8: 175-181.
- Ju KL, Zurakowski D, Kocher MS (2011) Differentiating between methicillin-resistant and methicillin-sensitive staphylococcus aureus osteomyelitis in children: An evidence-based clinical prediction algorithm. J Bone Joint Surg Am 93: 1693-1701.
- McCarthy JJ, Dormans JP, Kozin SH, et al. (2005) Musculoskeletal infections in children: Basic treatment principles and recent advancements. Instr Course Lect 54: 515-528.
- Mortia M, Nakamura H, Kitano T (2009) Comparison of clinical outcome after treatment of hip arthritis caused by MRSA with that caused by non-MRSA in infants. J Pediatr Orthop B 18: 1-5.
- Liu C, Bayer A, Cosgrove SE, et al. (2011) clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children. Clin Infect Dis 52: e18-e55.
- Spellberg B, Guidos R, Gilbert D, et al. (2008) The epidemic of antibiotic-resistant infections: A call to action for the medical community from the infectious diseases society of america. Clin Infect Dis 46: 155-164.
- Sarkissian EJ, Gans I, Gunderson MA, et al. (2016) Community-acquired methicillin-resistant staphylococcus aureus musculoskeletal infections: Emerging trends over the past decade. J Pediatr Orthop 36: 323-327.
- Ezzat A LJ, Alexander K (2016) Antimicrobial Stewardship in the Management of Acute Osteomyelitis and Septic Arthritis in Childreon. British Bone and Joint Journal.
- Nandi SK, Bandyopadhyay S, Das P, et al. (2016) Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol Adv 34: 1305-1317.
- Singer M, Deutschman CS, Seymour CW, et al. (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801-810.
- Bode K, Haggerty C, Tokish JT, et al. (2011) In vivo intra-articular negative pressure wound therapy effect on cartilage in a goat model. J Surg Orthop Adv 20: 44-49.
- Hamdy RC, Lawton L, Carey T, et al. (1996) Subacute hematogenous osteomyelitis: Are biopsy and surgery always indicated? J Pediatr Orthop 16: 220-223.
- Peltola H, Paakkonen M, Kallio P, et al. (2010) Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: Prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 29: 1123-1128.
- Peltola H, Unkila-Kallio L, Kallio MJ (1997) Simplified treatment of acute staphylococcal osteomyelitis of childhood. the finnish study group. Pediatrics 99: 846-850.
- RB G (1982) Management of open fractures and their complication. Philadelphia.
- Wong K, Liu X (2010) Silver nanoparticles- the real "silver bullet" in clinical medicine. Med Chem Commun 1: 125-131.
- Thomsen I, Creech CB (2011) Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep 13: 451-460.
- Liu T, Yang F, Li Z, et al. (2014) A prospective pilot study to evaluate wound outcomes and levels of serum C-reactive protein and interleukin-6 in the wound fluid of patients with trauma-related chronic wounds. Ostomy Wound Manage 60: 30-37.
- Morykwas MJ, Simpson J, Punger K, et al. (2006) Vacuum-assisted closure: State of basic research and physiologic foundation. Plast Reconstr Surg 117: S121-S126.
- Halvorson J, Jinnah R, Kulp B, et al. (2011) Use of vacuum-assisted closure in pediatric open fractures with a focus on the rate of infection. Orthopedics 34: E256-E260.
- Gabriel A, Shores J, Bernstein B, et al. (2009) A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: Experience and case series. Int Wound J 6: 1-25.
- Baharestani M, Amjad I, Bookout K, et al. (2009) V.A.C. Therapy in the management of paediatric wounds: Clinical review and experience. Int Wound J 6: 1-26.
- De Franzo AJ, Argenta LC, Marks MW, et al. (2001) The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg 108: 1184-1191.
- Flynn JM SD, Morrissy RT (2006) Staying out of trouble with orthopaedic infections. Philadelphia.
- Hung NN (2010) Cortical bone fenestrations with continuous antibiotic irrigation to mediate hematogenous tibial osteomyelitis in children. J Pediatr Orthop B 19: 497-506.
- Plikaitis CM, Molnar JA (2006) Subatmospheric pressure wound therapy and the vacuum-assisted closure device: basic science and current clinical successes. Expert Rev Med Devices 3: 175-184.
- Yang C, Wang S, Li CC, et al. (2017) A high-vacuum wound drainage system reduces pain and length of treatment for pediatric soft tissue abscesses. Eur J Pediatr 176: 261-267.
- Ruebner R, Keren R, Coffin S, et al. (2006) Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics 117: 1210-1215.
Corresponding Author
Barbara Minkowitz, Department of Orthopaedic Surgery, Children's Orthopaedic and Sports Medicine Center, Atlantic Health Morristown Medical Center, 261 James Street, Suite 3C, Morristown, New Jersey 07960, USA, Tel: 973-206-1033.
Copyright
© 2019 Minkowitz B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.