Is a Minimal Invasive Autologous Chondrocyte Implantation (ACI) in the Hip Possible? A Feasibility and Safety Study of Arthroscopic Treatment of Full Thickness Acetabular Cartilage Defects with an Injectable ACI
Abstract
Introduction
Injectable autologous chondrocyte implantation (ACI) offers a new method for the minimal invasive treatment of acetabular cartilage defects. The feasibility of the procedure has not been objectified yet. Our goal was to evaluate the feasibility and safety of this arthroscopic technique.
Materials and methods
The technical feasibility was evaluated by the rate of successfully implanted chondrocytes into the defect area. Therefore, the application process was fully video documented and analyzed by two independent observers. Furthermore, all perioperative complications were recorded.
Results and discussion
Twenty six patients (4 females, 22 males, 33 years) were treated with an ACI in a 3-year period. 19 cases (73%) could be performed with a 100% application rate into the defect area. In 7 cases a maximum of 5 spheroids were lost during application process creating a maximum loss of spheroids of 4.7%. No intraoperative complications or postoperative adverse events could be recorded.
Conclusion
The injectable ACI is a technically feasible and safe procedure in the hip joint and opens a new minimal invasive opportunity in treating large full thickness focal acetabular cartilage defects.
Keywords
Autologous chondrocyte implantation, Hip, Cartilage defect, Feasibility, Safety
Introduction
New concepts of biomechanical dynamic conflicts in the hip joint and the profound gain of knowledge in reconstructive hip surgery have led to a surge interest in hip preservation surgery.
Surgical dislocation of the hip joint with trochanteric osteotomy was the gold standard in treating intra articular hip pathology for many years. Hip arthroscopy as a minimal invasive method has improved markedly in the last decade and is an attractive option because of a reduced complication rate [1], a faster rehabilitation [2] and an equal precision in femoroacetabular impingement (FAI) surgery [3].
Cartilage defects play the key role in the progress of secondary degenerative joint disease and are the most important prognostic factor for joint preserving surgery [4]. Cartilage tissue has very limited recovery potential, and even after correcting the underlying biomechanical causes no improvement is seen in chondral defects of the hip joint [5]. Since many of these patients are young the surgeon is challenged not only by treating the deformity but also by addressing the consecutive chondral damage, because specific treatment of the cartilage defects results in superior clinical outcome [6].
Surgical options include total hip arthroplasty, microfracture, articular cartilage repair, autologous chondrocyte implantation (ACI), mosaicplasty, and osteochondral allograft implantation [7]. In the knee joint ACI has received credit for its histological outcome [8] and is recommended for cartilage restoration of larger defects (> 2 cm) [9].
With the rapidly growing techniques in hip arthroscopy and the development of injectable ACI products nowadays even a minimal invasive arthroscopic approach of this promising cell based treatment is conceivable in the hip joint for larger defects.
The safe application is thereby of paramount interest, since the ACI is only effective if a sufficient number of cells are really applied directly into the cartilage defect. This is technically demanding, because the typical acetabular cartilage defect is located in the anterolateral marginal area. In the general setting for hip arthroscopy in supine position this area is located in a steeply sloping surface or even in an overhang situation and has to be applied in an operative setting without the normal fluid pressure making a major loss of the spheroids during application process possible.
In addition, the procedure is associated with a mandatory second surgery, putting the patients in jeopardy after a relative short interval of 6-8 weeks after the initial surgery.
The goal of the study was to define the feasibility of a minimal invasive arthroscopic application and to examine potential complications associated with the required second surgery in ACI of the hip joint.
Materials and Methods
Patients with symptomatic FAI persistent after conservative therapy were offered an ACI procedure in case of a suspected cartilage defect in the routinely performed preoperative MRI (1.5 Tsl direct or 3.0 Tsl indirect arthro-MRI). In case of an intraoperative confirmation of a cartilage defect ≥ 2 cm2 an ACI procedure was initiated and cartilage was harvested for further cell cultivation.
Inclusion criteria
Active patients younger than 50 years with a single full-thickness acetabular cartilage defect ≥ 2 cm2 in a contained defect situation.
Exclusion criteria
Patients older than 50 years, patients unwilling to undergo the two-step ACI procedure. Patients with hip dysplasia (Lateral center-edge angle < 25°, Acetabular index > 10°) and patients with advanced degenerative changes (Kellgren and Lawrence Score > 1).
Surgical technique
The patient was placed in supine position using a carbon radiolucent extension device (MAQUET, Rastatt, Germany) with a thorough padded perineal post and shoe.
The central compartment was safely accessed [10] and the chondrolabral damage was carefully evaluated in a diagnostic round. In case of a full thickness cartilage defect fulfilling the defined inclusion and exclusion criteria a two-step ACI procedure war initiated. In the cartilage defect all unstable parts were debrided using a curette. Concomitant labral tears, if present, were treated with a labral base or loop refixation technique depending on the labral quality [11,12]. The labral repair is important to create a "contained defect" situation for further cartilage therapy. All related pathologies like a cam deformity were assessed and treated completely.
Three cartilage-bone-cylinders were harvested from a non-weight-bearing area of the head-neck junction using an arthroscopic punch (Karl Storz, Tuttlingen, Germany). The cartilage cylinders along with patient serum were sent to the company (co.don AG, Teltow, Germany) for further cultivation of the cells.
After cultivation of the cells the chondrocytes were implanted directly into the defect area in a second hip arthroscopy usually 6-8 weeks later. Each spheroid consists of approximately 200.000 chondrocyte agglomerates without the need of a scaffold material. The spheroids were claimed to have a high primary adhesion that should allow application in so difficult areas like the anterolateral acetabulum.
At the time of the ACI the contained cartilage defect situation was confirmed again. Fibrous tissue in the defect area was removed, the subchondral bone was exposed and calcified layer of the subchondral bone was debrided using a curette.
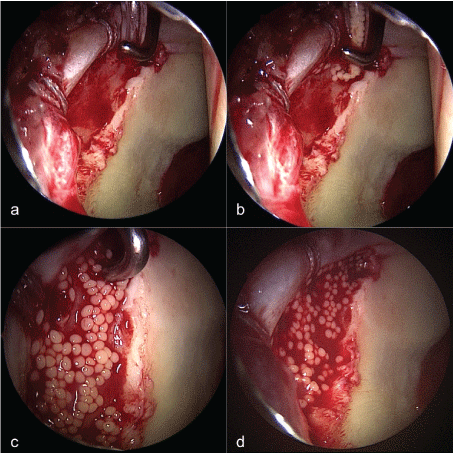
For the injection of the spheroids the camera was switched to the posterolateral portal for visualization and the whole application process was completely video documented.
The joint was cleared of fluid, the defect area dried and the cells were implanted using the provided applicator via the mid anterior portal (Figure 1). An arthroscopic probe in the anterior portal was used for directing the applicator in the defect area for a controlled complete application of the spheroids and a consistent distribution. The applicator was controlled visually to ensure no spheroids were left in the applicator. The joint was kept dry under traction for another 20 minutes to ensure unimpaired primary adhesion of the spheroids in the defect area. Finally, traction was released and the portals closed.
The postoperative rehabilitation protocol included physiotherapy and continuous passive motion (CPM) or static cycling from the first postoperative day. Mobilization was performed with 15 kg of partial weight bearing for 6 weeks postoperatively.
Prophylaxis for heterotopic ossification was carried out with Indomethacin for 3 weeks postoperatively.
Products and regulatory status
Codonchondrosphere® is granted a license by national authorization pursuant to Section 4b Arzneimittelgesetz (German Medicinal Products Act: AMG, PEI.A.11507.01.1) and the holder of authorization is co.don AG, Teltow, Germany (http://www.pei.de/EN/medicinal-products/advanced-therapy-medicinal-products-atmp/advanced-therapy-medicinal-products-atmp-node.html).
Evaluation
The evaluation of the feasibility of the injectable ACI was performed by monitoring of the number of spheroids successfully implanted in the defect area. Therefore, the full video documentation of the application process was retrospectively analyzed. The video was replayed in 50% slow motion and in a first step the number of successfully applied spheroids into the defect area was counted. The second step was a counting of the spheroids that were lost in the joint and could not successfully be implanted in the defect area. Afterwards the total spheroid count (successfully + not successfully applied) was compared with the official number of spheroids provided by the laboratory to ensure a correct evaluation process. The percentage of successfully applied spheroids was calculated. Two independent observers (DK, MB) working in consensus performed the analysis.
The size of the spheroids was checked for any influence on the effectiveness of the implantation process. As the spheroids are not always perfectly round, the largest diameter as determined by the laboratory was used for the calculation. The correlation coefficient of the size of the spheroids, the number of applicators provided and the percentage of successfully implanted spheroids was calculated.
Intraoperative complications such as iatrogenic chondral damage, broken instrumentation, traction related and portal related nerve injuries as well as abdominal compartment by fluid extravasation were monitored [13-16]. Postoperative adverse events like femoral neck fracture, infection, heterotopic ossifications (HO), thromboembolic events and death were documented [17-20]. At the 3-month follow-up the patients were examined for traction or portal related neural damages and evaluated for heterotopic ossifications using conventional radiographs. If present, heterotopic ossifications were graded using the Brooker classification [21].
The statistical analysis was performed using the IBM SPSS Statistics software (IBM Corp. Released 2013. IBM SPSS Statistics, Version 22.0. Armonk, NY: IBM Corp.). The correlation coefficient r was calculated for exploration of the number of applicators used, the size of the spheroids and the size of the cartilage defect on the amount of spheroids lost. The level of significance was set at p < 0.05.
The study was approved by the local institutional review board (EA2/154/14).
Results
We analyzed 26 patients treated between 2012 and 2015 with an autologous chondrocyte implantation (ACI) for full thickness acetabular cartilage defects. The patients consisted of 4 female and 22 male patients. The mean age was 33 (18-49) years. The right hip was affected in 15, the left in 11 cases and the mean acetabular cartilage defect size was 4.9 (2-6) cm2. The average number of delivered spheroids was 107 (42-179) in 1-3 application devices. The average maximum diameter of the spheroids was 690 (396-970) μm.
During application process of the spheroids 19 cases (73%) could be performed with a 100% application rate into the defect area. In 7 cases a maximum of 5 spheroids was lost during application process creating a maximum total loss of spheroids of 4.7% (Table 1). This resulted in a calculated concentration of 21.5 (9-40) spheroids per square cm in the defect area. In only one patient (3.8%) the intended concentration of 10-70 spheroids per square cm was just not achieved (only 9 spheroids per square cm) due to loss of some spheroids.
71% (5 out of 7) of the cases without a 100% application rate happened to be within the first 10 cases of the ACI procedure. Most common mechanism of the loss was flushing of the spheroids above the margins of the defect area due to too much pressure used at the applicator. An outflow of the cells after primary adhesion could not be seen.
No correlation was found between the amount of spheroids lost and the number of applicators (r = -0.04), the size of the spheroids (maximum diameter, r = 0.04) or the size of the cartilage defect (r = 0.01).
There were no intraoperative complications like iatrogenic chondral damages or broken instruments. Abdominal compartment syndromes by fluid extravasation could not be seen. No traction or portal related nerve injuries were seen at the three-month follow-up.
There were no postoperative adverse events like femoral neck fracture, infection, heterotopic ossifications, thromboembolic events and death.
Though not surgery related, there were two patients (7.7%) with failed cell cultivation to unknown reason. The initially vital cells unfortunately showed no proliferation after the first stage of cell cultivation. Both patients decided for a renewed extraction of cartilage-bone-cylinders. The second cell cultivation succeeded so that finally the ACI could be performed.
Discussion
Cartilage defects are the most relevant prognostic outcome factor in the mostly young and active patient population with FAI [4]. The best treatment of acetabular full thickness cartilage defects is still a matter of debate, as there is only very limited data for all of the different cartilage therapies.
The matrix associated chondrocyte implantation consisting of chondrospheres in a NaCl fluid solution offers a new treatment option for cartilage defects without the need for a membrane as scaffold material. It has a potentially easier application in the setting of hip arthroscopy [22] but challenges the surgeon to applicate the cells in a fluid solution on the steep sloping surface of the acetabulum. A "contained defect" situation is important in cases of cartilage therapy and might require labral repair.
The application is of paramount interest, since the ACI is only effective if a sufficient number of cells are really applied directly into the cartilage defect. The procedure is associated with a mandatory second surgery in a relative short interval of 6-8 weeks after initial surgery, a latency in rehabilitation and increased health care costs. Therefore, feasibility and safety of the ACI procedure are a crucial requirement for interpretation of the value of this type of ACI procedure.
We evaluated the feasibility of this procedure by analyzing the amount of spheroids that were successfully placed in the defect area.
We could show that in a high percentage of patients a successful implantation of the spheroids was possible without any loss of cells. In 7 cases a maximum of 5% of the spheroids were lost during application process. Still, 5 out of 7 cases without a 100% application rate were seen within the first 10 applications. This illustrates a reasonable short learning curve for the injectable ACI for surgeons experienced in hip arthroscopy. Special care has to be taken not to use too much pressure on the applicator to prevent flushing of the spheroids above the defect area, because this was the most frequent problem. We could not detect an outflow of cells out of the defect area after primary adhesion. This confirms the claimed high adhesion forces of the spheroids even in an overhang application in the anterolateral acetabulum.
The use of the chondrosphere implantation in the hip joint was described before in studies presenting short-term follow-up of a limited number of patients treated with FAI correction, but neither of the studies really objectified the technical feasibility of the injectable ACI in this difficult area. Fickert, et al. reported a statistically significant improvement of all 6 patients in the functional outcome scores used [23]. Körsmeier and colleagues also claimed that significant improvement in the NAHS and WOMAC scores after 16 months postoperatively [24].
The size of the spheroids varies due to the cultivation process. Still we could not see difficulties in the application due to smaller or lager spheroids. A correlation between the size of the spheroids and the amount of spheroids lost could not be seen.
Hip arthroscopy is a safe procedure but still complications have to be considered especially in a procedure that requires two arthroscopic interventions within a short period. Therefore, we analyzed pre- and postoperative complications and adverse events. In our patient population we did not see traction or portal related neural damages. Körsmeier, et al. reported on no intraoperative complications in 16 patients with ACI for acetabular cartilage defects [24]. They found a transient pudendal nerve neuropraxia in 2 cases and two patients were treated with arthroscopic arthrolysis due to capsular adhesions.
Heterotopic ossification (HO) is a common complication after hip arthroscopy with a rate up to 44% without prophylaxis [25]. Using different prophylaxis protocols the overall rate of HO after hip arthroscopy in a larger population has been reported to be around 5% [26]. Especially the repetitive trauma by the consecutive second arthroscopy within a short time might put the patient at an increased risk for the development of HO. In our patient population we could not detect any signs for HO at the 3-month follow up in plain radiographs. This might be a hint for the efficiency of NSAID prophylaxis even in short time repetitive hip arthroscopy. To our knowledge this is the first paper mentioning the rate of HO in patients with repeated hip arthroscopy.
In this study we could show that the injectable ACI is a technically feasible and safe procedure for the treatment of full-thickness acetabular cartilage defects. Since the hip joint is not comparable to the knee joint barely any long-term data for ACI procedures of the hip is available [27]. Therefore, the literature is still not sufficiently robust to draw firm conclusions regarding best practices for chondral defects in the hip joint. Additional research is needed to expand our knowledge of ACI procedures in the hip joint and to develop guidelines for management of chondral injuries of the hip.
Limitations
Due to the study design we were only able to evaluate the efficiency of the chondrosphere application. Neither clinical data like patient reported outcomes nor the histological outcome of the ACI was evaluated in this study. However, the aim of the investigation was to evaluate the technical feasibility of the application and the complications directly associated to the application surgery.
The fact that we could not detect any signs of HO in our population might also be contributed by the relatively small sample size of 26 cases.
Conclusions
The injectable ACI is a technically feasible and safe procedure in the hip joint and opens a new minimal invasive opportunity in treating large full thickness focal acetabular cartilage defects.
References
- Matsuda DK, Carlisle JC, Arthurs SC, et al. (2011) Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy 27: 252-269.
- Botser IB, Smith TW Jr, Nasser R, et al. (2011) Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy 27: 270-278.
- Büchler L, Neumann M, Schwab JM, et al. (2013) Arthroscopic versus open cam resection in the treatment of femoroacetabular impingement. Arthroscopy 29: 653-660.
- Egerton T, Hinman RS, Amir Takla, et al. (2013) Intraoperative cartilage degeneration predicts outcome 12 months after hip arthroscopy. Clin Orthop Relat Res 471: 593-599.
- Suzuki C, Harada Y, Shigeru Mitsuhashi, et al. (2005) Repair of cartilage defects and torn acetabular labrum in hip joints after conventional osteotomy: evaluation by follow-up arthroscopy. Journal of Orthopaedic Science 10: 127-132.
- Haefeli PC, Steppacher S, Tannast M, et al. (2016) Treatment Of Cartilage Defects In Impingement Surgery Reduces The Risk Of Total Hip Arthroplasty At 10-Year Follow-Up. Switzerland.
- El Bitar YF, Lindner D, Timothy J Jackson, et al. (2014) Joint-preserving Surgical Options for Management of Chondral Injuries of the Hip. J Am Acad Orthop Surg 22: 46-56.
- DiBartola AC, Everhart JS, Magnussen RA, et al. (2016) Correlation between histological outcome and surgical cartilage repair technique in the knee: A meta-analysis. Knee 23: 344-349.
- Richter DL, Schenck RC, Wascher DC, et al. (2016) Knee Articular Cartilage Repair and Restoration Techniques A Review of the Literature. Sports Health 8: 153-160.
- Dienst M, Seil R, Kohn DM (2005) Safe arthroscopic access to the central compartment of the hip. Arthroscopy 2: 1510-1514.
- Fry R, Domb B (2010) Labral Base Refixation in the Hip: Rationale and Technique for an Anatomic Approach to Labral Repair. Arthroscopy 26: S81-S89.
- Jackson TJ, Hanypsiak B, Christine E Stake, et al. (2014) Arthroscopic Labral Base Repair in the Hip: Clinical Results of a Described Technique. Arthroscopy 30: 208-213.
- Fowler J, Owens BD (2010) Abdominal compartment syndrome after hip arthroscopy. Arthroscopy 26: 128-130.
- Horisberger M, Brunner A, Herzog RF (2010) Arthroscopic treatment of femoral acetabular impingement in patients with preoperative generalized degenerative changes. Arthroscopy 26: 623-629.
- Rupp R, Duggan B (2012) Peripheral versus central compartment starting point in hip arthroscopy for femoroacetabular impingement. Orthopedics 35: e148-e153.
- Sampson TG (2001) Complications of hip arthroscopy. Clin Sports Med 20: 831-835.
- Ayeni OR, Bedi A, Lorich DG, et al. (2011) Femoral neck fracture after arthroscopic management of femoroacetabular impingement: a case report. J Bone Joint Surg Am 93: e47.
- Bushnell BD, Dahners LE (2009) Fatal pulmonary embolism in a polytraumatized patient following hip arthroscopy. Orthopedics 32: 56.
- Matsuda DK (2009) Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy 25: 400-404.
- Salvo JP, Troxell CR, Duggan DP (2010) Incidence of Venous Thromboembolic Disease Following Hip Arthroscopy. Orthopedics 33: 664.
- Brooker AF, Bowerman JW, Robinson RA, et al. (1973) Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am 55: 1629-1632.
- Libera J, Luethi U, Alasevic OJ (2006) co. don chondrosphere® (co. don® AG): autologous matrix-induced engineered cartilage transplantation Basic science, clinical repair and reconstruction of articular defects: current status and prospects 1: 591-600.
- Fickert S, Schattenberg T, Niks M, et al. (2014) Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: a case series. Arch Orthop Trauma Surg 134: 971-978.
- Körsmeier K, Claßen T, Kamminga M, et al. (2016) Arthroscopic three-dimensional autologous chondrocyte transplantation using spheroids for the treatment of full-thickness cartilage defects of the hip joint. Knee Surg Sports Traumatol Arthrosc 24: 2032-2037.
- Rath E, Sherman H, Sampson TG, et al. (2013) The incidence of heterotopic ossification in hip arthroscopy. Arthroscopy 29: 427-433.
- Bedi A, Zbeda RM, Bueno VF, et al. (2012) The incidence of heterotopic ossification after hip arthroscopy. Am J Sports Med 40: 854-863.
- Mancini D, Fontana A (2014) Five-year results of arthroscopic techniques for the treatment of acetabular chondral lesions in femoroacetabular impingement. Int Orthop 38: 2057-2064.
Corresponding Author
Joerg H Schroeder, MD, Center for Musculoskeletal Surgery, Campus Virchow-Klinikum, Charité - Universitaetsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany, Tel: +49-30-450552065, Fax: +49-30-450552901.
Copyright
© 2017 Krueger DR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.