Clinicopathological Factors in Predicting Outcomes in Patients with Human Papilloma Virus (HPV) Associated Oropharyngeal Carcinoma (OPC) Treated with Primary Surgery
Abstract
Background: The purpose of this study was to determine the relevance of traditional adverse Clinicopathological factors in predicting outcomes in patients with Human Papilloma Virus (HPV) associated or pharyngeal carcinoma (OPC).
Methods: A retrospective chart review was undertaken of 107 patients with AJCC 7th edition Stage III or IV HPV associated OPC who were treated with curative intent surgery +/- adjuvant therapy from 2006 - 2015.
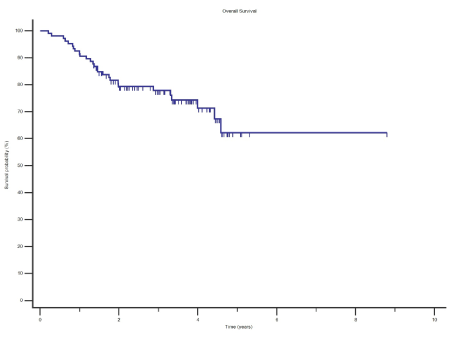
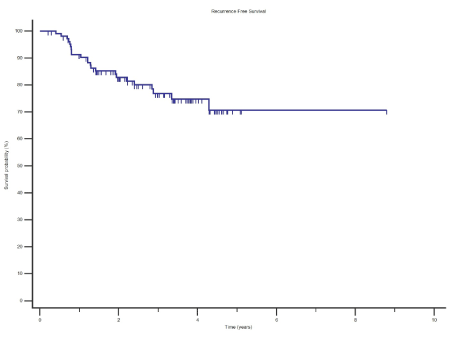
Results: With a median follow up duration of 2.9 years, the 2 year and 5 year OS and RFS estimates was 79.4% and 62.2% and 82.9% and 70.7% respectively. The recurrence rate was 21.5%. Factors which were significant for OS on multivariate analyses was age adjusted Charleston comorbidity index, pT stage and number of involved lymph nodes.
Conclusion: Some conventional adverse risk factors may be less relevant in the setting of HPV associated OPC.
Keywords
Oropharyngeal Cancer, Surgery, HPV, Risk Factors, Pathology
Background
The optimal management for locally advanced orpharyngeal cancer is variable, with multiple treatment options available [1]. Although no high level randomized comparisons exist between surgical and non-surgical approaches, outcomes are equivalent from some retrospective data [2-4]. Concurrent chemoradiation (CRT) has been the more common approach due to concerns regarding morbidity of open surgery [5,6,] however with the evolution of surgical techniques to less morbid transpolar approaches primary surgery with adjuvant therapy (if indicated) is gaining favour [7-9].
Failure post-surgery for head and neck cancer is high in patients with adverse risk factors [10]. Adjuvant radiotherapy has long been an integral component in the management of head and neck cancer, in an effort to improve outcomes. Risk factors typically accepted as indications for adjuvant radiotherapy include T3 or T4 primary, N2 or N3 nodal involvement, extracapsular extension (ECE), positive margins, lymphvascular invasion (LVI), perineural invasion (PNI), and nodal disease in levels IV or V with an oral cavity or oropharyngeal primary [11]. The additional benefit of concurrent chemotherapy to adjuvant radiotherapy has been established from 2 large randomized trials. The definition of high risk and inclusion criteria differed between the trials but pooled data showed an overall survival benefit for patients with extracapsular extension (ECE) and positive margins managed with CRT, which were risk factors common to both trials. Studies addressing prognostic factors and optimal adjuvant management strategies have typically included all subsites in the head and neck, and predate the identification of Human Papilloma Virus (HPV) as a significant prognostic factor in orpharyngeal carcinoma [12,13].
HPV is a well-established risk factor in the development of orpharyngeal carcinoma, with increasing incidence in the Western world [14,15]. HPV associated OPC differs from smoking related tumours in both demographic characteristics as well as treatment outcomes. Patients with HPV associated OPC are generally younger, more likely to have never smoked and display a significantly improved response to treatment and overall survival, irrespective of treatment modality [16-18]. Although there is not yet any difference in treatment approaches based on HPV status, de-intensification treatment strategies are actively being explored in an effort to reduce acute and late treatment related morbidity in this group of patients [19]. These strategies include using reduced radiation dose, less toxic chemotherapy schedules and less invasive surgical approaches.
The changes to the 8th edition AJCC staging system is an acknowledgement of the biological differences in OPC stratified by HPV status [20]. A distinct pathological staging now exists which differs from HPV negative OPC, which further calls into question the significance of traditional adverse pathological factors in directing adjuvant management. We therefore undertook a retrospective study to evaluate the prognostic impact of traditional risk factors on the outcomes of patients with locally advanced HPV associated OPC managed with primary surgery +/- adjuvant therapy.
Methodology
A retrospective review was performed of all patients with HPV positive OPC who were surgically treated with curative intent between January 2006 and December 2015. The initial list of all patients with Stage III-IVB squamous cell carcinoma of the or pharynx who were managed with curative intent was generated from the Alberta Cancer Registry and included 647 patients. Of these 245 patients were managed with curative intent surgery +/- adjuvant therapy. After excluding 26 patients with HPV negative status, 105 with an unknown status and a further 7 patients who did not have full pathological data recorded a final list of 107 patients was generated.
HPV status was determined by p16 immuno histochemistry. Smoking exposure was recorded by number of pack years. Smoking status was categorized as never smoker (less than 1 pack year smoking exposure), former smoker (smoking cessation was more than 1 year from diagnosis), and current smoker. Comorbidities was assessed using the age adjusted Charleston comorbidity index [21]. All patients had CT or PET-CT of the neck and chest to exclude metastatic disease prior to surgical resection of the primary tumor and unilateral or bilateral neck dissections. The specific type of surgical procedure open or transoral and subsites of tumor within the or pharynx was not uniformly recorded. Pathological staging was recorded as per the 7th edition AJCC staging system. Adjuvant radiotherapy was delivered by an IMRT approach to a dose of 6000cGy in 30 daily fractions. Local standard practice was to routinely offer concurrent chemotherapy for positive margins and ECE. The addition of chemotherapy to radiotherapy for other factors was individualized by patient risk profile and physician preference. Platinum-based chemotherapy schedules were utilized, when indicated.
Patient demographic and clinical characteristics, pathological data and treatment outcomes were recorded and summarized. Overall survival (OS) and recurrence free survival (RFS) was calculated for various Clinicopathological variables from date of diagnosis to death or recurrence. Survival estimates were obtained using the Kaplan-Meier analysis and compared using the log-rank test. Multivariate analysis was performed by the Cox proportional hazards regression analysis for Clinicopathological factors found to be significant on univariate analyses. A p value of less than 0.05 was considered significant. Analyses were performed using MedCalc for Windows, version 16.8.4 (MedCalc Software, Ostend, Belgium).
Ethics approval was obtained prior to initiating this study through the Human Research Ethics Board of Alberta – Cancer Committee (ETH #26196).
Results
Patient demographics and treatment characteristics Patient demographics and treatment parameters is summarized in Table 1. One hundred and seven patients were analyzed with a median follow-up duration of 2.9 years. Of the 16.8% who received no adjuvant therapy at all, half were due to patient declining further management, and the remainder was due to either patient demise prior to adjuvant management or being too unfit for further management.
There were 23 recurrences (21.5%) with a median time to recurrence of 2.39 years. Locoregional failure was identified in 10 patients (43.5%), distant failure in 7 (30.4%) and both locoregional and distant failure in 6 (26.1%) patients. Of the 23 patients who recurred, 5 (21.7%) were not offered adjuvant treatment. Adjuvant chemo radiation was received by 10 patients (43.5%) and radiotherapy by 7 patients (30.4%).
Pathological Data
Table 2 shows distribution of pathological data. Staged by AJCC 7th edition, 75.7% of patients were staged as IVA, but when reclassified by AJCC 8 staging almost half were restaged as stage I. Sixty seven percent of patients with nodal involvement had less than 4 nodes involved (pN1 by AJCC 8th edition staging). The presence of involved surgical margins, PNI, LVI and ECE was 14%, 26.2%, 38.3% and 36.6% respectively. Grade 3 was the commonest grade identified in 59.8% of patients.
Patients who were classified as pN2 by 8th edition AJCC staging had higher rates of involved margins (27.6% vs 9.7%), PNI (41.4% vs 18.1%), LVI (62.1% vs 30.6%), ECE (72.4 vs 25%) than those with pN1 stage however the highest rate of PNI (50%) and grade 3 tumor status (66.7%) was in the pN0 group. The prevalence of PNI and LVI increased as T stage increased: 10.3%, 17.5%, 41.7% and 57.1% and 31%, 32.5%, 50% and 50% respectively for T stage T1 to T4 [Table 3].
Survival Outcomes and Prognostic Variables
The 2 year and 5 year OS and RFS estimates for the whole study cohort was 79.4% and 62.2% and 82.9% and 70.7% respectively (Figures 1 and Figure 2).
Table 4 depicts the Univariate analysis performed on a variety of demographic, clinical and pathological factors to assess their impact on overall and recurrence free survival. The age adjusted Charleston co-morbidity index, 8th edition AJCC stage groupings, pT stage, number of involved lymph nodes characterized by the 8th edition AJCC pN stage, PNI, ECE and smoking exposure of ≥ 20 pack years was found to be prognostic for overall survival. Factors found to be prognostic for recurrence free survival was 8th edition AJCC stage groupings, pT stage, number of involved lymph nodes characterized by the 8th edition AJCC pN stage, the presence of involved margins, LVI, ECE and smoking exposure of ≥ 20 pack years.
Significant factors included in the multivariate analyses for overall survival was age adjusted Charleston comorbidity index, pT stage and pN stage by the 8th edition AJCC staging, PNI, LVI and smoking exposure of more than 20 pack years with only age adjusted Charleston co-morbidity index, pT stage and smoking exposure of ≥ 20 pack years remaining significant. For multivariate analyses for factors affecting recurrence free survival the AJCC 8th edition pT and pN stage, involved margins, LVI, ECE and and smoking exposure of more than 20 pack years were included with pT stage, involved margin status and smoking exposure of ≥ 20 pack years remaining significant [Table 5]. The AJCC stage groupings and 7th edition pT stage were considered duplicate factors and not included in the multivariate analysis.
Discussion
The optimal management for HPV associated oropharyngeal cancer is under investigation. HPV has emerged as a significant prognostic factor in oropharyngeal cancer, with excellent response to treatment and survival outcomes but this was not adequately reflected by the 7th edition TNM staging system [22-25]. In an effort to address this, the newest 8th edition staging manual has assigned a distinct staging system for HPV associated OPC based on prognostic data pooled from multi-institutional reviews. For patients managed with primary surgery neither lymph node size nor the presence of contralateral nodal involvement (previously N3 and N2c respectively) was predictive of survival. The number of pathologically involved lymph nodes was most prognostic with more than four involved nodes conferring the worst prognosis. Due to difficulty correlating nodal prognostic factors from surgical and CRT series, a separate staging was proposed based on treatment modality [20].
Adjuvant (chemo) radiotherapy post-surgery is standardly recommended in locally advanced head and neck cancer in an effort to reduce the high rates of failure [1,12,13]. However, the improved outcomes with HPV associated OPC has called into question optimal management of these patients. There is significant interest in de-escalation strategies aimed at reducing toxicity while maintaining Oncological outcomes [19]. For HPV associated OPC managed with surgery, this requires a review of the relevance of pathological prognostic factors which direct adjuvant management.
The 5 year OS of 62.2% in this study is lower the 76.4% previously reported in a similarly managed cohort of patients from this institution [26]. That study, which reported outcomes for patients managed in an earlier time period between 1998 and 2009, had preserved tissue reanalyzed for HPV status. In this study, retrospective pathological analyses for HPV status was not conducted, and almost half the surgically managed patients were excluded due to unknown HPV status. Patients who did not receive adjuvant therapy had been excluded from the previous analyses, whereas the 16.8% of patients who did not receive adjuvant therapy were included in this review. This difference in study population may account for some of the survival difference. Outcomes may also have been affected if a large proportion of the patients excluded were HPV positive with lower risk features. Indeed, it appeared that in this study there was a high prevalence of T3/T4 stage (35.5%), involved margins (14%), PNI (26.2%), LVI (38.3%) and recurrence rates (21.5%). Haughey et al reported a prevalence of T3/T4 stage, involved margins, PNI, LVI and recurrence rates as 25%, 8%, 12% and 15% and 7% respectively [27]. No additional pathological data was reported from the previous local publication to compare risk profiles between both cohorts to further explore potential reasons for the notable differences in outcome.
Pathological T stage was prognostic for OS and RFS in this study for both the 7th and 8th edition staging systems, in keeping with published literature [27,28]. Since the only change to the T category between the different versions of the staging system was the combination of T4a and T4b into as single stage, and this was relevant for only 2 patients, the similarity in outcomes for T category in both staging versions was not unexpected. The 7th edition AJCC nodal classification had no prognostic implication, but the number of pathologically involved lymph nodes was prognostic for both OS and RFS on Univariate analysis, reflecting the changes made to the 8th edition. Similarly, the 8th edition (but not the 7th edition) stage groupings were prognostic for both OS and RFS on Univariate analysis, validating the new changes to the staging system.
ECE is considered a high risk factor in head and neck cancer and its presence is usually an indication for concurrent systemic chemotherapy [12,13]. However, in HPV associated OPC, ECE does not seem to have the same prognostic implication [29-30]. While ECE was prognostic for both OS and RFS on Univariate analysis, this did not retain significance on multivariate analyses. The presence of PNI and LVI has also been reported to portend poorer outcomes in the setting of HPV associated OPC28,31. On Univariate analysis, PNI was found to be prognostic for OS and LVI for RFS, but neither was significant on multivariate analysis. Involved margin status was prognostic for RFS and remained significant when controlling for other factors. There was a high correlation between the pN2 stage and rates of involved margins (27.6% vs 9.7%), PNI (41.4% vs 18.1%), LVI (62.1% vs 30.6%), ECE (72.4 vs 25%) than those with pN1 stage. This may explain why factors that was prognostic for OS (pN stage, AJCC 8th edition stage, PNI, ECE) and RFS (pN stage, AJCC 8th edition stage, LVI, ECE) on Univariate analysis lost significance on multivariate analyses.
Comorbidity in patients with head and neck cancer are reported to be associated with adverse outcomes due to factors that influence clinical decision-making regarding treatment as well as competing causes for death [32,33]. In this study, higher age-adjusted Charleston comorbidity index was prognostic for OS but not RFS. The prognostic implication of smoking on HPV-associated OPC is also debatable with some studies, in both surgical and non-surgical series, reporting the improved outcomes associated with HPV may be compromised by smoking exposure [28-34]. Ang et al found a cut off of 10 pack-years to be prognostic in a non-surgical group [23]. In this study, smoking exposure of more than 20 pack-years was significant for both OS and RFS on Univariate analysis and maintained on multivariate analyses, with no prognostic impact noted for lower smoking exposure.
This study is limited by its retrospective nature and inherent biases related to patient selection for surgical management. Specifics regarding type of surgery (open vs transoral) and subsite of disease within the oropharynx are lacking. This is a small patient cohort with a limited follow up time, and all data is derived from a single institution.
Conclusion
The improved outcomes in HPV associated OPC has prompted a review of prognostic risk factors which may impact new management strategies. Some risk factors (LVI, PNI, and ECE) had poorer prognosis on univariate analyses, but when pooled together on multivariate analyses lost significance. Traditional risk factors which confer poorer prognosis in head and neck cancer may be less relevant in the setting of HPV. This suggests a case for modification of indications for adjuvant therapy, but caution must be exercised when de-intensifying treatment as outcomes, while improved in HPV associated OPC, have been attained with conventional management. Prospective randomized data is required prior to any changes in clinical practice.
Conflict of Interest
There are no conflicts of interest identified.
References
- NCCN practice guidelines for head and neck version 2.2017.
- Boscolo-Rizzo P, Gava A, Baggio V, et al. (2011) Matched survival analysis in patients with loco regionally advanced respectable orpharyngeal carcinoma: Platinum-based induction and concurrent chemo radiotherapy versus primary surgical resection. International Journal of Radiation Oncology Biology Physics 80:154-160.
- Kano S, Homma A, Hayashi R, et al. (2013) Matched-pair analysis in patients with advanced oropharyngeal cancer: Surgery versus concurrent chemo radiotherapy. Oncology 84: 290-298.
- Park G, Lee S, Kim Sy, et al. (2013) Can concurrent chemoradiotherapy replace surgery and postoperative radiation for locally advanced stage III/IV tonsillar squamous cell carcinoma? Anticancer Research 33: 1237-1243.
- Mendenhall WM, Stringer SP, Amdur RJ, et al. (2000) Is radiation therapy a preferred alternative to surgery for squamous cell carcinoma of the base of tongue? JCO 18: 35-42.
- Parsons JT, Mendenhall WM, Stringer SP, et al. (2002) Squamous cell carcinoma of the oropharynx Cancer 94: 2967-2980.
- Rich JT, Milov S, Lewis JS, et al. (2009) Transoral laser microsurgery (TLM) ± adjuvant therapy for advanced stage oropharyngeal cancer. Laryngoscope 119: 1709-1719.
- Haughey BH, Hinni ML, Salassa JR, et al. (2011) Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: A united states multicenter study. Head Neck 33:1683-1694.
- Cohen MA, Weinstein GS, O'Malley BW, et al. (2011) Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck. 33: 573-580.
- Cooper JS, Pajak TF, Forastiere A, et al. (1998) Precisely defining high-risk operable head and neck tumours based on rtog #85-03 and #88-24: Targets for postoperative radio chemotherapy? Head Neck. 20: 588-594.
- Mendenhall WM, Hinerman RW, Amdur RJ, et al. (2006) Postoperative radiotherapy for squamous cell carcinoma of the head and neck. Clinical Medicine & Research 4: 200-208.
- Cooper JS, Pajak TF, Forastiere AA, et al. (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 350: 1937-1944.
- Bernier J, Domenge C, Ozsahin M, et al. (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350: 1945-1952.
- Kreimer AR, Clifford GM, Boyle P, et al. (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev 14: 467-475.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. (2013) Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. JCO. 31: 4550-4559.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the united states. JCO. 29: 4294-4301.
- Fakhry C, Westra W,H, Li S, et al. (2008) Improved survival of patients with human Papillomavirus–Positive head and neck squamous cell carcinoma in a prospective clinical trial. JNCI: Journal of the National Cancer Institute 100: 261-269.
- Dayyani F, Etzel CJ, Liu M, et al. (2010) Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2: 15.
- Masterson L, Moualed D, Liu ZW, et al. (2014) De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of current clinical trials. Eur J Cancer 50: 2636-2648.
- Amin MB. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
- Charlson ME, Pompei P, Ales KL, et al. (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases 40: 373-383.
- Ragin CCR, Taioli E (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. International Journal of Cancer. 121 :1813-1820.
- Ang KK, Harris J, Wheeler R, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24-35.
- Edge SB (2010) AJCC cancer staging manual.
- Fischer CA, Zlobec I, Green E, et al. (2010) Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? International Journal of Cancer 126: 1256-1262.
- Seikaly H, Biron VL, Zhang H, et al. (2016) Role of primary surgery in the treatment of advanced oropharyngeal cancer. Head Neck 38 : 571-579.
- Haughey BH, Sinha P (2012) Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope 122: 13-33.
- Haughey BH, Sinha P, Kallogjeri D, et al. 2016) Pathology-based staging for HPV-positive squamous carcinoma of the or pharynx. Oral Oncol 62: 11-19.
- Maxwell JH, Ferris RL, Gooding W, et al. (2013) Extracapsular spread in head and neck carcinoma: Impact of site and human papillomavirus status. Cancer 119: 3302-3308.
- Sinha P, Lewis JS, Piccirillo JF, et al. (2012) Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer 118: 3519-3530.
- Albergotti WG, Schwarzbach HL, Abberbock S, et al.(2017) Defining the prevalence and prognostic value of perineural invasion and angiolymphatic invasion in human papillomavirus–positive oropharyngeal carcinoma. JAMA Otolaryngology–Head & Neck Surgery pages 143: 1236-1243.
- Rose BS, Jeong J, Nath SK,et al. (2011) Population-based study of competing mortality in head and neck cancer. JCO 29:3503-3509.
- Datema FR, Ferrier MB, van der Schroeff, et al. (2010) Impact of comorbidity on short-term mortality and overall survival of head and neck cancer patients. Head Neck 32: 728-736.
- Gillison ML, Zhang Q, Jordan R, et al. (2012) Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. JCO 30: 2102-2111.
Corresponding Author
Dr. Brock Debenham, MD, FRCPC Department of Oncology, Cross Cancer Institute, University of Alberta, 11560 University Ave NW, Edmonton, Alberta, Canada, Tel: 780-432-8754.
Copyright
© 2022 Vawda N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.