Acute Kidney Injury After Cardiac Surgery - Risk Factors for Renal Replacement Therapy
Abstract
Introduction
Acute kidney injury (AKI) occurs in up to 50% of patients after open cardiac surgery, and it is associated with significantly increased morbidity and mortality. Up to 3% of patients need renal replacement therapy (RRT) due to severe AKI. The need for RRT further impairs outcome of those patients. The aim of this study was to investigate risks for RRT after open cardiac surgery in Finland.
Methods
Our retrospective study included 80 cardiac surgery patients with severe postoperative AKI treated with RRT from Helsinki University Hospital between 2008 and 2010.
Findings
80 patients received RRT during their postoperative ICU admission. 45 patients (56%) had been scheduled for emergency surgery. 41 patients (51%) had at least one major adverse event (cardiac, neurological, pulmonary, infection), which was associated with increased mortality (p = 0.013).
Discussion
The presence of major adverse events is a significant risk for postoperative RRT in this patient cohort. Also, emergency surgery and blood transfusions are associated with impaired outcome.
Keywords
Acute kidney injury (AKI), Renal replacement therapy (RRT), Risk factor, Cardiac surgery, Major adverse event (MAE)
Introduction
Acute kidney injury (AKI) is a frequent complication after open heart surgery. According to previous data, AKI develops in up to 50% of patients after open cardiac surgery [1]. In the majority of these cases, kidney injury is mild or moderate, and it does not require large scale of interventions. However, 1-3% of patients develops severe renal failure and requires renal replacement therapy (RRT) [2]. AKI after cardiac surgery is associated with increased mortality and prolonged hospital stay. Mortality rate increases with the severity of AKI [1-3]. Postoperative initiation of RRT prolongs intensive care unit (ICU) stay, worsens patients' outcomes, and raises hospital cost [3].
Previous studies suggest that the several risk factors are associated with development of AKI in cardiac surgery patients: decreased glomerular filtration rate, decreased left ventricular ejection fraction, and red blood cell transfusions. Also emergency surgery and the patients' chronic illnesses such as diabetes mellitus and chronic obstructive pulmonary disease are reported to impair the patients' postoperative outcome [2-4]. Recent reported model of predict AKI development requiring RRT after cardiac surgery includes additionally increased haemo-globin concentration, and proteinuria [5].
The prevention of postoperative AKI is crucial to reduce mortality, shorten hospital course, decrease complica-tions, and lower treatment costs.
We aimed to determine the risks for post-operative RRT, and factors associated with mortality in a cohort of Finnish cardiac surgery patients.
Methods
Patient population, data source and study period
This study includes 80 patients scheduled for on-pump cardiac surgery in Helsinki University Hospital between 2008 and 2010. All study patients suffered from severe postoperative AKI required RRT (KDIGO stage 3) within 1 week after surgery. All the patients received RRT from this time period were included. We collected data from the automated database of the cardiac surgery ICU. (Caresuite 8.2, PiCIS Inc., San Francisco, USA). Cardiopulmonary bypass (CPB) was performed using a nonpulsatile pump and a membrane oxygenator. Prime solution consisted of 1500 ml of Ringer's acetate and 5000 IU of Heparin. Additionally to Ringer's solution HES 130/0.4 and Albumin (4 and 10%) solutions were used for fluid therapy perioperatively.
In 2012, 1111 cardiac surgery patients were operated in Helsinki University hospital and they could be considered as a reference group.
Study type and parameters examined
This study was a quantitative retrospective evaluation of risk factors of AKI related to open heart surgery. Several characteristics of the patients were recorded: age, gender, comorbidities, preoperative haemoglobin, postoperative major adverse events (MAE): multi organ failure, postoperative infection, stroke, acute respiratory distress syndrome, severe bleeding, and resternotomia; the amount of 4% albumin solution and synthetic colloids administered prior to RRT, fluid balance, emergency surgery (when patient should be operated immediately because of haemodynamical instability), amount of transfused red blood cells, serum creatinine, ischemia time, time on cardiopulmonary bypass (CPB), postoperative creatine kinase, muscle-brain fraction (CK-Mb), length of stay in ICU and in-hospital mortality.
Statistical analysis
Data were analysed by SPSS version 19 (SPSS, Chicago, Ill., USA). Normally distributed data (Kolmogorov-Smirnov test) are presented as mean values ± standard deviation or percentages. Non-normally distributed data are reported as medians and 25 and 75 percentiles (*). For paired comparisons, T-test or Wilcoxon test was used. Spearman correlation test was used for finding out potential correlations. Kruskall-Wallis or Mann-Whitney test was used for comparison of distributions across groups. A p value < 0.05 was considered as statistically significant.
Results
Totally, 2984 cardiac surgical patients were treated in the cardiac surgery ICU between 2008 and 2010. 80 (2.7%) patients developed severe AKI (KDIGO 3) with need for RRT. 3/80 patients was on RRT preoperatively. In 7 patients (8.8%), RRT was started as intermittent haemodialysis, rest of patients needed continuous RRT. The mean age of study patients was 64.5 ± 14 years and it didn't differ between men and women, p = 0.86. Table 1 shows the demographic data of the study patients. Types of the surgery are presented in table 2. Preoperative mean left ventricle ejection fraction was 36.8 ± 14.0%.
In hospital mortality in study patients was 33.8% (27 patients). 18 patients (22.5%) were discharged home directly from Helsinki University Hospital, and 35 patients (43.8%) were transferred to the secondary or tertiary hospital for further treatment. One year mortality was 47.5% (38 patients).
56% of the patients had scheduled for emergency surgery whereas amount of non-elective surgery in whole cardiac surgical population in Helsinki University hospital was 34.4% in 2012. Among these patients, mortality rate was 35.6% compared with 31.4% in patients scheduled for elective surgery, p = 0.7. Mortality in females was 24% compared to this in males (38.2%), p = 0.217.
Postoperative MAE:s occurred in 41/80 (51.3%). Mortality was 51.2% among patients with MAE:s compared to 15.4% in patients without MAE:s, p = 0.001. 19 patients (23.8%) suffered from an infection. Logistic regression analysis showed that postoperative MAE:s were associated with mortality (p = 0.013).
Data about red blood cells transfusions are presented in table 3. The total median amount of red blood cells transfused postoperatively within one week in survived patients was 995 (550-1807) ml compared to 1309 (797-2366) ml in non-survivors, p = 0.16.
Within patients who didn't receive albumin solutions, mortality was 23.8%, whereas it was 37.3% in patients who did receive it (p = 0.265). The perioperative median net fluid balance was 7472 (5034-9433) ml (presented per person's body weight kilogram 87.1 (58.7-117.3) ml), and there were no differences between survivors and non-survivors.
The mean aortic cross clamp time was 113.7 ± 65.5 min and CPB time 185 ± 77 min. The median CK-Mb on the first postoperative day was 66.5 (33-128.5*).
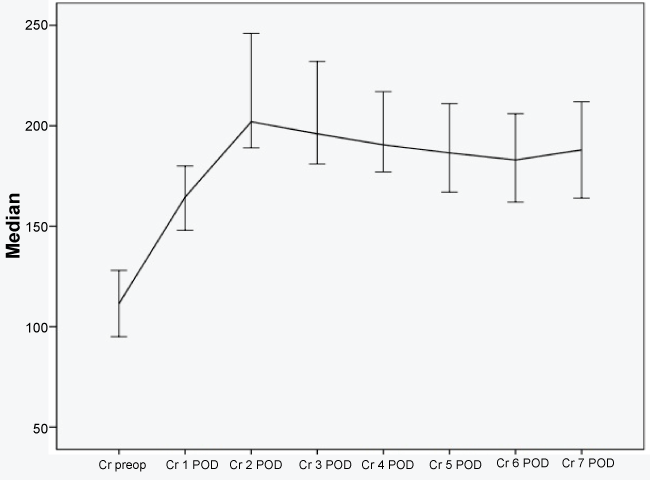
All pre-operative laboratory values of the study patients were within normal ranges except for serum creatinine (SCr). The median SCr was 108 (80.5-160.5*) μmol/l. SCr preoperatively was below 80 μmol/l in 20 patients (25%), between 81 and 150 μmol/l in 37 patients (46.3%), between 151 and 350 μmol/l in 19 patients (23.7%), and higher than 350 μmol/l in 4 patients (5%). SCr increased to 162.5 (123-213*) μmol/l on the 1st post-operative day and to 200.5 (146-284.5*) μmol/l on the 2nd day after surgery. From the 3rd to 5th day the concentration of SCr decreased significantly (p < 0.05), but did not change further up till the 7th post-operative day (Figure 1).
The preoperative SCr was not associated with mortality. The median preoperative SCr of non-survivors was 115 (93-166) μmol/l, compared with 106 (80-157.5) μmol/l in survivors, p = 0.83.
The mean preoperative haemoglobin value was similar both in survived (120.5 ± 21.2 g/l) and non-survived patients (119.7 ± 20.0 g/l), p = 0.872.
Discussion
In this study we showed that non-elective surgery was an important risk factor for RRT. More than half (56%) of the study patients underwent urgent or emergency surgery. This fact supports previous study demonstrated that critical preoperative state is a risk factor for postoperative development of severe AKI [4,5]. Among 1111 patients in the reference group, only 34.4% were scheduled for non-elective surgery. Therefore, compared to the whole cardiac surgical population, those patients who underwent non-elective surgery developed AKI more often than other patients. However, in hospital mortality in patients scheduled for urgent cardiac surgery was similar with those who were treated electively (35.6% vs. 31.4%, respectively).
Our study showed high in-hospital mortality in patients with RRT (33.8%), which is in agree with previous studies [6]. However, further increase in mortality after one year was only moderate (11 patients died). After cardiac surgery, pathophysiology of AKI has several causes. CPB and ischemia during aortic cross-clamping play important role in its development. Our study demonstrated that patients with RRT survived in early period after cardiac surgery may have rather benign prognosis in comparison to patients with severe sepsis (with higher 1-year mortality) [7].
Gender was reported as a possibly risk factor for AKI [2]. In our study, 31% of patients were females. Approximately one third of patients undergoing cardiac surgery are women [1,3,8]. Surprisingly, in our study mortality rate among male patients was more than 38%, whereas only 24% in females (not statistically significant).
Old age is a known risk factor for AKI [9]. In our study age seems to be a risk factor for AKI development, since our patients with AKI were relatively old (mean value 64.5 years). This finding is supported by study of Ristikankare, et al. [10], where 56% of cardiac surgery patients over 70 years developed AKI.
MAE:s and postoperative red blood cell transfusions had a considerable effect on mortality in this study. 50% of the patients needed RRT had at least one MAE after surgery. Especially, amount of postoperative infections in our study cohort was considerably high, 23.8%. Our study demonstrated that the presence of MAE is a risk factor for postoperative severe AKI (KDIGO 3) development. Our study showed that the amount of postoperatively red blood cells transfused is associated with increased postoperative mortality. On the first postoperative day, more than 70% of patients were transfused, and on the second and third postoperative day, more than 50% (Table 3). Only 49% of patients scheduled for cardiac surgery in 2012 in Helsinki University hospital, have been transfused. In previous studies [3,11]. perioperative transfusions are considered as a risk factor for AKI, and our study confirms this finding.
The amount of colloid solution albumin administered was comparable between survived and non-survived patients. However, we observed a trend to higher mortality in patients received albumin (37.3%) compared to those who did not (23.8%), p = 0.265. The lack of statistical significance could be explained by relatively small amount of patients studied. Another possible explanation is the fact that the use of albumin is recommended primarily for critically ill and sick patients.
Increased pre-operative creatinine has been mentioned as a risk factor for AKI [2]. In our study increased preoperative SCr didn't correlate with mortality. The changes of SCr in this study were similar with those generally after cardiac surgery.
According to study from 2009 [3], a low haemoglobin level is a risk factor for RRT. In our study, the mean preoperative haemoglobin was near normal range, and it was similar in survivors and non-survivors. Consequently, in this study preoperative haemoglobin did not seem to associate with the outcome of the patients.
We conclude that the risk factor for severe AKI requiring RRT in patients undergoing cardiac surgery is MAE. Also emergency surgery and blood transfusions were associated with increased mortality. The results of larger prospective study may provide additional information for postoperative severe AKI prevention.
Strengths and limitation
The strength of this study is that we included all cardiac surgery patients treated in the cardiac ICU receiving RRT, whereas majority of studies investigated patients with defined kind of surgery [1,12].
This study has several limitations. First, our study cohort consists only of patients with severe AKI (KDIGO stage 3) treated with RRT, and therefore no comparison to patients without RRT was possible. Instead, we compared our results to previous studies. Secondly, the size of our study was relatively small, only 80 patients.
References
- Machado MN, Miranda RC, Takakura IT, et al. (2009) Acute kidney injury after on-pump coronary artery bypass graft surgery. Arq Bras Cardiol 93: 247-252.
- Rosner MH, Okusa MD (2006) Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1: 19-32.
- Perez-Valdivieso JR, Monedero P, Vives M, et al. (2009) Cardiac-surgery associated acute kidney injury requiring renal replacement therapy. A Spanish retrospective case-cohort study. BMC Nephrol 10: 27.
- Muralidhar K, Bhagyashri K, Guptha R, et al. (2013) Determinants of renal replacement therapy after adult cardiac surgery. Asian Cardiovasc Thorac Ann 21: 533-538.
- Pannu N, Graham M, Klarenbach S, et al. (2016) A new model to predict acute kidney injury requiring renal replacement therapy after cardiac surgery. CMAJ 188: 1076-1083.
- Schneider AG, Eastwood GM, Seevanayagam S, et al. (2012) A risk, injury, failure, loss, and end-stage renal failure score-based trigger for renal replacement therapy and survival after cardiac surgery. J Crit Care 27: 488-495.
- Ng KP, Chanouzas D, Fallouh B, et al. (2012) Short and long-term outcome of patients with severe acute kidney injury requiring renel replacement therapy. QJM 105: 33-39.
- Mitter N, Shah A, Yuh D, et al. (2010) Renal injury is associated with operative mortality after cardiac surgery for women and men. J Thorac Cardiovasc Surg 140: 1367-1373.
- Nisula S, Kaukonen K-M, Vaara ST, et al. (2013) Incidence, risk factors and 90-day mortality of patients with acute kidney injury in finnish intensive care units: the FINNAKI study. Intensive Care Med 39: 420-428.
- Ristikankare A, Poyhia R, Kuitunen A, et al. (2010) Serum cystatin C in elderly cardiac surgery patients. Ann Thorac Surg 89: 689-695.
- Coleman MD, Shaefi S, Sladen RN, et al. (2011) Preventing acute kidney injury after cardiac surgery. Curr Opin Anaesthesiol 24: 70-76.
- Leacche M, Rawn JD, Mihaljevic T, et al. (2004) Outcomes in patients with normal serum creatinine and with artificial renal support for acute renal failure developing after coronary artery bypass grafting. Am J Cardiol 93: 353-356.
Corresponding Author
AA Schramko, Department of Anaesthesiology and Intensive Care Medicine, Helsinki University Hospital, Helsinki University, Helsinki, PO Box 340, FI-00029 HUS, Finland, Tel: +358-947-176-476, Fax: +358-947-176-477.
Copyright
© 2017 Lahdenperä N-1, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.