Laparoscopic-Endoscopic Surgery for a Non-Ampullary Neuroendocrine Tumor after Incomplete Endoscopical Resection
Abstract
Background
Much controversy exists for the treatment of small low-grade non-ampullary duodenal NETs and endoscopically incomplete resected non-ampullary duodenal NETs. In recent years, the development of minimally invasive surgery has spawned a less aggressive treatment option for these patients. We present a case of a 21-year-old patient that was incidentally diagnosed with duodenal NET and treated with laparoscopic-endoscopic salvage surgery after incomplete endoscopic NET resection.
Methods
A 21-year-old man was admitted to our hospital for further evaluation and treatment after an incomplete endoscopic resection of a NET in the third portion of the duodenum. The histological examination revealed a moderately differentiated NET with 20 mitoses in 10 high power fields and a Ki-67 index of 3-20%. The tumor extended to the resection margin. The patient was scheduled for a laparoscopic endoscopic full-thickness excision of the tumor remnant. The histological analysis of the surgical specimen revealed fibrotic tissue after previous endoscopic mucosal resection with no residual tumor cells. The patient was discharged on the fifth postoperative day.
Conclusion
We showed that in experienced hands the trauma of open surgery can be reduced with a minimally invasive approach without exposing the patients to higher morbidity and excellent long term functional results. Therefore, we believe that in the future laparoscopic-endoscopic cooperative surgery will firmly be established as the treatment of choice for small low-grade and incompletely resected NETs of the non-ampullary duodenum.
Keywords
Non-ampullary duodenal NET, Laparoscopy, Endoscopy
Introduction
Although the incidence of duodenal NETs has been slowly rising due to better imaging modalities and wider use of endoscopy [1-6], the experience in dealing with this tumors is relatively small. The duodenal NETs are classified as ampullary or non-ampullary because of their different biological behavior [1]. The ampullary duodenal NETs are generally more aggressive, so the choice for the therapy is relatively straight forward. In most cases, a cephalic pancreaticoduodenectomy is advocated [1,3]. The non-ampullary duodenal NETs however, can behave relatively indolent. The choice for a therapeutic approach in non-ampullary NETs depends on the size of the tumor.
The latest Neuroendocrine Tumor Society (ENETS) guidelines proposed that a surgical resection should be recommended for non-ampullary duodenal NETs > 20 mm, whereas non-ampullary duodenal NETs < 10 mm without lymph node metastases and confined to submucosal layer should be resected endoscopically [1]. Much more controversy exists for non-ampullary tumors smaller than 20 mm. Additionally, the choice of salvage treatment after incomplete endoscopic resection of small non-ampullary NETs is also a matter of debate.
Because of the small incidences of duodenal NETs, it is relatively difficult to obtain enough experience for endoscopically borderline resectable or endoscopically unresectable duodenal NETs. Given the relative indolent behavior of these tumors, it seems that open surgery in these cases presents overtreatment for these patients and exposes them to a highly morbid surgery. In recent years the development of minimally invasive surgery has spawned a third and less aggressive treatment option for patients with small non-ampullary duodenal NETs. We present a case of a 21-year-old patient that was incidentally diagnosed with duodenal NET and treated with laparoscopic-endoscopic salvage surgery after incomplete endoscopic NET resection.
Case Report
A 21-year-old man was admitted to our hospital for further evaluation and treatment after an incomplete endoscopic resection of a duodenal NET. His previous medical history was uneventful apart from dyspeptic pain in the epigastrium and reflux, which he had been suffering for six months before hospitalization. He was admitted to another hospital for endoscopic evaluation of the dyspeptic problems. An Oesophago-gastro-duodenoscopy was performed disclosing multiple superficial erosions in the esophagus and a small hiatal hernia. The endoscopy was carried out in the second portion of the duodenum, where a 7 mm flat and broad-based polyp on the lateral wall was found. A polypectomy was performed with the endoscopic mucosal resection and the specimen was sent for histology. The histological results confirmed that the resected polyp was a duodenal NET with moderately differentiated cells with 2 to 20 mitoses on 10 fields of high magnification and a Ki-67 index of 3% to 20%. The radial resection borders were free of tumor cells, but the tumor extended to the base of the specimen suggesting a R1 resection. The patient was sent to octreo-scan and a SPECT CT with both negative results, excluding possible multifocal disease or distant metastases. The patient was then transferred to our hospital for further treatment. The site of the scar was marked with carbon dye before the operation. His medical records had been re-evaluated and we decided to perform a laparoscopic-endoscopic resection of the excisional scar. The procedure was discussed with the patient and he agreed to the operation. During the operation, the identification of the scar was facilitated with the endoscopic transillumination. The resected specimen was sent for histological analysis, the defect was closed with a running suture and a drain was placed behind the duodenum. After the procedure, the patient was admitted for observation to our intensive care unit for two days. On the first postoperative day, the nasogastric tube was removed and the patient began with slow sips of fluid. On the second postoperative day, the enteral feeding was increased to 100 ml of fluids per day and the patient was transferred on the ward. On day two the patient started to pass stool, we began to slowly increase the enteral feeding to day five when the patient was allowed solid food. The abdominal drain had been removed and oral analgesics started. After an uneventful recovery the patient was discharged on day six. The histological analysis of the surgical specimen revealed fibrotic tissue and intramural bleeding after polypectomy with no sign of residual NET on the last follow-up 6 months after the operation (Figure 1). The patient was well with no functional disturbances and no sign of disease recurrence.
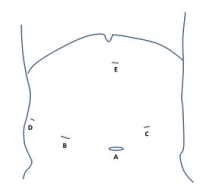
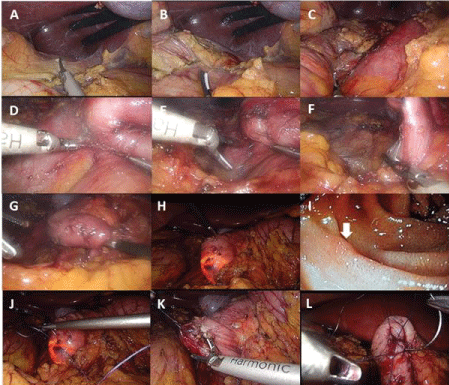
The patient was placed into the prone position with his legs abducted. After the preparation of the surgical field and draping, a small incision was placed supraumbilically and pneumoperitoneum was established with a Veress needle. For pneumoperitoneum CO2 insufflation was used. Optical and working trocars were placed as described elsewhere (Figure 2). The surgeon was positioned between the legs with the first assistant on the patient's right and the second assistant on the patient's left leg. After the exploration of the abdominal cavity, the hepatocolic ligament was incised and the hepatic flexure of the colon was mobilized caudally until the prerenal fascia was visible. The preparation continued towards left. When the second portion of the duodenum was well visible, we began with the kocherisation of the duodenum. The kocherisation was carried out from right to left, with the ultrasonic scalpel held in the surgeon's left hand while the right hand retracted the duodenum with a non-traumatic forceps toward the patient's left side. The second assistant continuously exerted traction on the transverse colon toward the left, the first assistant retracted the hepatic flexure caudally. The kocherisation was continued until the second and the third portion of the duodenum were completely mobilized from the inferior vena cava. As the final step, the third portion of the duodenum was mobilized from the transverse mesocolon. After the duodenum was freed from the retroperitoneum, an intraoperative duodenoscopy was performed. Endoscopic air insufflation was used for visualization of the duodenal lumen. The tumor site was clearly visible with the transillumination of the spotted tumor site with the endoscope. The site was marked with a 2-0 holding suture under endoscopic supervision. The site was completely excised with an ultrasonic endoscalpel and placed into an endobasket. The defect was closed up with two 3-0 resorbable running sutures. After the suturing, the duodenum was tested for leakage with gentle endoscopic insufflation. A 15 French drain was placed behind the duodenum and the specimen extracted from the supraumbilical incision. The duration of the operation was 260 minutes (Figure 3).
Discussion
In recent years, a slow increase in incidence of duodenal NETs has been noted [1-5]. This rise has been attributed to a more widespread use of endoscopy, therefore clinicians are more often faced with a difficult decision on how to treat these relatively indolent tumors. The treatment for small and low-malignant NETs and incomplete excised NETs of the duodenum, however, is still highly debatable. For ampullary NETs, the latest ENETS guidelines propose a surgical resection [1,3]. For non-ampullary NETs the decision is much more difficult. Neuroendocrine tumors larger than 20 mm should be resected because of the high LN metastases probability. Neuroendocrine tumors smaller than 10 mm can be treated with endoscopy, but the case is not that clear on NETs ranging from 10 mm to 20 mm and those not resectable with endoscopy. In cases of unsuccessful treatment, the only option has traditionally been open surgery, which potentially exposes patients with an indolent lesion to harmful complications and long-term functional disabilities [2,7-10]. In this paper, we present a case of a 22-years-old man with an incomplete NET excision of the third portion of the non-ampullary duodenum.
Small NETs of the non-ampullary region of the duodenum have a small probability of lymph node metastases [1,3,11]. Given their accessibility with endoscopy, they should ideally be treated with endoscopic mucosal or endoscopic submucosal resection. But in contrast to the stomach, where the organ walls are thick and the working place is large, the duodenum poses specific difficulties. Because of the thin duodenal wall, endoscopic resections have a high risk of complications occurring in 30% with early and late perforation described as the most dangerous ones [2,7-10]. Once perforation has occurred, it can rarely be controlled endoscopically and the patient has to be operated and subjected further to intensive care because of the severe peritonitis and sepsis. Another problem is the narrow working space in the duodenum that makes the endoscopic maneuvering and the resection difficult [2,7-10]. An even more difficult problem for endoscopic treatment are incomplete tumor resections in the duodenal region. After an incomplete resection, only a submucosal resection can obtain adequate microscopic margins. However, salvage resections are extremely difficult in duodenum. The thin duodenal walls pose a great risk for perforation after endoscopic submucosal resection and many salvage resections can therefore not be performed. In their study of more than 62 patients, Kim, et al. could obtain a complete resection with submucosal dissection only in 37.5% of cases [12]. Therefore in most cases of incomplete endoscopic tumor resection a surgical treatment is the only valid alternative. The development of laparoscopy allowed that in patients with low-malignant or endoscopically unresectable NETs an open surgical procedure can be avoided.
The laparoscopy allows a better visualization of the duodenum and reduces the risks of early and late perforations common to endoscopic mucosal and submucosal resections in the duodenum. The defect can be closed up with the safe suturing of the whole wall. If the tumors are located on the anterior wall of the second part of the duodenum, the excision is straightforward. However, tumors in every other region are inaccessible without the mobilization of the duodenum. Laparoscopic kocherisation takes considerable laparoscopic skills. Additionally, the suturing of the wall defect after the excision of the lateral or the posterior duodenal wall can be exceedingly difficult. A great challenge remains the excision of the tumor with sufficient clear resection margins. The tumor is often not clearly visible even if the site has preoperatively been marked with carbon dye. Furthermore, the resection margins cannot be safely determined intraoperatively.
To deal with these difficulties a laparoscopically assisted duodenal excision was proposed by Abe, et al. [10]. With this approach, the surgeon mobilizes the duodenum laparoscopically so it can be lifted towards a small midline laparotomy. The procedure is than carried out with an open approach. Some proponents of laparoscopically assisted surgery claim that this type of surgery carries significant advantages to total laparoscopic full-thickness excisions of the duodenal wall, because extracorporeal suturing eliminates the risk of postoperative hemorrhage, anastomotic insufficiency, surgical site infection, and duodenal deformity [10]. However, the mobilization of the duodenum towards the laparotomy, as proposed by Abe, et al., could prove very difficult or even impossible in obese patients [10]. This approach is therefore limited to lean patients with the tumor located in the second portion of the duodenum. In our case, the tumor was located on the lateral wall of the third part of the duodenum, so the laparoscopically assisted approach would probably be unsuccessful. We therefore decided to perform a laparoscopic-endoscopic cooperative surgery.
This type of surgery was first described by Hiki, et al. and was developed to overcome the drawbacks of the laparoscopically assisted procedures [13]. In laparoscopic-endoscopic surgery the duodenum is kocherized laparoscopically, but then the tumor site is marked or even excised intraoperatively endoscopically. Even more, full-thickness wall excisions can be performed laparoscopically under endoscopic view. The defect can be safely sutured, preventing leakage after endoscopic excisions. In our case laparoscopic-endoscopic surgery was performed. We also performed a full thickness wall excision, since endoscopic excision had already been previously performed in our patient. The tumor was located on the lateral wall on the D3 portion in our patient and was safely extracted. This location would have been relatively inaccessible, if a laparoscopically assisted procedure had been done with the duodenum mobilized to the level of laparotomy. Totally laparoscopic-endoscopic full-thickness excision has the advantage that it can safely be performed in obese patients since the mobilization to the level of laparotomy can be avoided.
The opponents of total laparoscopic approach claim that laparoscopic suturing in this region is prone to leakage. Our patient, however, did not have any leakage, and furthermore, reports from experienced centers support that laparoscopic suturing is safe with an extremely low morbidity [2]. Abe, et al. claim that with the laparoscopic full-thickness excision of the duodenal wall, there is a significant risk for intraperitoneal seeding [10]. Duodenal NETs smaller than 20 mm are very indolent in their behavior, therefore the risk of seeding is negligible [14]. Moreover, none of the authors that reported their long-term experience with full-thickness excision of low-grade duodenal NETs have reported intra-abdominal recurrences due to intraoperative seeding [14]. Therefore, we feel that seeding of low-grade tumors after R0 full-thickness excision is exceedingly rare, since no intra-abdominal implants after full-thickness excisions have ever been recorded in previous reports [6,15]. Additionally, the incidences of LN metastases in small low-grade gastrointestinal NETs, that would necessitate a more aggressive surgical approach, are reported to range from 7% to 15% [1,3,11,15]. Due to the low rate of LN metastases and low recurrence rates of low-grade duodenal NETs, we are convinced that the whole thickness excision does not predispose the patients for locoregional recurrences and is sufficient for small low-grade and incompletely resectable NTEs of the non-ampullary duodenum. On the last follow-up 6 months after surgery, our patient was free from disease further supporting the safety of the laparoscopic-endoscopic full-thickness excisions.
The case of laparoscopic-endoscopic non-ampullary duodenal NET excision presented in this paper is an example of minimally invasive approach for treatment of low malignant or endoscopically unresectable NETS of duodenum. We showed that in experienced hands the trauma caused by open surgery for these indolent tumors can be reduced without exposing the patients to higher morbidity and excellent long term functional results. Therefore, we believe that in the future laparoscopic-endoscopic surgery will firmly be established as the treatment of choice for small low malignant and incompletely excised NETs of the non-ampullary duodenum.
References
- Hatta W, Koike T, Iilima K, et al. (2017) The risk factors for metastases in non-ampullary duodenal neuroendocrine tumors measuring 20 mm or less in diameter. Digestion 95: 201-209.
- Tsujimota H, Ichiikura T, Nagao S, et al. (2010) Minimally invasive surgery for resection of duodenal carcinoid tumors: endoscopic full-thickness resection under laparoscopic observation. Surg Endosc 24: 471-475.
- Fukasawa H, Tounou S, Nabetani M, et al. (2017) Endoscopic resection of ampullary neuroendocrine tumor. Intern Med 56: 499-503.
- Uppin MS, Uppin SG, Sunil CSPV, et al. (2017) Clinicopathologic study of neuroendocrine tumors of gastroenteropancreatic tract: a single institutional experience. J Gastrointest Oncol 8: 139-147.
- Ulla Rocha JL, Salgado A, Sardina Ferreiro R, et al. (2017) Neuroendocrine Gastroenteropancreatic Tumors: Where Are We? Surg Laparosc Endosc Percutan Tech 27: 36-41.
- Kim SH, Park CH, Ki HS, et al. (2013) Endoscopic treatment of duodenal neuroendocrine tumors. Clin Endosc 46: 656-661.
- Kyuno D, Ohno K, Katsuki S, et al. (2015) Laparoscopic-endoscopic cooperative surgery is safe and effective treatment for superficial nonampullary duodenal tumors. Asian J Endosc Surg 8: 461-464.
- Tsushimi T, Mori H, Harada T, et al. (2014) Laparoscopic and endoscopic cooperative surgery for duodenal neuroendocrine tumor (NET) G1: Report of a case. Int J Surg Case Rep 5: 1021-1024.
- Ichiikawa D, Komatsu S, Dohi O, et al. (2016) Laparoscopic and endoscopic co-operative surgery for non-ampullary duodenal tumors. World J Gastroenterol 22: 10424-10431.
- Abe N, Takeuchi H, Hashimoto Y, et al. (2015) Laparoscopy-assisted transduodenal excision of superficial non-ampullary duodenal epithelial tumors. Asian Journal of Endoscopic Surgery 8: 310-315.
- Zhang S, Zheng C, Chen Z, et al. (2017) Clinicopathologic features, surgical treatments, and outcomes of small bowel tumors: A retrospective study in China. International Journal of Surgery 43: 145-154.
- Kim TW, Kim GH, Park DY, et al. (2017) Endoscopic resection for duodenal subepithelial tumors: a single-centre experience. Surg Endosc 31: 1936-1946.
- Hiki N, Yamamoto Y, Fukunaga T, et al. (2008) Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 22: 1729-1735.
- Iwasaki T, Nara S, Kishi Y, et al. (2017) Surgical treatment of neuroendocrine tumors in the second portion of the duodenum: a single center experience and systematic review of the literature. Langenbecks Archives of Surgery 402: 925-933.
- Matsuhashi N, Takahashi T, Tomita H, et al. (2017) Evaluation of tratment for rectal neuroendocrine tumors sized under 20 mm in comparison with the WHO 2010 guidelines. Mol Clin Oncol 7: 476-480.
Corresponding Author
Jagric T, Department for Abdominal and General Surgery, University Medical Centre Maribor, Ljubljanska ulica 5, Maribor, Slovenia.
Copyright
© 2018 Jagric T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.