Molecular Diagnosis and Identification of Carbapenemase Producing Acinetobacter baumannii among ICU Patients, in Khartoum State-Sudan
Abstract
Background: Carbapenemase producing-Acinetobacter baumannii (CP-A.baumanii) has become a significant nosocomial pathogen because of its remarkable ability to acquire antibiotic resistance. The aim of this study was to investigate the molecular characterization of CP-A.baumanii isolated from intensive care unit (ICU), in Khartoum state sudan.
Methods: Hundred isolates of CP-A.baumanii were collected from ICU patients, from the Royal Care International Hospital and the National Ribbat Hospital. The disc diffusion of antimicrobial susceptibility testing of the isolates against common antibiotics were determined. The carbapenemase-encoding resistance genes of these isolates using the primer were detected by PCR.
Result: Thirty nine isolates were found to carry multiple drug resistance Among the 39 A.baumanii isolates, 22 were carbapenemase producing-A.baumanii (CP- A.baumanii), for an overall carbapenemase producing rate of 56.4%.
Conclusion: This is the first report of the molecular mechanisms of CRAB in Khartoum state. A very high level of carbapenem non-susceptibility was detected in these Sudanese hospitals over a period of two years form ICU patients. The predominant Carbapenemase in RCIH and NRH hospitals were NDM and GES and OXAs. As blaOXA-23, blaOXA-51, blaNDM-1 and blaGES producing CRAB isolates were prevalent in the ICU. The coexisted genes (OXA-23/51 and blaNDM-1+blaOXA-23/51/143) were also associated with increased virulence as compared to other OXAs. Here, we detected an emergent OXA subclass identified in two A. baumannii strains OXA-143 which reported as High-Risk Clones among MDR and XDR A. baumannii.
Introduction
Acinetobacter baumannii is an important opportunistic pathogen often involved in various nosocomial infections, such as bacteremia, urinary tract infection, secondary meningitis, surgical site infection, and ventilator-associated pneumonia, especially in patients admitted to intensive care and burn units [1]. A. baumannii is notorious for its remarkable innate and acquired resistance to multiple antimicrobial classes, including extended spectrum cephalosporins and carbapenems. The emergence of carbapenem-resistant A. baumannii has been described as the sentinel event of antimicrobials resistance [2,3].
Despite the high burden of antibiotic resistance in Sudan, there are very limited reports on the epidemiology of resistance among A. baumanii isolates in our region. Recent reports have indicated that carbapenem resistance mainly OXA-type genes may have their natural reservoirs and endemic in some countries (There is little known about the spread and clinical importance of A.baumanii antibiotic resistance and carbapenemase producing blagenes at Khartoum state among hospitalized patients). A study on the epidemiology of A.baumanii in Egypt showed that 70% resistant isolates, with acquired the three OXA carbapenemases in different clones within one year [4]. Elsewhere; CP-AB in hospital settings ranged from 2.3% to 67.7% in North Africa and from 9% to 60% in sub-Saharan Africa and the major bla genes were OXA-23, OXA-58, OXA-48, NDM-1 and VIM-2 associated with A.baumanii isolates of hospitalized patients during the years between 2010-2018 [5,6]. More alarmingly, there was record of Extreme drug resistance A.baumanii with intermediate resistance to colistin. The lack of systematically collected data on the Sudan area contributes to a poor understanding of antimicrobial resistance and limits an effective response to the problem. Consequently, there is a critical need to conduct research to estimate the burden of carbapenemase genes underlying the attributed resistance.
Materials and Methods
One hundred A. baumannii isolates were collected during the period 2017 to June 2019 from Microbiology laboratory department at Royal Care International Hospital (RCIH) and National Ribat Hospital (NRH) as identified by both laboratories, both hospitals located in Khartoum city, Sudan. The clinical specimens were sputum, blood, urine, wound swab, central-line catheter and tips then plated out on MacConkey agar.
Isolation and identification of A. baumannii: were carried out based on cultural characteristics, Gram stains, oxidase test and conventional biochemical tests following standard assay of gram-negative rods at microbiology laboratory at both hospitals. Then genotypes identification of isolate was performed by amplification DNA-based testing PCR was used to confirm the identification of Acinetobacter species and A. baumannii from another gram negative.
Antimicrobial susceptibility was performed by disc diffusion method as per the (CLSI) guidelines [7], using Muller-Hinton agar (Hi-Media, Mumbai) and antimicrobial discs (bioanalyse, Turkey and Hi-Media, Mumbai). The following antimicrobial agents (μg/ml) were used: Ceftazidim (30), cefuroxime (30), gentamicin (10), cefixime (30), ciprofloxacin (5), amoxiclav (30), meropenem (10), ceftriaxone (30) and colistin (10). The diameter of inhibition zones was measured and reported as susceptible or resistant. Quality control of the disks were checked by using reference strains. For meropenem resistant strains the presence of the carbapenemases (blaNDM1, blaVIM, blaIMP, blaOXA, blaGES and blaKPC genes)was screened by Multiplex PCR.( On the other hand 11 samples of A.baumanii were identifying carbapenemase genes blaOXA and blaNDM sub-types by specific primers).
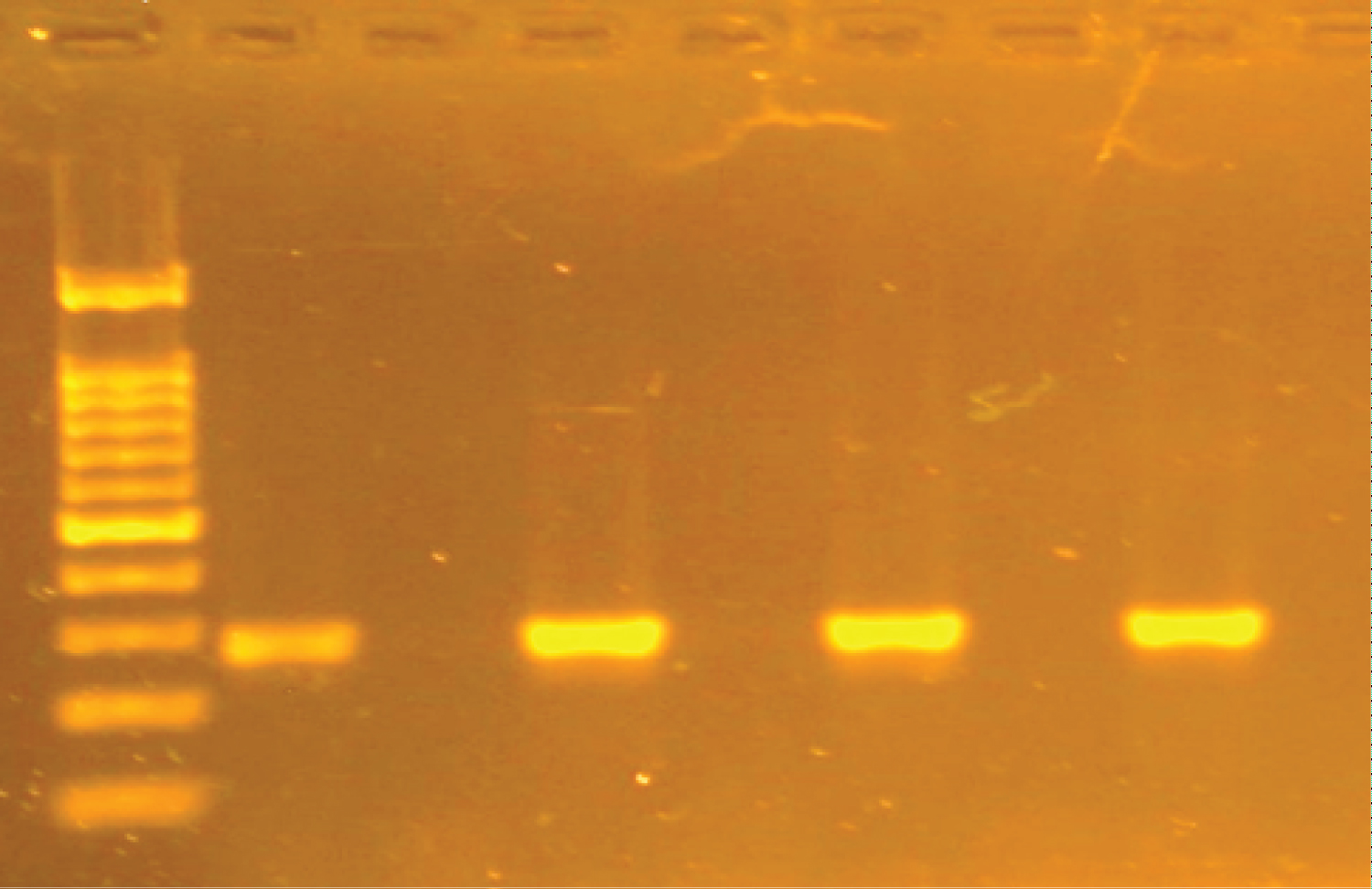
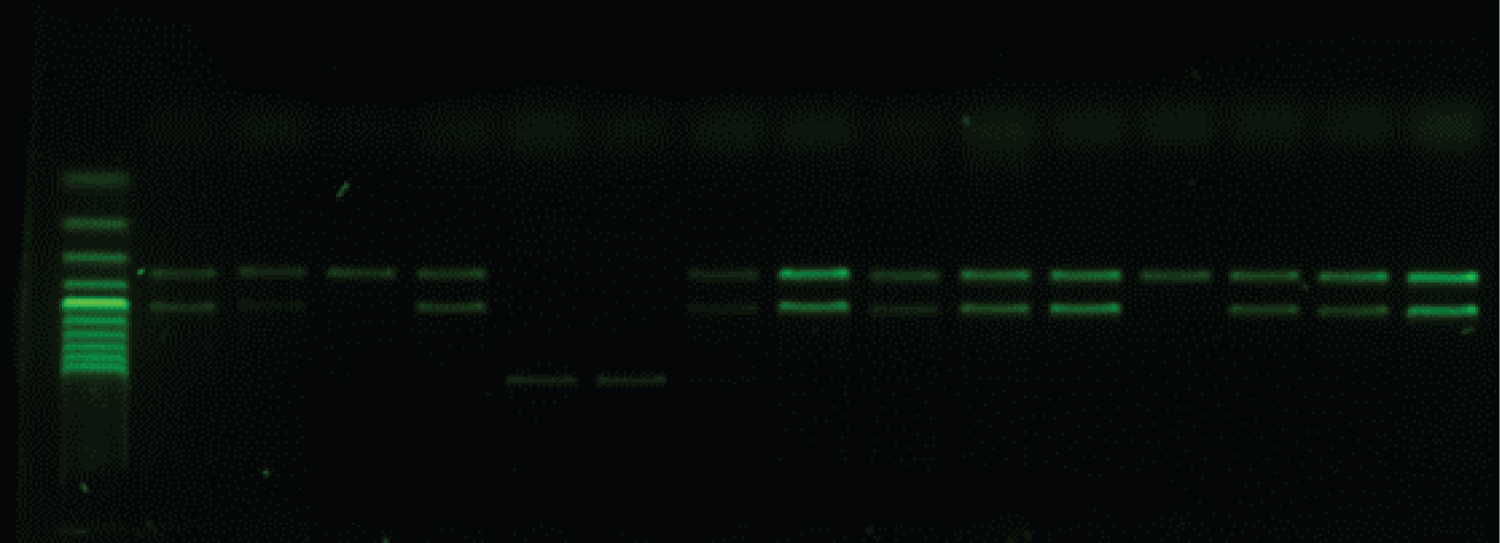
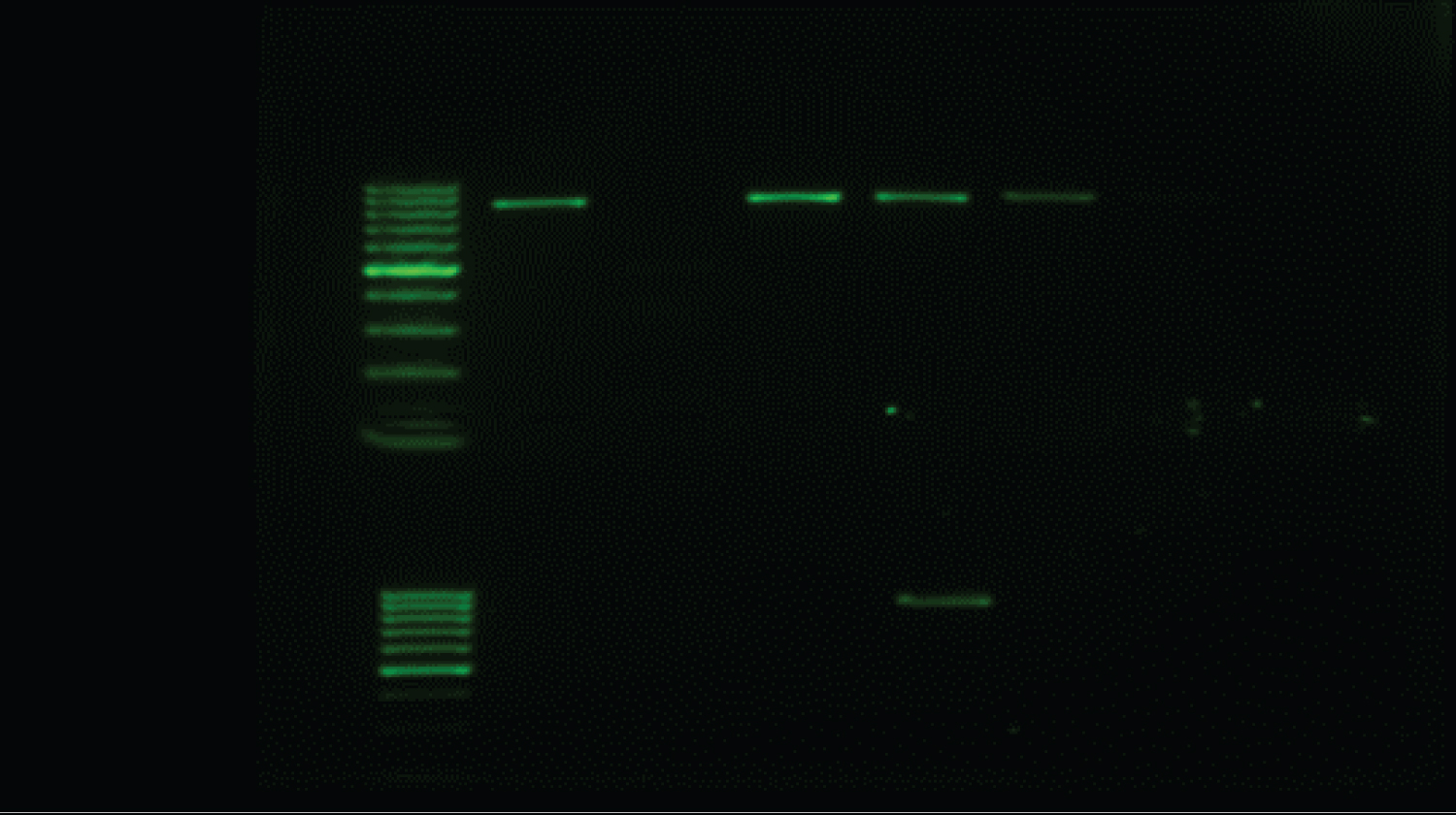
Genotypic characterization of AC-CP: The multiplex PCR assay (using eight primers) amplified fragments of blaNDM [8] blaIMP, blaKPC, blaVIM, blaOXA and blaGES [9] and for blaOXA-23, blaOXA-24 , bla OXA-143 and blaOXA-51 [10] and blaNDM-1 [11]; alleles encoding each of the three multiplex PCR to detect the carbapenemases genes, Table 1 and Table 2. PCR-Reaction conditions were prepared by using ready master mix (APSLABS, India), 0.5 μl of each primer and 1 μl of template DNA (about 10 ng) in a total 25 μl. PCR thermal profile for NDM comprised of initial denaturation at 94 °C for 10 min followed by 30 cycles of 1 min denaturation at 94 °C, 1 min annealing at 60 °C and 1 min extension at 72 °C and final extension step of 10 min at 72 °C. The PCR amplification for multiplex (VIM, IMP and KPC) and (GES and OXA) carried out as a following: initial denaturation at 94 °C for 10 min; 30 cycles of 94 °C for 40 s, 55 °C for 40 s and 72 °C for 1 min; and a final elongation step at 72 °C for 7 min. The annealing temperature of multiplex GES and OXA was optimal at 57 °C instead of 55 °C. The PCR products were analyzed by gel electrophoresis.
Results
Collection of a total of 100 non-fermenting Gram-negative coccobacilli cultures that obtained from hospital microbiology laboratory was done and were re-identified as Acinetobacter spp. A.baumannii was identified in 39 isolates and Acinetobacter species in 13 isolates while the remaining 48 were identified as non-Acinetobacter species .antibiotic susceptibility results of the 39 isolates and the 22 carbapenem resistant isolates shown in Table 3.
Genotyping identification by restriction analysis of the 16s - 23s, served to identify the Acinetobacter species as well as confirm the A. baumannii isolates. similarities shared by isolates in the A. baumannii and non-Acinetobacter species as routine biochemical tests for identification Gram negative bacteria was poorly distinguish between them. Molecular methods; on the other hand, are specifically tailored for accurate identification. A total of thirty-nine non-duplicate A. baumannii isolates were used in the study and 13 Acinetobacter species were exclude. Among the 39 A.baumanii isolates, 22 were carbapenem resistant A. baumanii detection of the carbapenemase genes were confirmed by the produce of the carbapenemase resistant genes.
All 22 of the carbapenem-harbouring A. baumannii isolates carried either single blagenes or more than one genes per (multiple blagenes (12/22; 54.5%). The prevalent genes seen to be associated with the A. baumannii isolates is shown in Table 4. The common single blagenes detected were OXA (5/22; 22.7%) followed by NDM (4/22; 18.2%) then NDM-1 and GES (1/22; 4.5%). Whereas Eleven A. baumanii isolates were re-identified by specific primers toward NDM-1, OXA-23 and OXA-51. The majority (n = 17) of the 22 CP-AB isolates were from a sputum source (n = 5) produce both OXA and NDM + OXA followed by NDM (n = 4), then OXA-23/51 (n = 3). The triple gene CP-AB was NDM-1 + OXA-23/51/143 was encountered from sputum and catheter tips (Table 4) (Figure 1, Figure 2 and Figure 3).
Discussion
A. baumanii is an important opportunistic pathogen that is responsible for health-care infection specially the MDR. A. baumannii are highly resistant to commonly used antibiotics such as penicillins, cephalosporins, aminoglycosides and fluoroquinolones by intrinsic and acquired mechanisms. They are also gradually becoming resistant to carbapenems. The isolation of MDR A. baumannii from ICU samples had been reported earlier by researchers [12].
Hospitals have long served as reservoirs for the transmission of pathogenic bacteria, and. Among the source of the isolates in these study the vast majority of positive cultures were from respiratory specimens (74.4%) followed by urine and tip specimens, consistent with other studies [13-15]. Infections with A. baumannii affecting mainly the respiratory tract, urinary tract, wound infections and sometimes local infections may develop bacteraemia as almost all cases received (mechanical ventilation or endotracheal tube and catheters during their ICU stay, all these findings may support by [16].
The results of the present study show that there was an extreme increase in the resistance rate of A. baumannii to meropenem, from 89% in 2015 to 100% in 2019 [17]. In addition, the resistance rate of A. baumannii to colistin was 59%, which is higher than in previous reports in Khartoum state and other studies [18-20]. The present study showed 100% resistant rates of the most clinically applicable antibiotics for the treatment of infections caused by A. baumannii, except for colistin, which may be used as the final options in the management of infections caused by this bacterium. In this study, the high resistance rate of A. baumannii against carbapenems may indicate the outcome of overuse and misuse of carbapenems in our hospital.
Overall, blaOXA-51 genes were the most prevalent subgroup, which is consistent with the view that they are intrinsic to A. baumannii [21]. These genes were detected in 7 of 11 isolates, irrespective of levels of carbapenem susceptibility or resistance, these alleles does not correlate with the level of carbapenem resistance of the host isolate. Thus, resistance to carbapenems cannot be inferred from detection of blaOXA-51-like alleles.
In contrast, alleles encoding OXA-23-like, OXA-24-like, and OXA-58-like enzymes were consistently associated with resistance or, at least, with reduced susceptibility.
The blaOXA-23 carbapenemase-producing A. baumannii are becoming widespread globally in Europe, South America, and Asia [22]. In this study, blaOXA-23 carbapenemase was detected in 6 (15.4%) of the 39 carbapenem-resistant isolates and as in terms of carbapenem non-susceptibility, an alarmingly high rate of 75.0% over 2 years was detected, this high rate is similar to that reported by Perez, et al. [23] This rate, however; is much higher than that reported for other African countries [13] revealing a worrisome situation in this country. Alleles encoding OXA-24 (OXA-40)-like enzymes were not detected in any of the Sudanese clinical isolates; these enzymes are most often found in Portugal, Spain, Poland, Iran, the United States and Asia [15]. In Saudi Arabia, the blaOXA-24 gene was detected at a rate of 4-45% in Acinetobacter species isolates [24], we first report blaNDM-1-positive A.baumanii isolates in Sudan. In contrast to in other countries where blaNDM-1 was mostly carried by Enterobacteriaceae; all the blaNDM-1-positive A. baumannii isolates, which suggests that this species, which has a robust survival capability, can easily acquire foreign resistance genes such as blaNDM-1 [25].
Recently the Ambler class A of the GES (carbapenemase) types have also been reported for A. baumannii [26]. The blaGES genes are usually carried on integrons found in various species, predominantly Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa, and these resistance determinants have been reported in several countries in Europe, Asia, South America, and South Africa [27]. Our data further point out the fact that one of CR-AB is producing GES gene, that might now be emerging independently in different areas in the world and indicate that, were also recently reported in an Acinetobacter isolate from Kuwait [28], as an additional mechanism of resistance to carbapenems in A. baumannii. Only GES-type carbapenemase was reported in Mediterranean countries [27].
Acknowledgements
I wish to express my warm gratitude to all individuals who had contributed in making this thesis possible and supported me during this fascinating and absorbing period.
Ethical Clearance
This study was approved by the ethics committee of Alribat national university-graduate collage. The informed consent was obtained from all the participants, and informed consent obtained was written.
References
- Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter bauman-nii: Emergence of a successful pathogen. Clin Microbiol Rev 21: 538-582.
- Gales AC, Jones RN, Sader HS (2011) Contemporary activity of colistin and polymyxin B against a worldwide collec-tion of Gram-negative pathogens: Results from the SENTRYAntimicrobial Surveillance Program (2006-09). J Antimicrob Chemother 66: 2070-2074.
- Kuo HY, Chang KC, Kuo JW, et al. (2012) Imipenem: A potent inducer of multidrug resistance in Acinetobacterbaumannii. Int J Antimicrob Agents 39: 33-38.
- Al-Agamy MH, Khalaf NG, Tawfick MM, et al. (2014) Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int J Infect Dis 22: 49-54.
- Manenzhe RI, Zar HJ, Nicol MP, et al. (2014) The spread of carbapenemase-producing bacteria in Africa: A systematic review. J Antimicrob Chemother 70: 23-40.
- de Jager P, Chirwa T, Naidoo S, et al. (2015) Nosocomial outbreak of New Delhi metallo-β-lactamase-1-producing Gram-negative bacteria in South Africa: A case-control study. PLoS One 10: e0123337.
- Patel JB, Cockerill FR, Bradford PA (2015) Performance standards for antimicrobial susceptibility testing: twenty-fifth informational supplement.
- Murali S, Jambulingam M, Tiru V, et al. (2012) A study on isolation rate and prevalence of drug resistance among microorganisms isolated from multiorgan donor and donor corneal rim along with a report on existence of bla NDM-1 among Indian population. Curr Eye Res 37: 195-203.
- Dallenne C, Da Costa A, Decré D, et al. (2010) Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65: 490-495.
- Higgins PG, Pérez-Llarena FJ, Zander E, et al. (2013) OXA-235, A novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 57: 2121-2126.
- Pritsch M, Zeynudin A, Messerer M, et al. (2017) First report on bla NDM-1-producing Acinetobacter baumannii in three clinical isolates from Ethiopia. BMC Infect Dis 17: 180.
- Josheghani SB, Moniri R, Firoozeh F, et al. (2017) Emergence of bla OXA-Carrying Carbapenem Resistance in Multidrug-Resistant Acinetobacter baumannii in the Intensive Care Unit. Iranian Red Crescent Medical Journal.
- Sileem AE, Said AM, Meleha MS (2017) Acinetobacter baumannii in ICU patients: A prospective study highlighting their incidence, antibiotic sensitivity pattern and impact on ICU stay and mortality. Egypt J Chest Dis Tuberc 66: 693-698.
- Reddy D, Morrow BM, Argent AC (2015) Acinetobacter baumannii infections in a South African paediatric intensive care unit. J Trop Pediatr 61: 182-187.
- Elabd FM, Al-Ayed MS, Asaad AM, et al. (2015) Molecular characterization of oxacillinases among carbapenem-resistant Acinetobacter baumannii nosocomial isolates in a Saudi hospital. J Infect Public Health 8: 242-247.
- Hong KB, Oh HS, Song JS, et al. (2012) Investigation and control of an outbreak of imipenem-resistant Acinetobacter baumannii infection in a pediatric intensive care unit. Pediatr Infect Dis J 31: 685-690.
- Omer MI, Gumaa SA, Hassan AA, et al. (2015) Prevalence and resistance profile of acinetobacter baumannii clinical isolates from a private hospital in Khartoum, Sudan. Am J Microbiol Res 3: 76-79.
- Bakour S, Olaitan AO, Ammari H, et al. (2015) Emergence of colistin-and carbapenem-resistant Acinetobacter baumannii ST2 clinical isolate in Algeria: First case report. Microb Drug Resist 21: 279-285.
- Qureshi ZA, Hittle LE, O'Hara JA, et al. (2015) Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin Infect Dis 60: 1295-1303.
- Machado D, Antunes J, Simões A, et al. (2018) Contribution of efflux to colistin heteroresistance in a multidrug resistant Acinetobacter baumannii clinical isolate. J Med Microbiol 67: 740-749.
- Biglari S, Alfizah H, Ramliza R, et al. (2015) Molecular characterization of carbapenemase and cephalosporinase genes among clinical isolates of Acinetobacter baumannii in a tertiary medical centre in Malaysia. J Med Microbiol 64: 53-58.
- Cieslinski JM, Arend L, Tuon FF, et al. (2013) Molecular epidemiology characterization of OXA-23 carbapenemase-producing Acinetobacter baumannii isolated from 8 Brazilian hospitals using repetitive sequence–based PCR. Diagn Microbiol Infect Dis 77: 337-340.
- Tomaschek F, Higgins PG, Stefanik D, et al. (2016) Head-to-head comparison of two multi-locus sequence typing (MLST) schemes for characterization of Acinetobacter baumannii outbreak and sporadic isolates. PLoS One 11: e0153014.
- Al-Agamy MH, Shibl AM, Ali MS, et al. (2014) Distribution of β-lactamases in carbapenem-non-susceptible Acinetobacter baumannii in Riyadh, Saudi Arabia. J Glob Antimicrob Resist 2: 17-21.
- Khan AU, Maryam L, Zarrilli R (2017) Structure, genetics and worldwide spread of New Delhi metallo-β-lactamase (NDM): A threat to public health. BMC Microbiol 17: 101.
- Cicek AC, Saral A, Iraz M, et al. (2014) OXA-and GES-type β-lactamases predominate in extensively drug-resistant Acinetobacter baumannii isolates from a Turkish University Hospital. Clin Microbiol Infect 2: 410-415.
- Djahmi N, Dunyach-Remy C, Pantel A, et al. (2014) Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean countries. Biomed Res Int 2014: 305784.
- Bonnin RA, Rotimi VO, Al Hubail M, et al. (2013) Wide dissemination of GES-type carbapenemases in Acinetobacter baumannii isolates in Kuwait. J Antimicrob Chemother 57: 183-188.
Corresponding Author
Shirehan M Ibrahim, Department of Medical Microbiology, Faculty of Medical Laboratory Sciences, National Ribbat University, Khartoum, Sudan
Copyright
© 2022 Ibrahim SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.