Production and Optimisation of Citric Acid by Yeasts Isolated from Kola Nut Pod Husk
Abstract
Citric acid is an organic acid commonly used in food, pharmaceutical, chemical industries.Citric acid is a weak acid found in nearly all tissues; both in plants and animal and it is generally regarded as safe.
The work aimed to obtain yeast isolates capable of citric acid production from kola nut pod waste and to know optimum conditions that favour higher citric acid production. One hundred twenty seven (127) isolates were obtained out of which 46 were selected following primary screening for production of citric acid on compounded solid media. Nine (9) of these yeasts that had the highest potential for citric acid production having the largest acid production zones after primary and secondary screening were selected. KI8 identified as Candida rugosa, KI8 identified as Pichia kudriavzevii and KF16 identified as Rhodotorula rubra using molecular methods. Three (3) isolates with the highest citric acid were used for citric acid production and optimization. Box-Benkhen design was the statistical technique used for optimization of the experimental factors. Four factors:pH, carbon source (g/L), nitrogen source (g/L) and temperature were tested as the variables affecting citric acid production using Box-Benkhen design.
The results showed that pH (5.0), glucose concentration (7.5 g/L), ammonium sulphate concentration of (1.5 g/L) and temperature (30 ℃) were the best conditions for the highest yield of citric acid. The yield of citric acid yield was high at 17.8 g/L under the optimized conditions. Crude citric acid (4.00 g) was crystallized from the production medium. The citric acid recovered after recrystallization was 3.10%. The recovery was 78%. Hence, citric acid production through fermentation by yeasts can greatly increase acid availability for its various industrial applications.
Keywords
Box-Benkhen design, Citric acid, Kolanutpod husk, Optimization,Yeast
Introduction
Citric acid (C6H8O7) is an organic acid that is used in food, pharmaceutical and chemical industries. Citric acid is a weak acid found in nearly all tissues; both in plants and animal and it is generally regarded as safe. Citric acid is an intermediary in the tricarboxylic acid cycle [1]. Citric acid is one of the most common microbial fermentation-based commercial products for which there is a never ending demand. In 2008, global production of citric acid exceeded 1.6 million tons, and is only expected to increase in the future [1]. It is the number one organic acid which has various applications in foods, pharmaceutical agents, beverages, detergents and several other products. It is widely known for its various applications in production serving as antioxidant, preservative, anticoagulant (calcium chelating activity), buffer, acidulant, emulsifier, flavour enhancement inducer, plasticizer and synergistic agent [2].
The acid is soluble inwater, alcohol, ether, dimethyl sulfoxide and ethyl acetate. If citric acid is heated above 175 ℃, it decomposes through the loss of carbon dioxide and water.Citric acid is a colourless, odourless crystal and it has an acidic taste. It is highly soluble in water and has a resultant acidic taste. The possession of citric acid by some substances is responsible for the tart taste and flavor enhancement. The salts that are formed using citric acid are called citrates. The acid dissolves in absolute ethanol at 15 ℃ [3].
The factors that affect citric fermentation are; concentration of carbon source, cell viability,limitation of nitrogen and phosphate, Hydrogen ion concentration, aeration,concentration of oligo-elements, morphology of the producing microorganism. Some of the nutrients have to be in excess for example; sugars, protons and oxygen. Some are at limiting levels such as nitrogen and phosphate while some are below threshold values for example; trace metals especially manganese) [4].
A lots of yeasts that have the ability to produce citric acid from carbohydrates and n-alkanes, some of these species belong to the genera: Pichia, Torula, Yarrowia, Hansenula, Zygosaccharomyces, Candida, Debaromyces, Torulopsis, Kloekera, Saccharomyces and Yarrowia. Oil was from a cheap source while citric acid was produced by industrial means using Candida sp., including Candida tropicalis, Candida catenula, Candida guilliermondi and Candida intermediate [5].
Kola nut is known as an indigenous fruit from the kola tree found all over West Africa. The trees could reach heights of between 40 to 60 feet,and it produces a star-shaped fruit. Each fruit has between two and five kola nuts. Kola nut is almost the size of a chestnut, and it is packed with caffeine. The genus (kola) of trees are common in the tropical rainforests of Africa [6]. The fruit contains caffeine and the fruit from the tree is used as flavoring agents in beverages. Cola acuminata is a tree of about 20 metres in height, with long, ovoid leaves that are pointed at both ends and a leathery texture. The trees possess yellow flowers with purple spots, and has star-shaped fruit [7]. Within the fruit, about a dozen round or square seeds usually develop inside a white seed-shell. The smell of kola nut is sweet and also rose-like. It has a bitter taste but it becomes sweet when chewed.
The demand for citric acid has increased with the increasing population and industrialization. The demand for citric acid and the need for cheap materials as substrates in the recent time have resulted to the use of substrates that are viable economically. The aim of this research was to obtain yeast isolates capable of citric acid production from kola nut pod waste and to know the optimum conditions that favour higher citric acid production.
Materials and Methods
Sample collection
Samples of kola nut pod wastes were collected in a sterile polythene bags from Ifon (Osun State), Oja Oba and Oyo Town. The kolanut were aseptically taken to the laboratory for analyses.
Sample preparation
Kolanut pod husk were obtained, weighed (1 g) and crushed using a crucible. The crushed Kolanut pod husk was then dispensed into 90 ml of distilled water in a sterile sample bottle in order to obtain the stock from which 1 ml aliquot was used for serial dilution. The samples were analyzed daily.
Media preparation
Biomark yeast extract agar was used for isolation process. Sterilization of media was carried out at 121 ℃ for 15 minutes. Streptomycin (0.01 mg/ml) was put into the media to inhibit bacterial. Media was prepared according to the manufacturer’s description.
Isolation of yeasts
Pour plate method was adopted for the isolation of yeast cells from the samples obtained in order to isolate yeasts that grow on the surface of the media and within the media. One (1 g) of the kolanut pod waste was then poured in 9 ml of distilled water. One ml from dilution factors 2, 5, 7 and 9 was inoculated onto sterile petri-dishes. Incubation was done at 25℃ for 72 hrs [3]. Distinct colonies were sub-cultured onto new sterile agar plates applying streaking method in order to obtain pure cultures and also incubated for 48-72 hrs. Pure cultures obtained were transferred to sterile yeast extract broth and yeast extract agar slants for proper storage in duplicates and were maintained at 4℃ for subsequent analyses.
Screening of yeast ısolates for citric acid production using solid medium
Basal medium for the screening of citric acid was compounded; the yeast isolates obtained from the kolanut pod waste were grown on a medium containing the composition as follows (g): (NH4)2SO4 1.0 g; KH2PO4 1.0 g; K2HPO4 • 3H2O 0.16 g; MgSO4 •7H2O 0.70 g; NaCl 0.50 g; Ca(NO3) • 4H2O 0.40g; bromocresol green 0.40 g; glucose 20.0 g; agar 20.0 g and 1 Litre of distilled water. Pure cultures were inoculated onto the freshly prepared screening medium. Formation of yellow zones after incubation showed positive result [3].
Medium for fermentation and conditions
Fermentation was carried out in a small scale laboratory fermentor using 250 ml Erlenmeyer flasks that contain 100 ml of fermentation medium. Production medium was also compounded as follows (g): glucose 30 g, (NH4)2SO4 0.5 g, yeast extract (Fluka), nitrogen content: 7% (w/w) 0.5 g, KH2PO4 7 g, Na2HPO4 2.5 g, MgSO4.7H2O 1.5 g, CaCl2 0.15 g, FeCl3.6H2O 0.15 g, ZnSO4.7H2O 0.02 g, MnSO4.H2O 0.06 g and were incubated at 30℃ using a rotary shaker at 180 rpm for 7 days [3].
Citric acid measurement
Citric acid concentrationin in the fermentation medium was estimated using titration using NaOH and phenolphthalein. This was used to estimate the total titratable acidity and then followed by estimation of citric acid using pyridine-acetic anhydride method. The readings were taken at regular intervals during the fermentation process [1].
Citric acid assay
Fermentation process of citric acid was succeeded by centrifugation of the fermentation medium at 400 rpm for 15 minutes. Titration method was used to determine the total acid content of the fermentation medium. 0.1 M NaOH was used to titrate 10 mL of the filtrate and 2-3 drops of phenolphthalein was used as an indicator. When the endpoint was reached, the volume of NaOH used (titre) on the burette was read, the titration was completed. Acetic - anhydride method was used for quantitative analysis of citric acid content of the supernatant obtained [8].
One ml of 0.1 M NaOH was equivalent to 0.0064 g citric acid. The amount of citric acid is expressed in terms of (g/l) by citric acid factor (0.0064) formula. The titration was done in duplicates and the average titre was determined and used for the calculation using the equation below:
Optimization of fermentation medium
Optimization of the fermentation conditions using different parameters which include; temperature, pH, nitrogen sources and carbon sources. This was done for citric acid production, it showed the optimum conditions for citric acid production. Citric acid production was calculated after 7 days of incubation of the inoculation medium:
Temperature: The temperature ranges selected were; 30℃, 35℃, 40℃; and the fermentative media were allowed to produce citric acid using these temperature ranges [9,10].
pH: The pH of the fermentation medium was varied using citrate - phosphate buffer. pH; 4.5, 5.0, 5.5, 6.0, 6.5, 7 were the pH rang selected for citric acid production by the yeast isolates. The optimum pH was determined after citric acid quantitative assay using pyridine - acetic anhydride method.
Selection of carbon sources
Different carbon sources were varied during optimization and assayed for citric acid production. The selected carbon sources were based on the sugars fermented by the yeast isolates. They include; glucose, sucrose, maltose, mannitol and fructose.
Selection of nitrogen sources
Different nitrogen sources were varied during optimization and assayed for citric acid production. The selected nitrogen sources were based on the nutrogen source fermented by the yeast isolates. They include; Yeast extract, Sodium nitrate, Peptone, Ammonium sulphate and Urea.
Process of recovery of citric acid
Separation of pellet and supernatant was done by filtration. The filtrate was the source of citric acid. Lime (Ca (OH)2) was mixed with the fermentation broth so that precipitation of citric acid in the form of calcium citrate could occur. The precipitate formed by the mixture was recovered using centrifugation. The suspension was centrifuged at 3000 rpm for 10 minutes and was dried at room temperature [11].
The equation of the reaction is;
2C6H8O7 + 3Ca (OH)2 = (C6H5O7)2Ca34H2O+2H2O
The precipitate was treated with concentrated sulphuric acid (H2SO4) to precipitate insoluble calcium sulphate, and was then filtered, citric acid was then left in the solution.
(C6H5O7)2Ca34H2O+3H2SO4=2C6H8O7 + 3CaSO42H2O+2H2O
The resultant supernatant was decanted. This supernatant was assumed to contain citric acid only. The resultant supernatant was left in the refrigerator for 2 hours. The crystals that was formed were washed with water twice. It was then left to dry at room temperature and then weighed before the estimation of melting temperature.
Purity assessment of the crystals
Assessment of purity of the citric acid obtained was done using a melting point apparatus. Temperature of the citric acid crystals produced and the melting point was compared to the temperature at which pure anhydrous citric acid crystals melt .Melting point apparatus was used to assess the purity of the product obtained [7].
Determination of melting point
A glass capillary melting point tube was first obtained and one end was sealed. The open end was jabbed into the citric acid crystals. The crystals of citric acid were properly dried and pulverized to avoid water serving as impurities. The crystals were allowed to fall to closed end of the capillary tube. The crystals were closely packed to prevent shrinkage during melting. The capillary tube was put in a melting point apparatus and the crystals were -observed from the lens to determine the approximate range. The process was repeated to ensure an accurate interpretation. The melting point was compared with pure citric acid sample used as the standard [11].
The re-crystallization method
Several impurities and by-products are formed during citric acid production. Re-crystallization was done to remove these impurities and by-products that might be attached to the crystals. Most impurities are soluble and are therefore filtered out of the mixture obtained. Five (5) ml of hot distilled water was added to citric acid (3 g) to obtain the citric acid crystals and 45 ml of hot acetone was then added. It was placed on ice tubes in order to allow crystals settle out. Resultant mixture was then filtere [11].
Experimental design of citric acid optimization
The Box-Benkhen experimental design, a fractional factorial design used to reflect the relative importance of various physical and nutritional factors on the citric acid production in liquid cultures. A completely randomized design is set up and the selected fermentation factors are varied and optimization is carried out [12].
Box-Benkhen model is a statistical techniques used for the screening of the experimental factors. The factors that had a level above 95% (P < 0.05) are considered as significant and used for further optimization [13]. The designing and analyzing of these experiments was carried out using Minitab Software version 18.
Results
The total number of isolates obtained was 127 out of which 46 were selected after primary screening for production of citric acid. These 46 yeast isolates were from the four sampling points in the course of this research work. 10 isolates from Ifon (sample KI), 10 isolates from second sampling done at Ifon (sample KF), 9 isolates from Oja-Oba (sample KO) and 17 isolates from Oyo town (sample KY). Nine (9) of these yeasts that had the largest acid production zones after primary and secondary screening were selected for citric acid production. Three (3) isolates had the highest citric acid yield out of all the nine (9) isolates used for citric acid production and optimization.
The yeasts isolated from the samples were: Pichia guilliermondi, Candida rugosa, Pichia kudriavzevii, Saccharomyces cerevisiae, Rhodotorula rubra, Candida parapsilosis, Candida sp.
Table 1 is showing the diameter of zones of acid production (in mm) by the yeasts after primary and secondary screening. The yeast isolates exhibited significant zones of clearance from the secondary screening was carried out on the 46 isolates that were positive to the primary screening carried out. Of all the 9 yeasts that the highest acid production zones after secondary screening, KI18 had the largest diameter of 34.3 ± 0.3 mm while KY26 had the smallest diameter with 18.0 ± 0.2 mm.
Pichia, Candida and Rhodotorula were the most probable genera for the three (3) selected yeast isolates after subjecting them to morphological identification, several biochemical tests and oxidative characterization.
Pichia (KI18) showed the highest citric acid yield in the course of this research work. Molecular method was used to identify the Pichia (KI18) and the result of the molecular analysis showed that the yeast with code KI18 had 98.50% similarity with Pichia kudriavzevii.
The fermentation process being a factorial experiment was subjected to one-factor-at-a-time optimization as well as optimization using various statistical models to check the effect of each of the experiment on the yield and interactive effect of the factors on the quantity of citric acid produced.
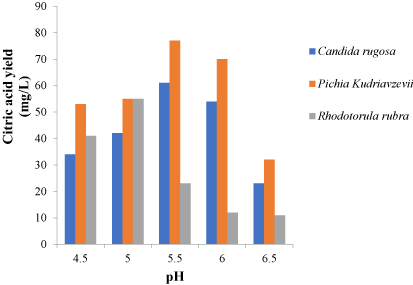
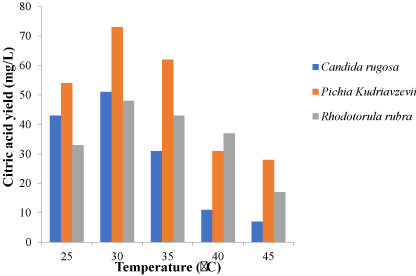
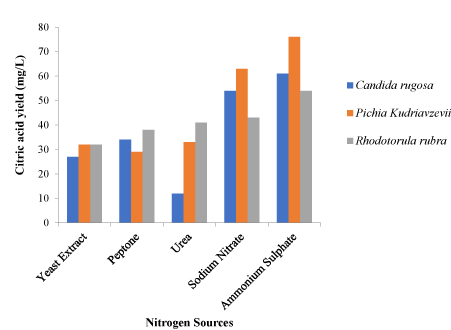
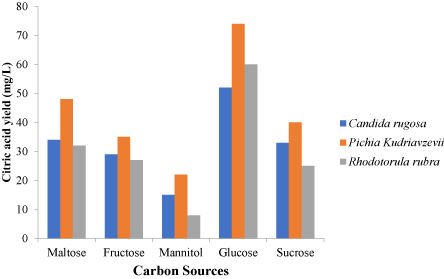
The factors varied include temperature, pH, nitrogen source and carbon source and were varied to chose the optimum conditions for each isolates. Different nitrogen sources such as urea, ammonium sulphate, sodium nitrate, peptone and yeast extract were used in the optimization process. Carbon sources; glucose, fructose, maltose, mannitol and sucrose were used. The incubation temperature was varied and 25 ℃, 30 ℃, 35 ℃, 40 ℃ and 45 ℃ were used in the optimization process. The pH of the fermentation medium was adjusted to 4.5, 5.0, 5.5, 6.0 and 6.5 for each fermentation setup during optimization (Figure 1).
These factors were varied and for the 3 isolates. An optimum pH was observed to be 5.5 for Pichiakudriavzevii and Candida rugosa. Rhodotorula rubra had an optimum pH of 5.0 as shown in Figure 1 while 30 ℃ was observed as the best temperature for the three yeast isolates (Figure 2) while the ammonium sulphate was the best nitrogen source after the optimization process as shown in Figure 3. In Figure 4, glucose was the best carbon source obtained although maltose also had a significant citric acid yield when compared to glucose. Three different setups were made for the three different isolates using the optimized conditions from the one-factor-at-a-time method of optimization.
The optimization process was also done statistically and the Box-Benkhen model was used. This model allows for three or more variables to be considered simultaneously. The upper and lower limit for the variables; carbon source, nitrogen source, temperature, pH were inputted into the Box-Benkhen design and a number of runs (25 runs) were generated which was then carried out in the lab for each of the fermentation organisms one after the other.
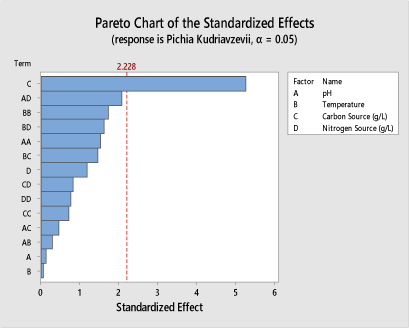
As shown in Figure 5, the result obtained on the Pareto chart generated from the Minitab 18 software used for the statistical optimization showed that at 95% confidence level for Pichia Kudriavzevii (KI18), glucose is responsible for the citric acid yield obtained. Although, at 90% confidence level, glucose concentration (carbon source), pH and nitrogen source are the factors responsible for the citric acid yield obtained.
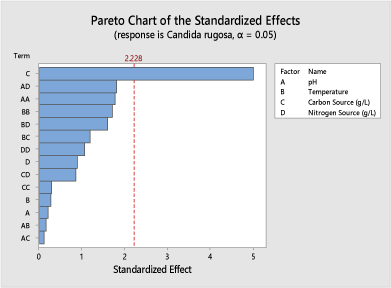
The result obtained on the Pareto chart shown in (Figure 6), for Candida rugosa (KI8) shows the carbon source concentration used in the fermentation setup as the main factor responsible for citric acid yield obtained. The pH of the fermentation medium, carbon source and the nitrogen source (ammonium sulphate) had a substantial impact on the citric acid yield at 90% confidence level.
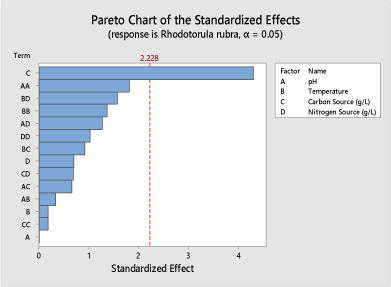
In (Figure 7), the Pareto chart generated for Rhodotorula rubra (KF16) at α = 0.05 shows that glucose is responsible for citric acid yield obtained. At α = 0.1 the carbon source and pH was significant on the yield of citric acid.
Various Pareto graphs obtained show the effect of each of the optimization parameters on the yield and synergistic effect of these factors on citric acid yield.
Pure substances have same melting point under any circumstance, before recrystallization, the melting point of the citric acid obtained from the fermentation medium was 142 ℃. After recrystallization was carried out on the citric acid obtained, the melting point obtained was 151 ℃ which is similar to the melting point of citric acid at 153 ℃. This is shown in Table 2.
Discussion
This study showed that one hundred and twenty-seven (127) yeast isolates were obtained from kola nut pod husk which is in line with the work of Antonucci [14] that wastes are well established sources of microbes with great industrial potentials. The result of the primary screening and secondary screening carried out on the yeast isolates was the basis for selection of the yeasts used for laboratory scale production of citric acid.
Pichia guilliermondi, Candida rugosa, Pichia kudriavzevii, Saccharomyces cerevisiae (3), Rhodotorula rubra, Candida sp. and Candida parapsilosiswere the nine (9) yeasts selected from the forty-seven (47) citric acid producers. These yeasts have been previously used by several researchers for producing citric acid. The yeasts isolated from the kolanut pod husk samples obtained in this research were also previously isolated by Karasu [15] that isolated yeasts from kolanut pod husk for bioethanol production. Out of the nine yeast isolates selected for citric acid production and optimization, Saccharomyces cerevisiae had the highest occurrence which corroborates the work of Sahasrabudhe [16] that found Saccharomyces cerevisiae to be the most occurring yeast in kolanut pod husk unlike [17] that claims Candida sp. are the most abundant yeasts in kolanut pod husk.
Contrary to the findings of Matapathi [18] that observed Saccharomyces sp. to be the most dominant and active yeast species during fermentation, Pichia kudriavzevii was found to produce citric acid best of all the yeasts isolated from kolanut pod husk in the course of this research work.
In the research work carried out by [19] yeasts isolated during the research work were also screened and used for other production processes in which some were able to produce several metabolites. Yeast isolates obtained from the kolanut pod husk samples used for this study showed clear zones during the primary and secondary screening carried out and this proves kolanut pod husk to be a source of citric acid producing yeasts and yeasts for other fermentation processes.
Various carbon sources were usedin other to get the best carbon source for production of citric acid by the selected yeasts. Maltose, fructose, mannitol, glucose and sucrose were the carbon sources varied in the optimization process. Pichia Kudriavzevii, Candida rugosa and Rhodotorula rubra utilize glucose as the carbon source with the highest citric acid yield. Pichia kudriavzevii had the highest yield of 74 mg/L, Candida rugosa had a yield of 52 mg/L andRhodotorula rubra had a yield of 60 mg/L. The results obtained were in agreement with the results of [20] who stated that during the first stage of the fermentation process, glucose was used as carbon source. Mannitol was the least citric acid yield among the carbon sources varied yielding 15 mg/L for Candida rugosa, 22 mg/L for Pichia kudriavzevii and 8 mg/L for Rhodotorula rubra.
30 ℃ was the temperature found to be the most favourable temperature for the production of citric acid production. Microbes are known to be more effective at the optimum temperature that supports their growth. Although, it is stated in different research works that a temperature between 25 ℃ and 35 ℃ is favourable for yeast fermentation, it is necessary to vary temperature in optimization process because optimum temperature could be different for different species and strain of microbes. At 30 ℃ Pichia kudriavzevii had the highest yield of 73 mg/L, Candida rugosa had a yield of 51mg/L and Rhodotorula rubra had a yield of 48 mg/L. This result obtained corroborates the work of [21] that had an optimum temperature of 30℃ for the yeast isolates used for fermentation of citric acid and contradicts the work of Fickers [22] with an optimum temperature of 25℃ for the yeasts used for the production of citric acid.
Various nitrogen sources were used to obtain the good nitrogen source for citric acid production by the yeasts being used for the fermentation process. Yeast extract, peptone, urea, sodium nitrate (NaNO3) and ammonium sulphate (NH4SO4) were the nitrogen sources varied in the optimization process. Pichia kudriavzevii, Candida rugosa and Rhodotorula rubra had ammonium sulphate (NH4SO4) as the nitrogen source with the highest citric acid yield. Pichia kudriavzevii had the highest yield of 76 mg/L, Candida rugosa had a yield of 61mg/L and Rhodotorula rubra had a yield of 54 mg/L. These results agreed with the results of [23] who demonstrated that fermentation processes carried out with yeasts have more yield when inorganic sources of nitrogen are components of the fermentation medium. They carried out fermentation with different organic and inorganic nitrogen sources and had sodium nitrate (NaNO3) being consumed exclusively as nitrogen source. Urea had the least citric acid yield of 12 mg/L for Candida rugosa, peptone had the least yield of 29 mg/L for Pichia kudriavzevii and yeast extract had the least yield of 32 mg/L for Rhodotorula rubra.
Optimum pH for microorganisms varies just as optimum temperature varies. After subjecting the fermentation process to different pH ranges of 4.5, 5.0, 5.5, 6.0, 6.5, the optimum pH for Pichia kudriavzevii yielding 77 mg/L and Candida rugosa yielding 61 mg/L was 5.5 while 5.0 was the optimum pH for Rhodotorula rubrawith a yield of 55 mg/L. pH of 6.5 had the least citric acid yield of 23 mg/L for Candida rugosa, 32 mg/L for Pichia kudriavzevii and 11 mg/L for Rhodotorula rubra.
Lye [24] stated in his research work that product inhibition has been a major drawback in fermentation owing to the fact that a large portion of the product formed further forms complexes with certain components. The crystals of citric acid from this work was 4.00g before recrystallization. 3.10g of pure citric acid was produced after the process of recrystallization which could be due to production inhibition in fermentation processes.
Owing to the fact that pure substances have same melting point under any circumstance, before recrystallization, the melting point of the citric acid obtained from the fermentation medium was 142 ℃. After recrystallization was carried out on the citric acid obtained, the melting point obtained was 151 ℃ which is very similar when compared with the standard melting point of citric acid which was 153℃. This further proves recrystallization suggested by many researchers such as [25], to be a very important process in obtaining pure substances.
Conclusions
This study shows the ability of yeasts isolated from kola nut pod husk to produce citric acid. Although, Saccharomyces and Candida have been widely used for citric production, this research work proves Pichia to be a good fermentative organism for citric acid production. However, for Pichia kudriavzevii, pH of 5.5, glucose of concentration 7.5 g/L, ammonium sulphate of 1.5 g/L and temperature 30 ℃ were the best parameters for citric acid production.
The substrates from cheap sources could reduce the cost of production. Hence, it is advisable to use economical sources like the kola nut pod husk used in the course of this research work. The conversion of waste by yeasts to valueable products is profitable.
The properties of citric acid produced in this research work suggests its possible use in different industrial processes and this makes kola nut pod husk a suitable source of citric acid producing yeasts. As observed in this study, statistical optimization helps to further increase citric acid yield when compared to one-factor-at-a-time optimization.
References
- Hesham AE-L, Mostafa YS, AlSharqia LEO (2020) Optimization of citric acid production by immobilized cells of novel yeast isolates. Mycobiology 48: 122-132.
- Kamzolova SV, Finogenova TV, Morgunov IG (2008) Microbial production of citric and isocitric acids from sunflower oil. Food Technol Biotechnol 46: 51-59.
- Papanikolaou S, Muniglia L, Chevalot I, et al. (2002) Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J Appl Microbiol 92: 737-744.
- Khosravi DK, Zoghi A (2008) Comparison of Pretreatment strategies of sugarcane baggase: Experimental design for citric acid production. Bioresour Technol 99: 6986-6993.
- Lotfy WA, Ghanem KM, El-Helow ER (2007) Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs. Bioresour Technol 98: 3470-3477.
- Amenaghawon NA, Areguamen SO, Agbroko NT, et al. (2013) Modelling and statistical optimisation of acid production from solid state fermentation of sugar cane bagasse using Aspergillus niger. Int J Sci 2: 56-62.
- Förster A, Aurich A, Mauersberger S, et al. (2007) Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl Microbiol Biotechnol 75: 1409-1417.
- Zia MA, Arnbreen S, Asad MJ, et al. (2012) Production of citric acid from waste bread by Aspergillus niger. J Biol Sci 1: 182-183.
- Marrier M, Boulet V (2003) Effect of biomass and sugar in citric acid production by Aspergillus niger using molasses and jackfruit as substrates. American Journal of Food and Nutrition 1: 1-6.
- Crolla A, Kennedy KJ (2001) Optimization of citric acid production from Candida lipolytica Y-1095 using n-paraffin. J Biotechnol 89: 27-40.
- Raspor P, Milek DM, Polanc J, et al. (2006) Yeasts isolated from three varieties of grapes cultivated in different locations of the Dolenjska vine-growing region, Slovenia. Int J Food Microbiol 109: 97-102.
- Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrica 33: 305-325.
- Montgomery DC (2005) Design and Analysis of Experiment. John Wiley and Sons, England.
- Antonucci S, Bravi M, Bubbico R, et al. (2001) Selectivity in citric acid production by Yarrowia lipolytica. Enzyme Microb Technol 28: 189-195.
- Yalcin SK, Bozdemir MT, Ozbas ZY (2010) Effects of different fermentation conditions on growth and citric acid production kinetics of two Yarrowia lipolytica Chemical and Biochemical Engineering Quarterly 24: 347-360.
- Sahasrabudhe NA, Sankpal NV (2001) Production of organic acids and metabolites of fungi for food industry. Applied Mycology and Biotechnology 1: 387-425.
- Sauer M, Porro D, Mattanovich D, et al. (2008) Microbial production of organic acids: Expanding the markets. Trends Biotechnol 26: 100-108.
- Munshi MK, Hossain MF, Huque R, et al. (2013) Effect of biomass and sugar in citric acid production by Aspergillus niger using molasses and jackfruit as substrates. American Journal of Food Nutrition1: 1-6.
- Kim JW (2004) Optimization citric acid production by Aspergillus niger: NRRL 567 in various fermentation systems. Department of Bioresource Engineering 41: 220-225.
- Ghanem NB, Yusef HH, Mahrouse HK (2000) Production of Aspergillus terreus xylanase in solid-state cultures: Application of the Plackett-Burman experimental design to evaluate nutritional requirements. Bioresour Biotechnol 73: 113-121.
- Karthikeyan A, Sivakumar N (2010) Citric acid production by koji fermentation using banana peel as a novel substrate. Bioresour Technol 101: 5552-5556.
- Kishore KA, Kumar MP, Krishna VR, et al. (2004) Optimization of process variables of citric acid production using Aspergillus niger in a batch fermentor. Engineering Letters 16: 572-573.
- Soccol CR, Vandenberghe LP, Rodrigues C, et al. (2006) New perspectives for citric acid production and application. Food Technol Biotechnol 44: 141-149.
- Lye V (1999) Studies on the submerged fermentation of citric acid by Aspergillus niger in stirred fermentor. University of Punjab Lahore, Pakistan 3:114-115.
- Tran CT, Mitchell DA (1995) Pineapple waste-a novel substrate for citric acid production by solid-state fermentation. Biotechnology Letters 17: 1107-1110.
Corresponding Author
Folake T Afolabi, Department of Microbiology, Faculty of Science, Industrial and Biotechnology Unit, University of Ibadan, Ibadan, Nigeria, Tel: +234034218552
Copyright
© 2022 Afolabi FT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.