Estimation of Clinical Comorbidities in COVID-19 Patients: A Systematic Review and Meta-analysis
Abstract
Background
The novel coronavirus or COVID-19 has become a catastrophic global crisis due to its accelerated upsurge in cases. Most hospital admissions and deaths are attributed to the underlying clinical conditions of the infected person.
Objective
This study aims to estimate the proportion of comorbidities in patients with confirmed COVID-19 serological diagnosis.
Methods
A systematic review was performed utilizing PubMed, EBSCO, and CINAHL databases prior to March 22, 2020.
Results
A total of 2028 COVID-19 patients were analyzed from the 11 studies. Nearly 33% of the patients had some form of clinical comorbidities. Hypertension (15%, 95% CI, 9-21%), diabetes mellitus (11%, 95% CI, 8-14%), cardiovascular disease (6%, 95% CI: 3-8%), cerebrovascular disease (3%, 95% CI: 1-4%), chronic lung disease (2%, 95% CI: 1-3) were the most prevalent comorbid conditions. The studies had significant heterogeneity between 49% to 93%.
Conclusion
Higher proportions of comorbidities in COVID-19 patients strengthen the conclusion that individuals with underlying chronic comorbidities could have a greater susceptibility to COVID-19 infections. A potential link between comorbid conditions and COVID-19 infection could protect vulnerable groups as well as help with risk assessment.
Keywords
COVID-19, SARS-CoV-2, Clinical Comorbidity, Systematic review, Meta-analysis
Introduction
A novel coronavirus referred to as coronavirus disease 2019 (COVID-19), originated from Wuhan, China in December 2019, and the World Health Organization (WHO) declared it as a public health emergency on January 30, 2020 [1]. On March 11, 2020, it was declared a global pandemic owing to the massive surge in cases [2]. This is not the first time the world has suffered infectious disease outbreaks of this potential. However, an unrestricted movement of people and the highly contagious nature of this virus has facilitated its rapid spread across the globe, making it challenging to manage [3,4].
As of April 23, 2020 a total of 2,544,792 confirmed COVID-19 cases were recorded worldwide with 1,75,694 deaths [5]. Many of the infections are in the aging population; people aged 50 years or above contributing 35-45% of the total infections [6]. Furthermore, the case fatality rate is also highest amongst this age group. Most of these deaths are attributed to the pre-existing comorbid conditions [6-8]. A study by Jain and Yuan reported pooled estimates of selected comorbidities as the strongest predictive for disease severity and intensive care unit (ICU) admission for COVID-19 patients [9]. However, there are no studies that provide an overall synthesis of evidence in terms of clinical comorbidities in patients with confirmed COVID-19. Therefore, we aim to estimates the prevalence of associated clinical comorbidities in COVID-19 patients, which could be useful for both the planning and management of the cases.
Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines [10]. The primary aim of the study is to report the associated clinical comorbidities in patients with confirmed COVID-19 serological diagnosis and estimate their pooled proportions.
Data sources and search strategy
We limited our search to three online databases: PubMed, EMBASE, and CINAHL. The last search was done on March 20, 2020. A systematic search strategy was employed without restrictions on language. Starting with a disease of interest, the search keywords included 'coronavirus disease 2019, SARS-CoV-2, 2019ncov, novel coronavirus 2019, Covid19, clinical characteristics, clinical outcomes, clinical comorbidity, comorbidity, clinical comorbidities, comorbidities, and chronic comorbidities. To avoid missing any relevant studies, we also applied morbidity specific keywords such as "hypertension, diabetes, cardiovascular disease, chronic lung disease, chronic liver disease, chronic kidney disease, and malignancy".
Study selection
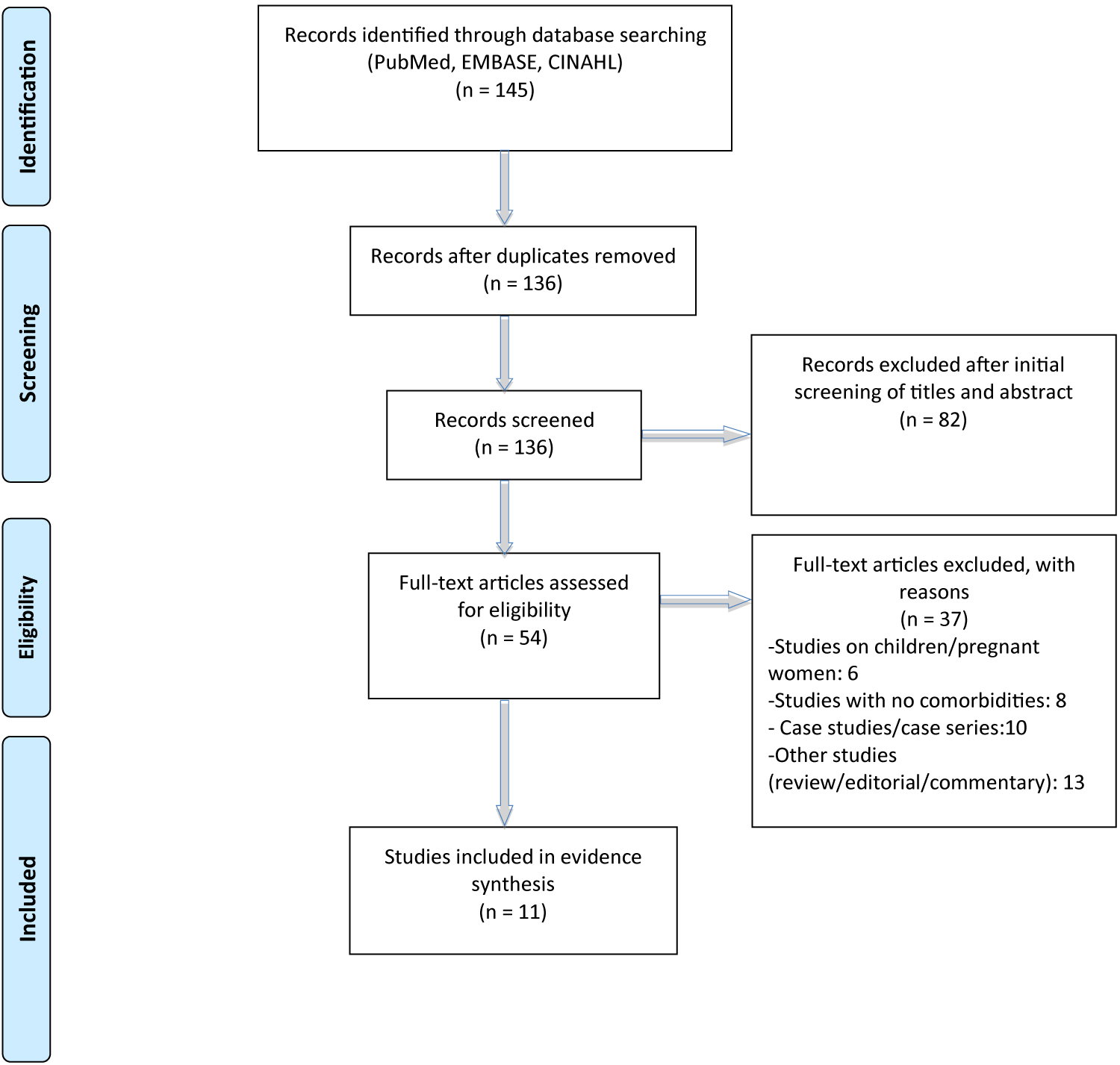
Study selection was performed by YA and AS independently. The studies were then assessed for final inclusion, followed by sorting of the duplicates, title and abstract screening, and full-text screening (Figure 1). Studies published as original articles, either observational or retrospective cohort in peer-reviewed journals from December 1, 2019 to March 20, 2020 were included in the study. Studies such as case studies or case series, editorial, review, or commentary and studies which did not have enough information on clinical morbidities were excluded. Similarly, studies conducted in the pediatric population and pregnant women were also excluded.
Data extraction
A data extraction form was developed in MS-excel, and three authors (AS, KS, LA) extracted data independently. Data extraction was limited to the study objectives and was verified by YA and MT. Any disagreement between authors was resolved by consensus. The data extraction was limited to author names, date of publication, journal, country, patient's age and gender, number of infected and hospitalized patients, medical staff, comorbidities, and deaths due to COVID-19.
Quality assessment
Quality of the included studies was assessed using the standard checklist produced by the US National Health Lung and Blood Institute for observational cohort and cross-sectional studies [11]. The focus of the quality assessment was based on the objective clarity, population definition, inclusion and exclusion criteria, clearly defined intervention, appropriate use of statistical methods and the outcome of interest, well-description of results, and consideration of the potential confounding variables and statistical adjustment for their impact. The outcome of the quality was assessed into four categories: Yes, No, NA (not applicable to the study) or NR (not reported in the study) (Supplementary Table 1).
Data analysis and statistical methods
We analyzed pooled estimates of the total clinical comorbidities and each specific morbidity where applicable. The pooled estimates were determined using a random effect model (DerSimonian-Laird). The pooled proportion below one percent was not presented in the table. Any inconsistencies in the study were assessed using the chi-square test for heterogeneity (I2). Higher the value of I2, the higher the inconsistency in the included studies. All statistical analyses and calculations were conducted using Minitab©, while the meta-analysis was performed in 'Open Meta Analyst©.' Data were presented in percentages, proportions, range, and confidence intervals as required. A p-value < 0.05 was considered statistically significant.
Results
Study selection
A total of 11 studies were included for the evidence synthesis out of the 145 extracted from the databases (Figure 1). During the title/abstract screening, 82 studies were excluded resulting in 54 studies for full-text screening. Of these, 37 were excluded based on our exclusion criteria; case studies/case series (10), studies in children and pregnant women (n = 6), studies without adequate data on comorbidities (n = 8), and studies either review, editorial, or commentary (n = 13).
Characteristics of included studies
Table 1 [12-22] describes the characteristic of the studies included in the study. A total of 2028 COVID-19 patients were included from 11 studies with the mean age of 50 years (range: 39 to 60 years). More than half (59%) of the patients were males. Similarly, more than half of the patients needed hospitalization. The overall case fatality rate (CFR) was 8.14% among all the patients.
Chronic clinical co-morbidities
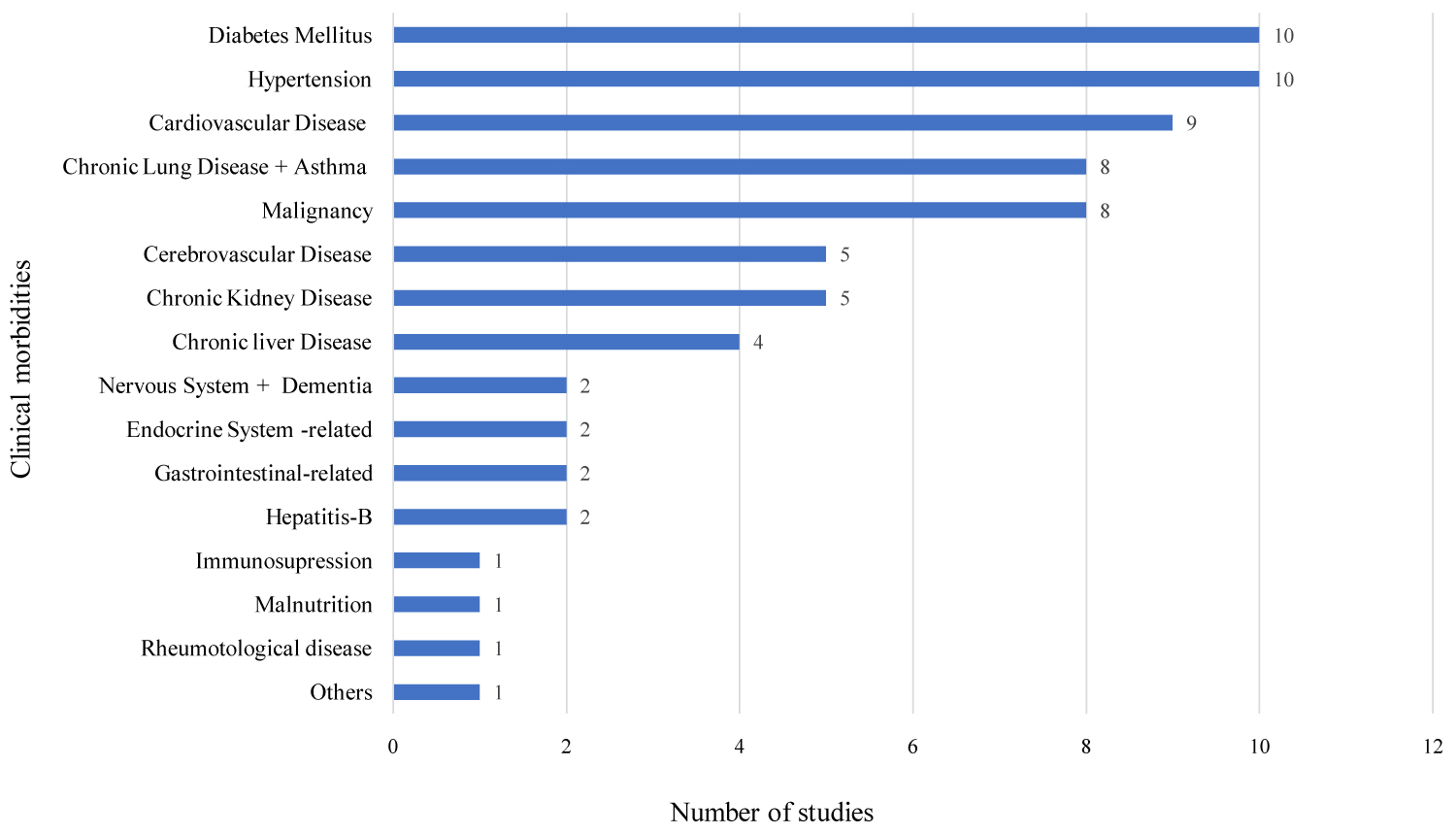
Out of 11 included studies, 10 studies reported Hypertension (HTN) and diabetes mellitus (DM), 9 cardiovascular disease (CVD), 8 each chronic lung diseases and malignancy, 5 each cerebrovascular diseases and chronic kidney diseases, and 4 chronic liver disease (Figure 2).
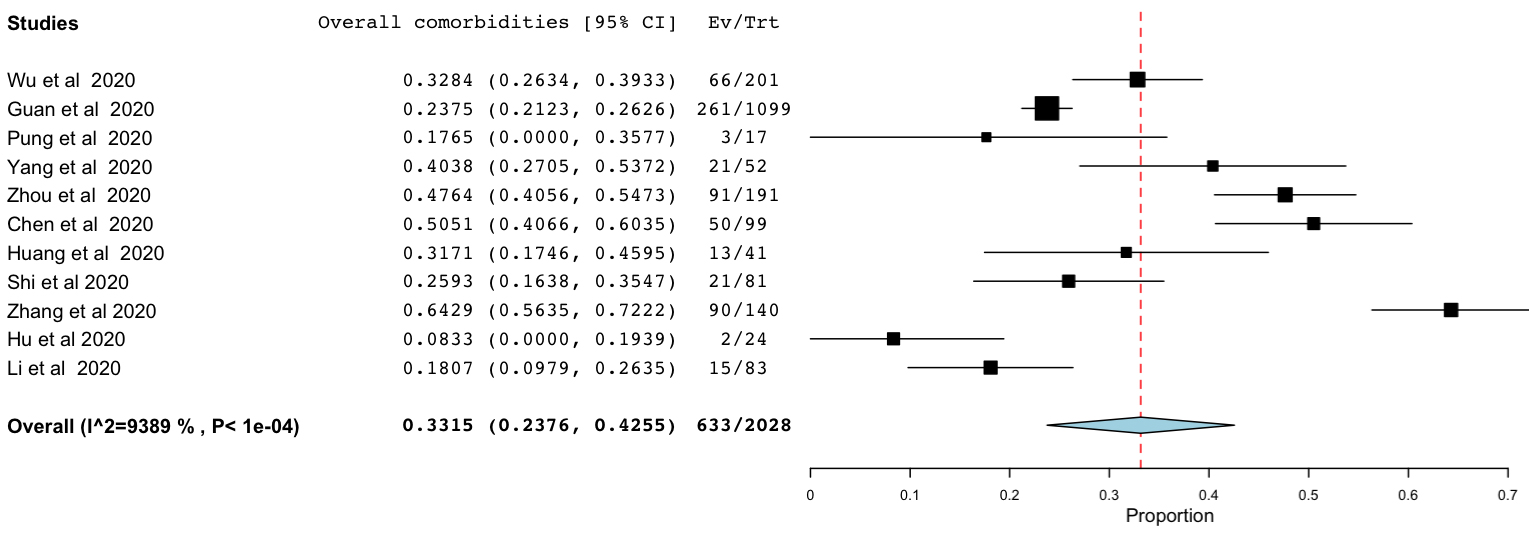
Of the total COVID-19 patients, 748 (33%, 95% CI 24-42%, I2 = 94%) reported having at least one or more of the associated comorbidities (Figure 3).
In Table 2, random-effect meta-analysis of specific morbidities showed 15% (95% CI, 9-21%) had HTN followed by 11% (95% CI, 8-14%) DM, 6% (95% CI, 3-8%) (CVD), 3% (95% CI, 1-4%) cerebrovascular disease and 2% (95% CI, 1-3%) chronic lung disease. More than one percent had malignancy and chronic liver disease; however, the pooled proportion was not calculated due to the small sample size. There was significant heterogeneity amongst the studies (48% to 93%).
Discussion
This systematic review and meta-analysis estimates the proportion of overall and specific morbidities in COVID-19 patients with a higher number of studies and patients population (11 studies, 2028 patients) than previously published reviews [9,23].
At present, there is no definitive treatment of COVID-19. The associated clinical comorbidities further complicate this absence of therapeutic alternatives in COVID-19. Our meta-analysis reported that 33% of the patient infected with COVID-19 had some forms of clinical comorbidities. Among them, HTN (15%), DM (11%), CVD (6%), cerebrovascular disease (3%), chronic lung diseases (2%) were the most common comorbidities. The findings resonate with two previously published systematic reviews [23,24]. However, an earlier study has reported relatively higher comorbidities (25% HTN and 24% CVD) [9,23]. Studies have associated HTN, DM, and CVD with COVID-19 related-mortality [25]. As the only option of COVID-19 recovery depends on the development of antibodies against the virus, multiple coexisting comorbidities can decrease the body host defense mechanism and increase complications [26]. Therefore, early recognition of patients with underlying comorbid conditions and concurrent management of co-morbidities is crucial to reduce complications, hospitalization, and deaths related to the infection.
Comorbidities can be seen at any age. However, it increases with age. Adult populations between 45-64-years had a 30% prevalence of comorbidities, while in those above 65 years, it is 96% [27,28]. Similarly, older people are more vulnerable and develop a severe form of infection with COVID-19. A study in China reported an infection rate of 3.3% and a case fatality rate of 6.4% in COVID-19 patients aged 60 years or above [29] Although we did not perform age-stratification and sub-group analysis, the higher case fatality rates in our study could be attributed to the number of patients with extremes of age in our study and the inclusion of the initial studies, which were mostly concentrated on the severe cases during the early unfolding of the disease. Also, 58.78% of our patients were males, and recent gender-disaggregation data suggest higher susceptibility and severity in males [30]. Similarly, alcohol intake and smoking behavior might have enhanced the severity of comorbidities contributing to the development of a severe form of infections and increased case fatality. Alcohol intake is an independent risk factor that accelerates morbidities [31] hence we suggest further exploration concerning COVID-19 diseases. Around 8.60% of our total patients were smokers. Unfortunately, we did not have information about alcohol consumption in our included studies.
The occurrence of multiple chronic conditions adds a layer of complexity to disease management. Infected individuals with underlying chronic comorbidities likely experience heightened infection risk with possible development of complications and poor prognosis. The reason for developing severe infections and deaths in COVID-19 could be the direct result of existing comorbidities before the onset of the infection. Alike COVID-19, patients with other respiratory infectious diseases reported similar comorbidities. For example, 51% of the MERS patients had diabetes, 48% hypertension, and 30% cardiac diseases [32]. Similarly, of all the comorbidities in SARS patients, 16% had diabetes, and 12% had cardiac disease [33]. These identified clinical comorbidities are proven to be associated with the deprived prognosis of the disease [8]. Our findings suggest that, like other severe acute respiratory outbreak comorbidities such as hypertension, diabetes, and CVD may predispose the adverse clinical outcome of COVID-19 patients in terms of disease severity and death [34]. Hence, the management of existing morbidities should be part of the COVID-19 treatment plan for a better outcome.
Study Limitations
We did not perform a comparative analysis to explore the association between comorbidities and disease severity or death, as many of the studies included were case series without an internal control group. Furthermore, the study was not able to stratify the proportion of comorbidities according to patient's characteristics such as age and gender, which would have provided valuable information on target populations for the intervention. Sex disaggregated pattern has been suggested in COVID-19, however, our study did not analyze the role of comorbidities in disease severity and deaths between males and females. Besides, our study has significant heterogeneity in study design and sample size, which needs to be considered while interpreting the results.
Conclusion
Hypertension, diabetes mellitus, and cardiovascular diseases are the most prevalent comorbid conditions in COVID-19. The higher proportion of comorbidities suggests the importance of early identification of underlying health conditions and intervention in COVID-19 management. A future study exploring the potential link between comorbidities and COVID-19 stratification by age group and gender would help in the risk assessment of vulnerable groups and formulate population-based strategies to mitigate the risk.
Authors Contribution
YA and AS design and conceive the study including data collection and management. KS and LA contributed in the data collection. YA performed data analysis. YA and MT interpreted the findings and support AS in initial drafting of the manuscript. YA and MT extensively revised and finalized the manuscript. All authors approved the final manuscript.
Conflict of Interest
Authors declare no conflict of interests.
Financial Disclosure
None.
References
- Cai J, Sun W, Huang J, (2020) Indirect Virus Transmission in Cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerg Infect Dis.
- (2019) Coronavirus disease 2019 (COVID-19): Situation summary. Center for Disease prevention and Control (CDC).
- Marco Cascella, Michael Rajnik, Di Napoli (2020) Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls.
- Weber L (2018) More dangerous outbreaks are happening. Why aren't we worried about the next epidemic? Health.
- (2020) COVID-19 coronavirus pandemic. Worldometer.
- Team TNCPERE (2020) The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) - China, 2020. China CDC Weekly 2: 113-122.
- Wang T, Du Z, Zhu F, et al. (2020) Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 395: E52.
- Guan W, Liang W, Zhao Y, et al. (2020) Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J 55: 2000547.
- Jain V, Yuan J-M (2020) Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. medRxiv.
- Liberati A, Altman DG, Tetzlaff J, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. In: Journal of Clinical Epidemiology 62: E1-E34.
- (2020) Study Quality Assessment Tools: For observational cohort, crossectional studies and case series. National Heart, Lung and Blood Institute.
- Chen N, Zhou M, Dong X, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A Descriptive study. Lancet 395: 507-513.
- Guan W, Ni Z, Hu Y, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382: 1708-1720.
- Huang C, Wang Y, Li X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506.
- Hu Z, Song C, Xu C, et al. (2020) Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 63: 706-711.
- Li K, Wu J, Wu F, et al. (2020) The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Invest Radiol.
- Pung R, Chiew CJ, Young BE, et al. (2020) Investigation of three clusters of COVID-19 in Singapore: Implications for surveillance and response measures. Lancet 395: 1039-1046.
- Shi H, Han X, Jiang N, et al. (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A Descriptive study. Lancet Infect Dis 425-434.
- Wu C, Chen X, Cai Y, et al. (2020) Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med.
- Yang X, Yu Y, Xu J, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A Single-centered, retrospective, observational study. Lancet Respir Med 475-481.
- Zhang J, Dong X, Cao Y, et al. (2020) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy.
- Zhou F, Yu T, Du R, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A Retrospective cohort study. Lancet 395: 1054-1062.
- Yang J, Zheng Y, Gou X, et al. (2020) Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A Systematic review and meta-analysis. Int J Infect Dis 94: 91-95.
- Li B, Yang J, Zhao F, et al. (2020) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109: 531-538.
- Alipio M (2020) Epidemiology and clinical characteristics of 50 death cases with COVID-2019 in the Philippines: A retrospective review. SSRN.
- Castle SC, Uyemura K, Rafi A, et al. (2005) Comorbidity is a better predictor of impaired immunity than chronological age in older adults. J Am Geriatr Soc 1565-1569.
- Gontijo Guerra S, Berbiche D, Vasiliadis HM (2019) Measuring multimorbidity in older adults: Comparing different data sources. BMC Geriatr 19: 166.
- Divo MJ, Martinez CH, Mannino DM (2014) Ageing and the epidemiology of multimorbidity. Eur Respir J 44: 1055-1068.
- Verity R, Okell LC, Dorigatti I, et al. (2020) Estimates of the severity of coronavirus disease 2019: A Model-based analysis. Lancet Infect Dis 669-677.
- Global Health 5050 (2020) COVID-19 sex-disaggregated data tracker Sex, gender and COVID-19.
- Woldesemayat EM, Kassa A, Gari T, et al. (2018) Chronic diseases multi-morbidity among adult patients at Hawassa University Comprehensive Specialized Hospital. BMC Public Health 18: 352.
- Badawi A, Ryoo SG (2016) Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A Systematic review and meta-analysis. Int J Infect Dis 49: 129-133.
- Booth CM, Matukas LM, Tomlinson GA, et al. (2003) Clinical Features and Short-term Outcomes of 144 Patients with SARS in the Greater Toronto Area. JAMA 289: 2801-2809.
- Chan J, Ng C, Chan Y, et al. (2003) Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 58: 686-689.
Corresponding Author
Meera Tandan, Cecil G Sheps Center for Health Service Research, University of North Carolina, Chapel Hill, USA.
Copyright
© 2020 Sayed A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.