Enhanced Production of Fibrinolytic Protease from Microalgae Chlorella Vulgaris using Glycerol and Corn Steep Liquor as Nutrient
Abstract
Cardiovascular diseases are the leading causes of death worldwide and may be caused by the accumulation of fibrin in the blood vessels. Microbial fibrinolytic enzymes have attracted much more attention than typical thrombolytic agents because of the expensive prices and the side effects. The objective was evaluated cell concentration (Xm), cell Productivity (Px) and Total Fibrinolytic activity (FTact) from the Chlorella Vulgaris microalgae using glycerol (Cgly) and corn steep liquor (Ccsl) by 22 plus star central composite experimental design combined with response surface methodology. In the optimized condition of the 0.9% of Cgly and 1.2% of Ccsl, Xm was 1520 mg L-1, 50% higher than in autotrophic conditions. Px increased with increase of Cgly and Ccsl, obtaining the highest value of 232 mg L-1 day-1. Maximum protease production of 21.7 U mL-1 was optimized in the condition of 1.1% Cgly and 0.9% Ccsl, while that to fibrinolytic to protease activity ratio (Fact/Pact) of 2.1 was using 2.0% Cgly and 0.8% Ccsl. The present study showed that C. vulgaris grown in culture medium containing glycerol and corn steep liquor byproducts, as well as to enhance protease and fibrinolytic enzyme production.
Keywords
Chlorella Vulgaris, Fibrinolytic enzyme, Corn steep liquor, Production
Introduction
According to the World Health Organization (2012), in 2030, almost 3.6 million people will die from Cardiovascular Diseases (CVDs), mainly from heart disease and stroke [1]. One of the pathophysiological Conditions which causes myocardial infarction, stroke and other CVDs, is the formation of a fibrin clot by the proteolytic action of thrombin on fibrinogen, and consequent accumulation of fibrin in the blood vessels usually leads to thrombosis [2].
Thrombolytic agents, anticoagulants, antiplatelet and direct thrombolytics are used for the treatment of thrombotic disorders [3]. Based upon their mechanism of activation of the fibrinolytic system, fibrinolytic agents are classified into two types. One is plasminogen activator such as Tissue-type Plasminogen Activator (t-PA) and urokinase [4,5]. The other one is plasmin-like fibrinolytic enzymes which can directly degrade the fibrin in blood clots, thereby dissolving the thrombi rapidly. However, all these thrombolytic agents still suffer significant shortcomings, including requirement of large therapeutic dose, short plasma half-life, limited fibrin specificity, reocclusion, antigenic reactions and bleeding complications [6].
Microalgae are a special group of microorganisms that have demonstrated to be excellent producers of new bioactive metabolites, such as antibiotics [7], anticancer [8] and anti-diabetic agents [9]. Few studies have been conducted on fibrinolytic enzymes biosynthesized by microalgae and no reports are available concerning statistical medium optimization from microalgae.
Obtaining a suitable medium for maximum production during the enzymatic bioprocess is of great importance since the elements that make up the culture medium significantly affect the yield of the product and the cost of production [10]. Enhanced productivity with economical viable cost is a key requisite for the fulfillment of market demand of fibrinolytic enzyme.
Photosynthetic microorganism can produces various useful metabolites under photoautotrophic and mixotrophic cultivation. Cellular concentrations in photoautotrophic cultures are usually very low, due to light limitation, with the consequent high cost of downstream processing. A feasible alternative for photoautotrophic culture is to use mixotrophic culture in which organic carbon sources and CO2 are simultaneously assimilated in the presence of light and both respiratory and photosynthetic metabolism operates concurrently [11-13].
Organic residues from agriculture and industries, e.g. soybean residue, cane molasses, glycerol and Monosodium Glutamate Waste Liquor (MGWL) had been increasingly exploited in bioprocesses because those were excellent substrates for heterotrophic microorganisms growth by supplying the essential nutrients [14] and reducing production costs. In general, these organic residues have been utilized to bacteria, yeast, fungi and, in limited number, microorganism photosynthetic cultivation.
The present study evaluated the fibrinolytic activity from cell extract of Chlorella Vulgaris microalgae cultivated in low-cost agro-industrial residues, initiating a new era of bioprospecting of fibrinolytic enzymes from microalgae. This is the first report about of fibrinolytic enzyme from C. vulgaris microalgae.
Materials and Methods
Microalgae and media
C. vulgaris (UTEX 1803) was obtained from the UTEX (University of Texas, Austin). Stock cultures were maintained in liquid Bold's Basal medium [15].
The culture medium was sterilized in autoclave at 121 ℃ for 20 min. Corn steep liquor (Corn Products Brazil, Cabo-PE, Brazil) was treated according to Liggett and Koffler [16]. Initially, the corn steep liquor was centrifuged at 8000 rpm for 10 minutes to remove the solid particles, and then concentrated NaOH was added to adjust pH 8 and autoclaved at 121 ℃. Corn steep liquor was then filtered and the filtrate was used in the cultures. The organic nutrients such as glycerol (Dynamic, PA - Purity = 99.5%) and treated corn steep liquor were separately sterilized in different bottles and aseptically added into the culture medium.
Culture conditions
C. vulgaris was grown autotrophically and mixotrophically on 400 mL of culture media (initial pH = 6.8) in 1000 mL Erlenmeyer flasks at 27 ± 1 ℃, under a constant fluorescent light intensity of approximately 74 µmol photons m-2 s-1 measured by a LI-250 Light Meter with a LI-190 quantum sensor (Li-Cor, Lincoln, NE, USA). Sparging air provided agitation and aeration during cell growth. Initial cell concentration was 50 mg L-1. Initially, preliminary tests were carried out with 0.5% glycerol and corn steep liquor (Table 1) to evaluate the influence of these nutrients on production of fibrinolytic enzymes. All experiments were duplicated. Then, experimental design was utilized to optimize the concentration of glycerol (Cgly) and corn steep liquor (Ccsl) on the response variables (Table 2) Periodic samples were taken from the flasks to determine the response variables.
Experimental design and results analysis
Response Surface Methodology (RSM) was used to determine the influence of the two independent variables, Glycerol Concentration (Cgly) and corn steep liquor concentration (Ccsl), on the response variables, namely maximum cell concentration (Xm), cell Productivity (Px), Total Protease activity (PTact), Specific Protease activity (SPact), Total Fibrinolytic activity (FTact), Specific Fibrinolytic activity (SFact) and Fibrinolytic to Protease activities ratio (Fact/Pact). To this purpose, multivariable regression analyses were done under experimental design called "star planning" proposed by Barros Neto, et al. [17] which consists of two Factors in five levels of independent variables. The central point was fourfold repeated to check the reproducibility of results (Table 2). The independent variables Cgly and Ccsl and its corresponding ranges were selected on the basis of the results of Liang, et al. and Mahboob, et al. [11,18] respectively.
A regression method was used to fit the second order polynomial equation (1) to the experimental data and to identify the relevant model terms p-values of less than 0.05 were considered to be statistically significant. RSM was applied to the experimental data using STATISTICA software (Version 5.5, 1999 Edition; Statsoft Inc., Tulsa, OK, USA). The same statistical software was used to generate the statistical and response plots.
Where, Y is the response, b0 is an intercept, bi, bii and bij are the coefficients of the linear, quadratic and interaction terms, respectively. xi and xj represent the coded independent variables. The fitted polynomial equation is expressed as surface plots in order to visualize the relationship between the response and experimental levels of each Factor.
Preparation of microalgae extracts
Lyophilized microalgae were homogenized in Phosphate Buffer (PB) 0.1 M pH 7.0, at cell concentration of 50 mg mL-1, in room temperature for 30 minutes. The homogenate was centrifuged at 15,000 rpm for 10 minutes at 4 ℃.
Analytical methods
Determination of cell density
Cell concentration was daily determined by measuring the optical density of samples at 685 nm using a spectrophotometer (Agilent 8453, P. R. China) [19]. The cell density was performed in duplicate. Cell productivity (Px, g L-1 day -1) was estimated by an equation (Equation 2) where X (g L-1) was the concentration of biomass at the end of the cultivation, X0 (g/L) was that at the beginning, and t was the duration of cultivation.
Protease activity
Protease activities assayed by spectrophotometric using azocasein as substrate. One unit (U) of enzyme activity was defined as the amount of enzyme able to hydrolyze azocasein giving an increase of 0.001 units of absorbance per minute, at 450 nm [20]. The activity was performed in duplicate.
Fibrinolytic activity
The specific enzyme activity was evaluated using an assay of fibrin degradation [21,22]. In this assay, one unit (Fibrin Degradation Unit, FU) of enzyme activity is defined as a 0.01-per minute increase in absorbance at 275 nm. The activity was performed in duplicate.
Protein concentration
Protein concentrations were determined by using BCA or Micro BCA protein assay reagent Kit (BCATM Protein Assay Kit, Thermo SCIENTIFIC). Bovine serum albumin was used as protein standard. The dosage was performed in triplicate.
Results and Discussion
Preliminary test of glycerol and corn steep liquor to protease and fibrinolytic enzymes productions by C. vulgaris
Protease and fibrinolytic activities of the cells extracts were showed in the Table 1. In the tests 1 and 4, without corn steep liquor addition, were observed low protease activity while that those with the corn steep liquor addition was obtained protease activity of 292 U mL-1 (Test 2; Table 1) and 1.48 U mL-1 (Test 3; Table 1). The removal of inorganic source (NaNO3) of BBM increased proteases production (Table 1) which is not feasible for the purpose of the work. One of characteristics of the ideal thrombolytic agent is fibrin speciefic, which allow direct degradation of fibrin at the clot surface. Furthermore, higher fibrin specificity would limit activation of circulating plasminogen and thus degradation of fibrinogen - attributes that would be expected to reduce the risk of bleeding [23]. The cell extract with corn steep liquor (0.5%) and BBM (with NaNO3) had low protease activity and high fibrinolytic activity (Table 1, Test 2). Therefore, BBM medium with NaNO3 was selected for further optimization study using statistical approaches.
From the results, Plackett-Burman statistical design and Response Surface Methodology (RSM) was performed to optimized glycerol and corn steep liquor concentrations added in standard BBM on dependents variables maximum cell concentration (Xm), cell Productivity (Px), Protease Total activity (PTact), Specific Protease activity (SPact), Total Fibrinolytic activity (FTact), Specific Fibrinolytic activity (SFact) and ratio to Fibrinolytic to Protease activities (Fact/Pact). High fibrinolytic enzyme concentration can reduce production costs and facilities the steps of downstream, since purification steps will be used in future.
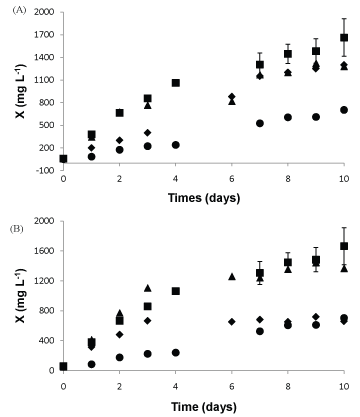
Effects of glycerol (Cgly) and corn steep liquor (Ccsl) concentrations on growth profiles of the C. vulgaris microalgae
Growth profiles of the C. vulgaris microalgae with 1% Cgly and different Ccsl are showed in Figure 1. The increasing of Ccsl results in higher Xm values, showing that C. vulgaris can use nutrients in corn steep liquor for its growth. In mixotrophic microalgae culture, the metabolism assimilates CO2 autotrophic and organic carbon sources provided to it. According to the literature, some microalgae show high cell concentration when mixotrophically cultivated which can be justified by the Fact that the additional carbon source minimizes the consequences generated by self-shading effect [24,25]. The assimilation of inorganic carbon is hampered due to low light availability caused by high cell density. Thus, corn steep liquor can be used as organic carbon source by microalgal since it is rich in protein and carbohydrates. In addition, exogenous protein supplement from corn steep liquor can induce protease production by microorganism [26]. Hence, protease activity in the culture without corn steep liquor was not detectable in this work. Similar results of protease induction by protein were observed by Dhar and Kaur [27] in Metarhizium anisopliae production using casein. Cultures with 1.0% and 1.7% of Ccsl showed similar cell concentration with a significant increase compared to 0.3% of Ccsl (Figure 1A). Stationary phase of growing using 0.3% Ccsl was after 3 days, obtained Xm of 665 mg L-1. In cultures with 1% of Ccsl, Xm was 1,305 mg L-1 after 7 days while that using 1.7% Ccsl, Xm was 1,097 mg L-1 after 6 days. C. vulgaris growth using 1% glycerol and 1% Ccsl obtained highest cell concentration and can be quite viable when applied on larger scale. Mahboob, et al. [18] related that corn steep liquor and urea, which are low cost nitrogen sources, were highly stable for C. vulgaris growth.
The growth profile of the C. vulgaris cultivates in BBM with 1% Ccsl and 1.7% Cgly exhibited a limited effect on the growth of microalgae (Figure 1B). Konh, et al. [28] showed that Chlorella vulgaris utilize glycerol as a sole carbon substrate for the production of biomass and biochemical components, such as photosynthetic pigments, lipids, soluble carbohydrates and proteins. The stationary phase of cell growth cultivated in 0.3% Cgly was after 6 days, achieving Xm values of 878 mg L-1. When C. vulgaris was cultivated in BBM with 1% and 1.7% Cgly, the stationary phase reached after 7 and 8 days, and Xm was of 1,305 and 1,191 mg L-1, respectively. Liang, et al. [11] observed an inhibitory effect on C. vulgaris growth using 2% glycerol, obtained Xm of 656.0 mg L-1 at 6 days of cultivation. Heredia-Arroyo [29] mixotrophic growth of C. vulgaris using 80: 20% glucose: glycerol, observed an increase in cell concentration, while increased levels of glycerol, about 15 g L-1 was inhibitory. Cheng, et al. [30] optimized the growth of C. protothecoides under three variables independents (NaNO3, MgSO4.7H2O and proteose concentrations) obtaining the maximum cell concentration of 1,190 mg L-1 after 11 days of cultivation, though use of proteose in industrial scale is not feasible. The present work showed that it is possible to use two agro industrial byproducts to obtain high microalgae concentration (1450 mg L-1) using 1% Cgly and 1% Ccsl since the costs for the cell production was minimal.
Protease and fibrinolytic productions
Extract of C. vulgaris obtained protease (12.15 U mL-1) and fibrinolytic (590.2 U mg-1) productions when cultivated in the BBM supplemented with 1.5% Cgly and 1.5% Ccsl (Test 4; Table 2). The best result was using 1.7% Cgly and 1% Ccsl (Test 5, Table 2) which obtained relatively low protease production of 17.58 U mL-1 and high fibrinolytic production of 662.6 U mg-1, showing that fibrinolytic protease produced has the ability to directly degrade fibrin. Fibrinolytic enzyme from Codium macro algae was obtained by Matsubara, et al. [31-33]. The enzyme extract of the C. intricatum macroalga showed specific activity of 691 U mg-1 for CIP-I and 533 U mg-1 for CIP-II C. divaricatum showed specific activities of 6.3 U mg-1 Fibrinolytic activities from C. vulgaris obtained in this study were comparatively higher than the earlier report of Codium macroalgae [33].
Currently, not there are papers reporting the use of enzymes produced by microalgae in combating cardiovascular disease. On the other hand, fibrinolytic enzymes produced by other organisms, mainly bacteria and fungi, have been widely studied and optimized the conditions of cultivation. Vijayaraghavan and Vincent [34] related that fibrinolytic activity (1,573 U mL-1) produced by Pseudoalteromonas sp. IND11 in optimized medium using cow dung substrate in solid-state culture three times higher than the unoptimized medium. Silva, et al. [35] showed that several other microorganisms may be producers of fibrinolytic enzymes, among which the Bacillus genus. Bacillus subtilis 168 (S1) produced 252 U/mg fibrinolytic enzymes [36] whereas B. licheniformis KJ-31 produced 242.8 U mg-1 [37]. Bacteria of the genus Streptomyces are also producers of fibrinolytic enzymes, with activity of 136.2 U mg-1 [38] and 19 U mg-1 [39]. Moreover, fungi of the genus Aspergillus [40] and Fusarium [41] may also be promising sources in the production of these enzymes. This present study obtained fibrinolytic activity from C. vulgaris microalgae varied between 143.0 to 662.6 U mL-1, proving to be a source future in combating cardiovascular disease, specifically in direct degradation of fibrin clot. These fibrinolytic enzymes may have wide application in pharmaceutical industry. Fibrinolytic enzymes from this kind of food grade organisms could effectively prevent and treat cardiovascular diseases [42].
Optimization of Cgly and Ccsl concentrations on response variables
Response surface methodology is an approach that combines various statistical and mathematical techniques and is useful for developing, improving and optimizing of process [43]. In the present study, Box-Behnken model for two independent variables Cgly (0.5-1.5%) and Ccsl (0.5-1.5%) with their low, medium and high levels, was used as the experimental design model for optimization of mixotrophic growth of C. vulgaris. Cell concentration (mg L-1), cell productivity (mg L-1 d-1) and total protease activity (U mL-1), specific protease activity (U mg-1), total fibrinolytic activity (U mL-1), specific fibrinolytic activity (U mg-1) and ratio to fibrinolytic to protease activities (U mg-1/U mg-1) were taken as response (Table 2). The goodness of fit of the model was evaluated by the coefficient determination (R2) and the Analysis of Variances (ANOVA).
Xm
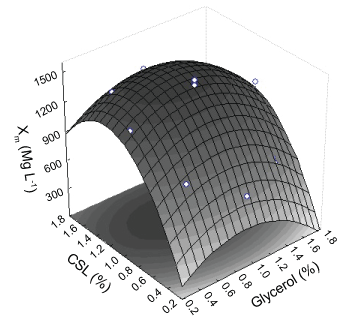
As showed in the Table 2, Xm varied between 650 to 1,480 mg L-1. Based in these values, the regression analysis was applied to Xm in function of both Cgly and Ccsl. It is possible to get a quadratic polynomial equation to express the relationship between Xm and the selected independent variables. To get better fitting of the model, the interaction coefficients between the independent variables were omitted, because they were proved not significant (p > 0.05).
Where Xm is the predicted response, i.e. the cell concentration (mg L-1), and Cgly and Ccsl are values of glycerol and corn step liquor concentrations, respectively.
The statistical significance of the model equation was evaluated by Analysis of Variance (ANOVA) which showed that adjust coefficient of determination (R2 = 0.95) of the regression was statistically significant (p < 0.05). This indicates that the model was adequate for prediction within the range of experimental variables.
Estimated Xm by the model was 1,516 mg L-1. Li, et al. [44] using 0.1% Cgly in photoheterotrophic cultivation of C. minutissima UTEX 2341 obtained Xm = 770 mg L-1 in 7 days of culture. Liang, et al. [11] noted that the growth of C. vulgaris under autotrophic conditions resulted 722 mg L-1 of algal biomass in 6 days. Carbon, nitrogen and phosphorous sources are three main influencing important nutrients microalgae growth [45] and the use of glycerol as a carbon source strongly stimulates its growth, since about 45% of microalgae organism is composed of carbon [46]. The great importance of glycerol on the production of biomass also is emphasized in the literature [47].
The present work presented about twice the Xm obtained by Li, et al. [44] and Liang, et al. [11] demonstrating that the use of glycerol and corn steep liquor, an agro industrial byproducts, improve Xm. Likewise, glycerol uptake for some Chlorella species has been described in other studies. For example, simultaneous utilization of multiple organic substrates, glycerol, glucose and B. peptone by Chlorella saccharophila was reported by Isleten-Hosoglu, et al. [48], where C. saccharophila was capable of utilizing glycerol as a carbon source but its cell growth on glucose is much better than that on glycerol.
Three-dimensional response surfaces were based on the model equation to investigate the interaction among the variables and to determine the optimum concentration of each Factor on Xm (Figure 2). The optimal conditions to maximize Xm estimated by deriving Equation (2) were Cgly = 0.9% e Ccsl = 1.2%.
Px
High Cgly and Ccsl values increase Px, obtaining higher values of 232 mg L-1 day-1 (Table 2 and Figure 3). This result was obtained since there was a decrease in the cultivation time in the experiment with high Cgly and Ccsl. By the way, cell productivity is calculated as function of cell concentration (Xm) and time cultivation, short time of cultivation provides higher cell productivity. Li, et al. [44] related that C. minutissima UTEX 2341 productivity of 110 mg L-1 day-1 was obtained by addition of 1 g L-1 of glycerol and Cheng, et al. [30] obtained C. protothecoides productivity of 108 mg L-1 day-1 using 0.25 g L-1 proteose, a product of hydrolysis of proteins. These values were 50% lower than the present work.
The second order polynomial equations fitted to the experimental data of the CCD for predicting Px, which showed p < 0.05 are given in Equation (4).
Where Px is the predicted response, i.e. the cell productivity (mg L-1 day-1), and Cgly and Ccsl are values of glycerol and corn step liquor concentrations, respectively.
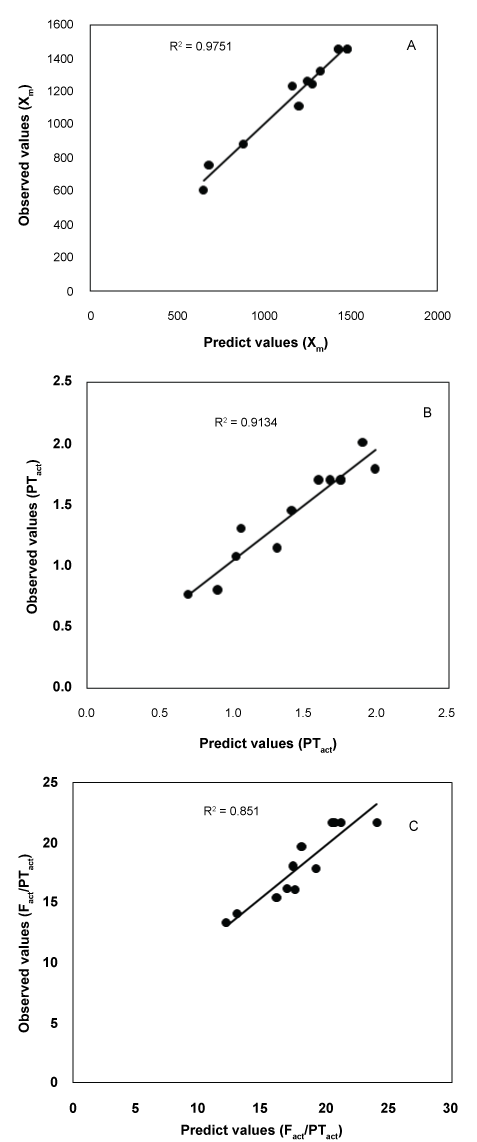
The values R2Adj = 0.80 (Equation 4) indicate a high degree of agreement between the observed and predicted values for cell productivity, suggesting that the proposed model equations provide satisfactory and accurate results (Figure 4A). ANOVA of the regression model demonstrates that the model is highly significant, as indicated by the calculated p-value < 0.05 for cell productivity.
Fibrinolytic and protease activities
A second-order polynomial regression model was employed dependent variables Total Protease activity (PTact), Specific Protease activity (SPact), Total Fibrinolytic activity (FTact), Specific Fibrinolytic activity (SFact) and Fibrinolytic to Protease activity ratio (Fact/Pact). It was observed lack of fit significant to SPact (p = 0.3899), FTact (p = 0.1665), SFact (p = 0.8182), suggesting that this model did not accurately represent data in the experimental region.
The experimental results of PTact and Fact/Pact were fitted to a second-order quadratic equation, giving two numerical correlations to estimate the responses PTact and Fact/Pact according to equation 5 and equation 6.
Where PTact or Fact/Pact is the predicted response, i.e. the Protease activity (U mL-1) or ratio between fibrinolytic and protease activity, and Cgly and Ccsl are values of glycerol and corn step liquor concentrations, respectively.
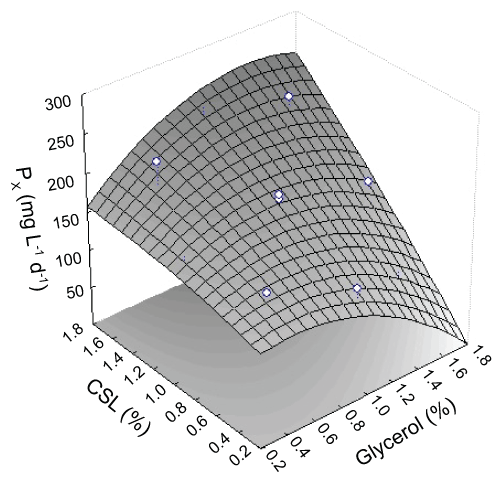
The results of the quadratic model for PTact and Fact/Pact in the form of Analysis of Variance (ANOVA). The values of R2 found also advocated a high correlation between the observed and predicted values. This means that regression model provides an excellent explanation of the relationship between the independent variables (glycerol and corn steep liquor concentrations) and the responses (PTact and Fact/Pact). This implies that 73% and 87% of the sample variation for PTact and Fact/Pact activities are explained by the independent variables. The examination of the fit summaries output revealed that the quadratic models are statistically significant for the responses and therefore these equations may be used for further analysis.
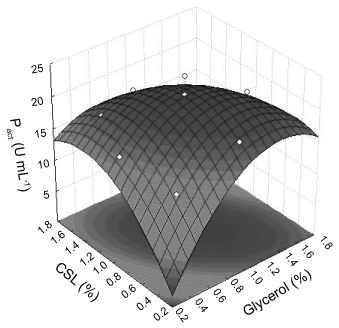
Figure 5 illustrate the three-dimensional response surface graphs of the quadratic model for protease total activities. As shown in the figure, it is also very easy and convenient to locate optimum levels of two variables. The best value of PTact (21.7 U mL-1) occurred close to the upper point indicating the values optimized of glycerol concentration and corn steep liquor was 1.06% and 0.89%, respectively.
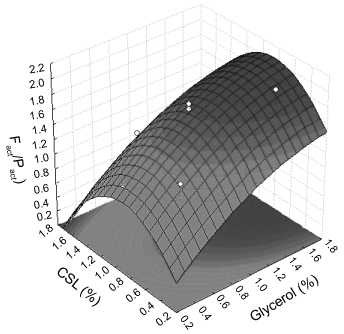
The effect of Ccsl and Cgly concentrations on the Fact/Pact ratio was investigated by using Response Surface Methodology (RSM) and were constructed for determining the optimum conditions for a required Fact/Pact values (Figure 6). The developed prediction equation shows that increasing of Cgly up to 1.2% increase Fact/Pact values, while that the increase of Cgly improve Fact/Pact values only in the high Ccsl, indicating that the Fact/Pact values is more dependent of Ccsl than Cgly. High proteins and peptides concentrations are presents in corn steep liquor [49] and it can induce the production of proteases by microalgae C. vulgaris. Ferrero, et al. [50] related that protease production by Bacillus licheniformis MIR 29 was repressed in the glycerol presence although cell growth had been observed.
The optimum operating conditions obtained from the quadratic form of the RSM were 2% of Cgly and 0.8% of Ccsl with 2.1 of predicted Fact/Pact ratio values.
The values of PTact and Fact/Pact experimentally obtained and predicted from the related empirical models showed in Figure 4B and Figure 4C indicated that for both independent variables, the calculated values of PTact and Fact/Pact agreed very well with the predicted values of PTact and Fact/ Pact at all concentration combinations studied.
Conclusion
The results showed that the use of corn steep liquor to production of fibrinolytic enzymes from the microalgae Chlorella Vulgaris UTEX 1803 is potentially viable. Statistical analysis proved to be a useful and powerful tool in developing optimum conditions. The statistical analysis based on a Box-Behnken design showed that 0.9% of glycerol and 1.2% of CSL concentrations were the best conditions to biomass production. Protease production was optimized in the condition of 1.1% of glycerol and 0.9% of corn steep liquor, while that to Fact/Pact ratio was 2.0% of glycerol and 0.8% of corn steep liquor. The agro-industrial byproducts proved to be good for increasing cell concentration, protease production, obtaining potential for future industrial and biotechnological applications in cardiovascular disease treatment.
Acknowledgements
The authors acknowledge supply of corn steep liquor by Corn Products Brazil, Cabo - PE, Brazil and the financial support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES.
References
-
Joshi R, Jan S, Wu Y, et al. (2008) Global inequalities in access to cardiovascular health care: Our greatest challenge. J Am Coll Cardiol 52: 1817-1825.
-
Kim HC, Choi BS, Sapkota K, et al. (2011) Purification and characterization of a novel, highly potent fibrinolytic enzyme from Paecilomycestenuipes. Process Biochemistry 46: 1545-1553.
-
Mackman N (2008) Triggers, targets and treatments for thrombosis. Nature 451: 914-918.
-
Mukhametova LI, Aisina RB, Glu L, et al. (2002) Characterization of urokinase type plasminogen activator modified by phenylglyoxal. Bioorg Khim 28: 308-314.
-
Collen D, Lijnen HR (2004) Tissue-type plasminogen activator: A historical perspective and personal account. J Thromb Haemost 2: 541-546.
-
Reddy DS (1998) Newer thrombolytic drugs for acute myocardial infarction. Indian J Exp Biol 36: 1-15.
-
Ferreira VS, Conz Ferreira ME, Lima LM, et al. (2017) Green production of microalgae-based silver chloride nanoparticles with antimicrobial activity against pathogenic bacteria. Enzyme Microb Technol 97: 114-121.
-
Kubatka P, Kapinová A, Kruzliak P, et al. (2015) Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition 31: 560-569.
-
Jeong H, Kwon HJ, Kim MK (2009) Hypoglycemic effect of Chlorella vulgaris intake in type 2 diabetic Goto-Kakizaki and normal Wistar rats. Nutr Res Pract 3: 23-30.
-
Sharma AK, Sahoo PK, Singhal S, et al. (2016) ImPact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 6: 116.
-
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31: 1043-1049.
-
Lam MK, Yusoff MI, Uemura Y, et al. (2017) Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renewable Energy 103: 197-207.
-
Salati S, D'Imporzano G, Menin B, et al. (2017) Mixotrophic cultivation of Chlorella for local protein production using agro-food by-products. Bioresour Technol 230: 82-89.
-
Pandey A, Soccol CR, Nigam P, et al. (2000) Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresource Technology 74: 69-80.
-
Bischoff HW, Bold HC (1963) Phycological studies IV. Some soil algae from enchantedrock and related algal species. Univ Texas Publ 6318.
-
Liggett RW, Koffler H (1948) Corn steep liquor in microbiology. Bacteriol Rev 12: 297-311.
-
Barros Neto B, Scarminio IS, Bruns RE (1996) Planejamento e otimização de experimentos. (2nd edn), Editora da UNICAMP, Campinas-SP, Brazil.
-
Mahboob S, Rauf A, Ashraf M, et al. (2012) High-density growth and crude protein productivity of a thermotolerantChlorella vulgaris: production kinetics and thermodynamics. Aquaculture International 20: 455-466.
-
Xu X-Y, Qian H-F, Chem W, et al. (2010) Establishment of real-time PCR for analysing mRNA abundance in Chlorella vulgaris exposed to xenobiotics. Acta Hydrobiologica Sinica 34: 139-143.
-
Alencar RB, Biondi MM, Paiva PMG, et al. (2003) Alkaline proteases from the digestive tract of four tropical fishes. Braz J Food Technol 6: 279-284.
-
Wu SY (2005) Optimization of nutritional conditions for Nattokinase production by a isolated Bacillus subtilis from natto health food. Thesis, Tatung University, Taiwan.
-
Wang SL, Wu YY, Liang TW (2010) Purification and biochemical characterization of a nattokinase by conversion of shrimp shell with Bacillus subtilis TKU007. New Biotechnology 28: 196-202.
-
Van de Werf FJ (1999) The ideal fibrinolytic: Can drug design improved clinical results? Eur Heart J 20: 1452-1458.
-
Fernándes Sevilla JM, Céron García MC, Sánchez Mirón A, et al. (2004) Pilot-plant-scale outdoor mixotrophic cultures of Phaeodactylumtricornutum using glycerol in vertical bubble column and airlift photobioreactors: studies in fed-batch mode. Biotechnol Progress 20: 728-736.
-
Lin TS, Wu JY (2015) Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour Technol 184: 100-107.
-
De Azeredo LAI, De Lima MB, Coelho RR, et al. (2006) A low-cost fermentation medium for thermophilic protease production by Streptomyces sp. 594 using feather meal and corn steep liquor. Curr Microbiol 53: 335-339.
-
Dhar P, Kaur G (2010) Cuticle-degrading proteases produced by metarhizium anisopliae and their induction in different media. Indian J Microbiol 50: 449-455.
-
Konh W-B, Yang H, Cao Y-T, et al. (2013) Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technol Biotechnol 51: 62-69.
-
Heredia-arroyo T, Wei W, Roger R, et al. (2011) Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass and Bioenergy 35: 2245-2253.
-
Cheng KC, Ren M, Ogden KL (2013) Statistical optimization of culture media for growth and lipid production of Chlorella protothecoides UTEX 250. Bioresour Technol 128: 44-48.
-
Matsubara K, Sumi H, Hori K, et al. (1998) Purification and characterization of two fibrinolytic enzymes from a marine green alga, Codiumintricatum. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 119: 177-181.
-
Matsubara K, Hori K, Matsuura Y, et al. (1999) A fibrinolytic enzyme from a marine green alga, Codiumlatum. Phytochemistry 52: 993-999.
-
Matsubara K, Hori K, Matsuura Y, et al. (2000) Purification and characterization of a fibrinolytic enzyme and identification of fibrinogen clotting enzyme in a marine green alga, Codiumdivaricatum. Comp Biochem Physiol B Biochem Mol Biol 125: 137-143.
-
Vijayaraghavan P, Vicent SGP (2014) Statistical optimization of fibrinolytic enzyme production by Pseudoalteromonassp. IND11 using cow dung substrate by response surface methodology. Springerplus 3: 60.
-
Silva PEC, Bezerra RP, Porto ALF (2016) An overview about fibrinolytic enzymes from microorganisms and algae: production and characterization. J Pharm Biol Sci 4: 1-11.
-
Chang C-T, Wang P-M, Hung Y-F, et al. (2012) Purification and biochemical properties of a fibrinolytic enzyme from Bacillus subtilis-fermented red bean. Food Chemistry 133: 1611-1617.
-
Hwang KJ, Choi KH, Kim MJ, et al. (2007) Purification and characterization of a new fibrinolytic enzyme of bacillus licheniformis KJ-31, isolated from korean traditional jeot-gal. J Microbiol Biotechnol 17: 1469-1476.
-
Uesugi Y, Usuki H, Iwabuchi M, et al. (2011) Highly potent fibrinolytic serine protease from Streptomyces. Enzyme Microb Technol 48: 7-12.
-
Simkhada JR, Mander P, Cho SS, et al. (2010) A novel fibrinolytic protease from Streptomyces sp. CS684 Process. Biochem 45: 88-93.
-
Shirasaka N, Naitou N, Okamura K, et al. (2012) Purification and characterization of a fibrinolytic protease from Aspergillusoryzae KSK-3. Mycoscience 53: 354-364.
-
Ueda M, Kubo T, Miyatake K, et al. (2007) Purification and characterization of fibrinolytic alkaline protease from Fusarium sp. Appl Microbiol Biotechnol 74: 331-338.
-
Mine Y, Wong AHK, Jiang B (2005) Fibrinolytic enzymes in Asian traditional fermented foods. Food Research International 38: 243-250.
-
Mandenius CF, Brundin A (2008) Bioprocess optimization using design-of-experiments methodology. Biotechnol Prog 24: 1191-1203.
-
Li ZS, Yuan HL, Yang JS, et al. (2011) Optimization of the biomass production of oil algae Chlorella minutissima UTEX 2341. Bioresour Technol 102: 9128-9134.
-
Chen G, Chen F (2006) Growing phototrophic cells without light. Biotechnol Lett 28: 607-616.
-
Singh A, Nigam PS, Murphy JD (2011) Mechanism and challenges in commercialization of algal biofuels. Bioresour Technol 102: 26-34.
-
Céron-García MC, Macías-Sánchez MD, Sáchez-Mirón A, et al. (2013) A process for biodiesel production involving the heterotrophic fermentation of Chlorella protothecoides with glycerol as the carbon source. Applied Energy 103: 341-349.
-
Isleten-Hosoglu M, Gultepe I, Elibol M (2012) Optimization of carbon and nitrogen sources for biomass and lipid production by Chlorella saccharophila under heterotrophic conditions and development of Nile red fluorescence based method for quantification of its neutral lipid content. Biochemical Engineering Journal 61: 11-19.
-
Hull SR, Yang BY, Venzke D, et al. (1996) Composition of corn steep water during steeping. J Agric Food Chem 44: 1857-1863.
-
Ferrero MA, Castro GR, Abate CM, et al. (1996) Thermostable alkaline proteases of Bacillus licheniformis MIR 29: isolation, production and characterization. Applied Microbiology and Biotechnology 45: 327-332.
Corresponding Author
Raquel Pedrosa Bezerra, Department of Morphology and Animal Physiology, Universidade Federal Rural de Pernambuco-UFRPE, Av. Dom Manoel de Medeiros s/n, 52171-900 Recife, PE, Brazil, Tel: +55-81-33206398, Fax: +55-11-21268485.
Copyright
© 2017 Páblo ECS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.