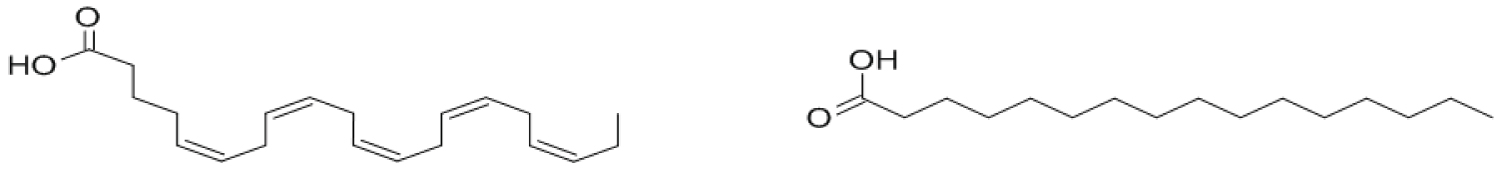Review of Presence, Induction and Isolation of Major Cellular Constituents From Porphyra Sensu Lato (Rhodophyceae), Including Mycosporine-Like Amino Acids (MAA's)
Abstract
Seaweeds from the genus Porphyra play a big economic role in seaweed aquaculture, mainly in Asia. In Europe, resources are put towards seaweed cultivation, but without attention to the Porphyra species which is also native to Europe. Different nomenclature and specifications are used to describe Porphyra, due to taxonomical reclassification and difficult phenotypical identification. Abiotic & biotic together with seasonal factors make for major variance in chemical compositions that are reported. This is also fueled by differences in chemical analytical methods and procedures followed. Combining taxonomical challenges, variance due to seasonal factors and differences in analysis, overviewing published research on Porphyra constituents such as protein, polysaccharides and fatty acids is warranted. Within this review, cellular consitutents found in Porphyra are discussed, including proteins, polysaccharides, fatty acids and mycosporine-like amino acids (MAA's). MAA's are considered amongst the strongest UV-photoprotectants found in nature and feature possible applications in cosmetics. As global interest in seaweeds as food, feed and industrial resource is emerging, opportunities for Porphyra constituents is rising.
Keywords
Porphyra, Bangiales, Polysaccharides, Proteins, Fatty Acids, Seasonal Variations, UV-photoprotectants, Red Seaweed
Introduction
General introduction
Red macroalgae belonging to the genus Porphyra, (class Bangiophyceae, order Bangiales, and family Bangiaceae [1,2]), commonly referred to as Nori or Laver, are economically important seaweed species used in aquaculture for food, feed and fine ingredients [3,4]. Porphyra is therefore researched for its primary metabolites and its fine chemicals from secondary metabolism [5].
The differentiation of bladed Bangiales species is problematic due to their simple morphology and low morphological variation within and between species [6,7]. Currently, both morphologic identification and genetic identification are used. With increasing availability and accessibility of genomic identification methods, genetic identification is likely to become the main identification method [8-11].
In 2011 Sutherland, et al. published a revision of the order of Bangiales, [12] resulting in a new identification, based on a two-gene phylogeny. This revision was needed due to increasing confusion and difficulty in Porphyra taxonomy [2,13]. The Sutherland, et al. (2011) review has resulted in a smaller pool of Porphyra species, making literature published before 2011 where taxonomical identification is based on older classifications, less reliable for comparison of species. The genus Porphyra encompasses, based on the revision of Sutherland, et al., the following species: P. purpurea, P. dioica, P. linearis, P. lucasii, P. mumfordii and Porphyra umbilicalis. Due to mixed terminology, the terms Porphyra sensu lato and Porphyra sensu stricto are often used. Porphyra sensu lato encompasses species that are closely related to Porphyra or until shortly belonged to the genus Porphyra such as Pyropia yezoensis, where Porphyra sensu stricto only applies to species currently belonging to the genus Porphyra [2,14-17]. In our review Porphyra sensu lato is reviewed as subject, due to the ambiguity of recently renamed and revised Porphyra species. For this review, we used the specie names that were used by referenced papers, resulting in the usage of both Pyropia (Py.) yezoensis and Porphyra (P.) yezoensis.
In Asia the use of bladed Bangiales (Porphyra sensu lato) and primarily Py. yezoensis is integrated for generations. Currently, Porphyra is mainly being cultivated in China, Japan and Korea where seaweeds are staple food [18]. The aquaculture of Py. yezoensis species has an estimated worth of about US $7.48 billion in 2017 [15]. Annual Chinese production was estimated at just over 100.000 metric ton of dried Porphyra, in 2015 [19]. Information on Chinese aquaculture and research towards Porphyra sensu lato is becoming increasingly available internationally [4,15,20]. Nowadays outside of Asia, thus also in Europe, interest in cultivation and usage of red macroalgae is increasing.
In Europe Porphyra is naturally found along the shores of the Atlantic Ocean [21-23], ranging from Portugal to Norway, in the upper parts of the intertidal zone. Europe had a total seaweed aquaculture production of 287.033 tons in 2019 of which 11.125 tons were from seaweed cultivation. No Porphyra was produced outside of Asia according to the Food and Agriculture Organization of the United Nations (FAO) data [24]. According to the FAO, there are opportunities for European production and European processing technologies of seaweeds, including red seaweeds [25]. Research towards cultivation and cultivation applications of seaweeds is increasing globally, also acknowledging the opportunities of red seaweeds [26-31].
Physological characteristics of Porphyra due to abiotic factors such as temperature, light intensity, depth and nutrient concentrations has been researched extensively [11,32-36]. Together with its importance in aquaculture, this has led to strain characterization and breeding programs [37,38]. Porphyra has been seen as model organism for red seaweeds and genetic research has been undertaken, including genomic sequencing [16,39-44].
Porphyra has been a part of human food for thousands of years, mainly in Asia [15,45]. However Porphyra is becoming increasingly popular globally for its health beneficial applications as nutraceutical and food additive [46-48]. Porphyra has a primary metabolite content consisting of, amongst others, high protein levels with a high concentration of essential amino acids. Due to this favorable protein fraction, interest towards food and feed applications is rising. In both applications, Porphyra has the benefit of being a saltwater crop, with low nutrient and no terrestrial land usage, when compared with current agricultural protein supplies. In feeding trials in aquaculture, Porphyra protein showed promising results when compared to current often-used protein sources [49-54]. Porphyra's lipid fraction, although being a small metabolite fraction, is rich in the polyunsaturated fatty acid eicosapentaenoic acid (EPA), which is found in fish oil and has beneficial effects on cardiovascular activity [55-57].
Furthermore, Porphyra has considerable concentrations of health-beneficial secondary metabolites such as antioxidants, vitamins and inorganic elements [45,58,59]. One such secondary metabolite that is of great interest are the mycosporine-like amino acids (MAA's), because of their UV-absorbing capacity, probably the highest absorption known in nature [60,61]. Besides that, MAA's are also of interest for having other cell beneficial capacities such as cell proliferation and renewal next to UV-absorbing capabilities [62-69].
In this review we provide an overview of the reported constituents and fine chemicals found in Porphyra species sensu lato, focusing on presence, induction and isolation of Porphyra constituents, with special attention for the MAA's (Figure 1).
Porphyra Constituents
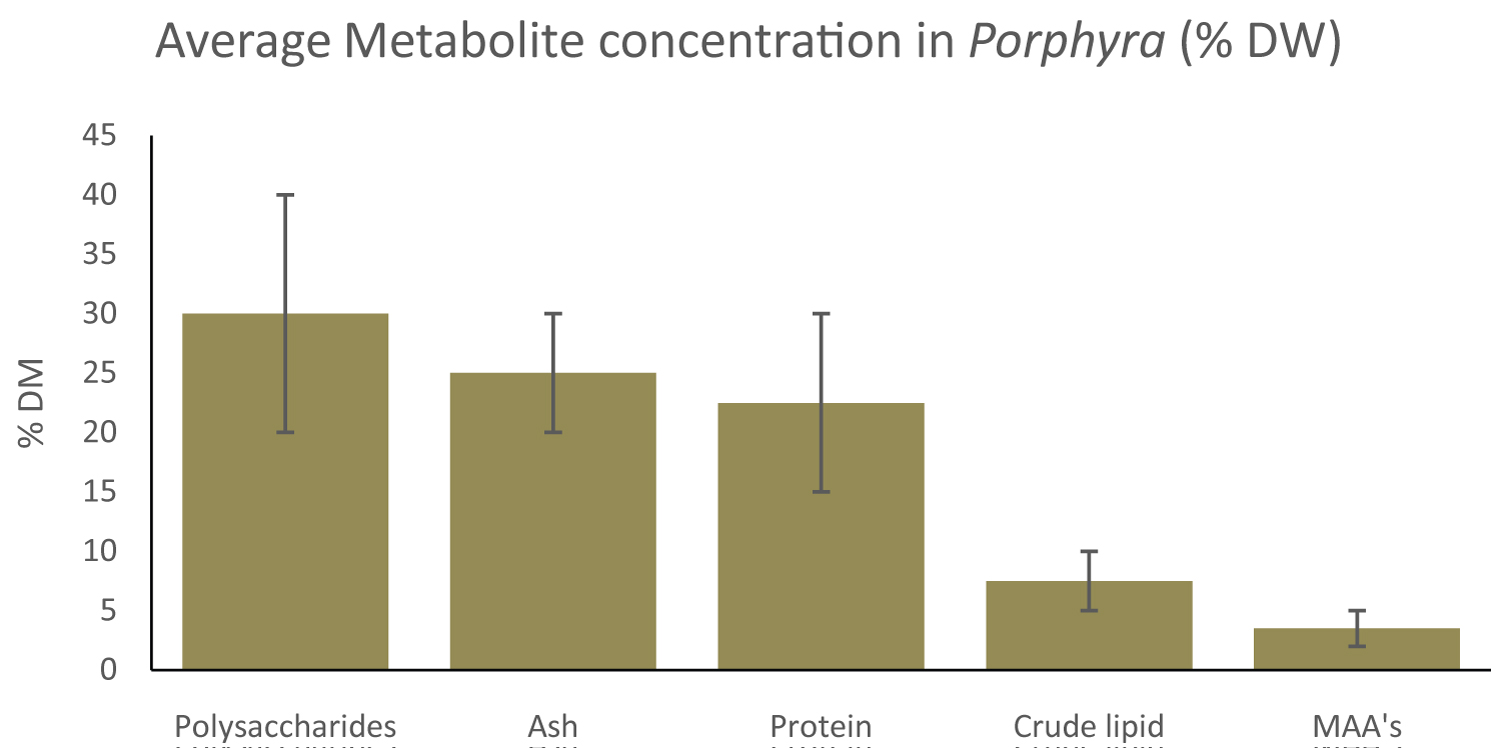
Porphyra constituents have been a topic for research, for their multiple application in for instance feed, food and cosmetics [14,45,51,52,62,70-72]. The composition of seaweed constituents in general is influenced by seasonal/environmental and abiotic factors [54,73,74]. Variation in isolation and analysis methods are also accountable for differences in concentrations. An overview of constituents in Porphyra, based on our literature review, will be discussed and an approximation is given in Figure 2.
Proteins and amino acids
Presence: Protein contents between 10-35% DW are most commonly reported for Porphyra, with huge variations found caused by amongst, seasonal variabilities, differences in extractions and choices in analytical techniques [9,75]. Due to these high protein contents, Porphyra has potential in food and feed applications [50,52,76]. The essential amino acid concentration of total amino acids of Porphyra can be similar to fish meal and therefore can be used as a feed additive [28,49,77]. Total protein content of Porphyra is shown in Table 1 and amino acid compositions are shown in Table 2.
Essential amino acid composed 57% of the total protein content for Porphyra sp, with a total protein content of 24% DW in January 2007 at the São Miguel Island, in the Azores Archipelago from the littoral zone [78]. Total amino acid content in P. dioica, P. purpurea and P. umbilicalis of respectively 24.2, 15.9 and 17.7 g/100g DW was found, in samples from October 2014 in Northern Norway [79]. For P. acanthophora a total amino acid 18.6 g/100g DW was reported, in samples from June and September 1998 from around Cabo Frio, Brazil [80]. For P. umbilicalis, cultivated in at the Sven Loven Center for Marine Infrastructure at Tjärnö, Sweden and supplied with filtered seawater, a total amino acid content of 31.8 g/100g DW was recorded [81].
Peptides from Porphyra have multiple bioactive characteristics, including antioxidant, anti-inflammatory and anticoagulant activity [82-84]. P. dioica protein hydrolysate showed high antioxidant activity of Tyr-Leu-Val-Ala peptide chains, which is found in the phycobiliprotein C-phycocyanin. Antioxidant activity was also found in peptide chains from the phycobiliproteins C-allophycocyanin and β-phycoerythrin [85]. A potent and novel anticoagulant peptide was isolated from processed nori sheets of Py. yezoensis, which is stable at room temperature and non-cytotoxic [86]. Peptides from respectively P. haitanensis and Py. haitanensis showed anti-proliferating activity on human [87,88]. Enzymatically hydrolysed peptides from Porphyra spp. showed inhibition of α-amylase enzyme, thus lowering blood glucose levels [89]. Proteomics analysis identified 30 proteins from P. haitanensis with antioxidant activity [90].
Induction: Protein content in P. umbilicalis blades is affected by light intensity and duration of light exposure. P. umbilicalis showed the highest protein levels at 30 µmol photons m-2 s-1 and decreasing with increasing lighting levels. Structural protein levels were observed to be highest under an 8:16 light:dark regime [11]. Seasonal variations show that protein levels of P. dioica more than double in winter, to 21.52% DW [91]. It was published that Porphyra protein levels from samples collected in New Zealand were alleviated during winter when maximal growth is reached [75]. Tissue nitrogen content of P. umbilicalis was 3.89% DW and 6.76% DW when cultivated for two weeks at respectively 25 μM and 250 μM ammonium at 10 ℃ [33]. When P. haitanensis was UV-B induced for one hour on two consecutive days, protein content peaked when induced at 0.5 W m-2 UV-B and total amino acid content peaked at 0.5-1.0 W m-2 UV-B [92]. This is shown in Table 1.
Analysis: In recent years, numerous publications have addressed the different direct and indirect analysis methods for determination of protein concentration, such as Kjeldahl, Lowry and total amino acid analysis (TAA) [79,93,94]. With the Kjeldahl method, samples are digested with sulfuric acid (H2SO4) and a catalyst in order to form ammonium (NH4). By acid distillation ammonium (NH4) is converted to ammonia (NH3), forming ammonium salts with a standard acid, which is titrated. Nitrogen concentration is then determined by acid-base titration [78-95]. Nowadays, this process is fully automated, and nitrogen to protein conversion factors (NPCF) are used to determine protein content [96]. Meta studies, analysing other research papers, found that different analysis methods are still being used throughout different fields of expertise, showing that nitrogen quantification with a nitrogen to protein conversion factor (NPCF) of 6.25 was the most commonly used method between 2009 and 2015 [97]. For, P. dioica, P. purpurea and P. umbilicalis NPCF's of respectively, 4.15, 4.69 and 3.92 were found for samples collected in October 2014 in Norwegian waters [79]. An universal seaweed n-protein factor of 5 was proposed to be used when accurate data was not available [97].
With total amino acid analysis, the crude protein fraction is hydrolysed under acidic conditions after which all amino acids are quantified separately using liquid chromatography, with their sum representing the total protein fraction. This can be done by hydrophilic interaction LC-MS [98], by derivatization for fluorescence using o-phthal-dialdehyde (OPA) or other pre-column reagents [99,100] or by ion exchange chromatography [80,93]. The downside of total amino acid analysis is the relatively high cost compared to indirect spectrophotometric analyses. It should also be noted that during acid hydrolysis, tryptophan, is destroyed completely and methionine is destroyed partially [78,80,101,102]. The sulfur-containing amino acids cysteine and its dimer cystine and methionine require additional derivation after acid hydrolysis before analysis is possible and are therefore sometimes omitted in amino acid analysis [103]. Overall, in spite of higher practical and financial costs, determination of total protein content summing up all individual amino acids is deemed to be the most accurate representation of total protein content.
The Lowry and Bradfort analyses are both spectrophotometric indirect biuret protein analyses. The Lowry method uses copper sulfate to form cuprous peptide complexes in combination with Folin-Ciocalteu reagents (phosphomolybdic and phosphotungstic acid) to cause interaction between cuprous compounds and the amino acids tyrosine, tryptophan, and cysteine [104]. The Bradford method relies on the interaction of the protein with Coomassie Brilliant Blue G-250 colourant [105]. Under acidic conditions, usually the addition of phosphoric acid, protonated Coomassie Brilliant Blue reacts primarily with arginine and to lesser extend with amino acids with positively charged side chains and aromatic side chains [93,98,104,106,105]. It was shown that the often used indirect protein analysis methods such Bradfort and Lowry (and Kjeldahl) can show differences in protein content of up to 30% or higher in underling comparison and in comparison to direct total amino acid analysis. Another downside is the usage of the animal protein BSA as standard, therefore partly missing plant proteins and unsoluble proteins. Upsides for these indirect protein analyses are their low cost, quickness and easiness.
Conclusion and recommendations: Protein, peptide and amino acid analyses in Porphyra are hampered by biological and analytical hurdles. The species used, growth conditions (light/nutrient availability), and season have major impact on the concentrations and composition of proteins, peptides and amino acids. Also, there are multiple analytical issues in accurate determination of the protein, peptides and amino acid concentration in Porphyra. This is problematic, as reliable protein analyses are needed to evaluate macroalgae as protein source [81,93,97,107-113]. Multiple differently analysed protein contents for Porphyra are shown in Table 1. attention should be given to which determination method is used. Throughout a multitude of analysis methods, sampling strategies and Porphyra species a protein concentration between 9-33% DW is shown.
It is recommended in future research to always state sampling strategy, including date, environment and season. Furthermore, protein determination based on total amino acid analysis is recommended for accurate results. Research focusing on the effect of abiotic factors on amino acid composition is recommended to increase viability for use as feed and food. Clearly there is need for standardisation of analytical methods as well as the availability of reference materials. Bioactivity of protein fractions should be taken into consideration when determining protein usage in food and feed applications.
Polysaccharides
Presence & bioactivity: Polysaccharides make up a large part, 20-40% of the total dry weight mass of Porphyra, commonly found in the extracellular matrix and cell structures [114]. Porphyra dry weight consists for 65% w/w of cell wall material, comprising mostly fibrillar cellulose, glycoproteins and sulphated galactans as phycocolloids [76]. Algal polysaccharides, including those from Porphyra are only partly digestible by the human digestion system. Undigested polysaccharides are deemed qualitative dietary fibres in food and feed applications, and hence deemed important for a proper functioning of the human digestive track. Often, the soluble carbohydrates and the dietary fibres are quantified since they are of interest for their application in food, cosmetics and pharmaceuticals [115] or for their antioxidant properties [116]. For the Hawaiian seaweed P. vietnamensis 30.5 g/100g DW of soluble carbohydrates was reported [108]. For Porphyra sp. from the Azores a soluble carbohydrate analysis of 25.37 g/100g DW was reported [78]. This in contrast to soluble polysaccharide concentrations of 3% DW found in Py. Yezoensis after hot water extraction [117]. This is shown in Table 3.
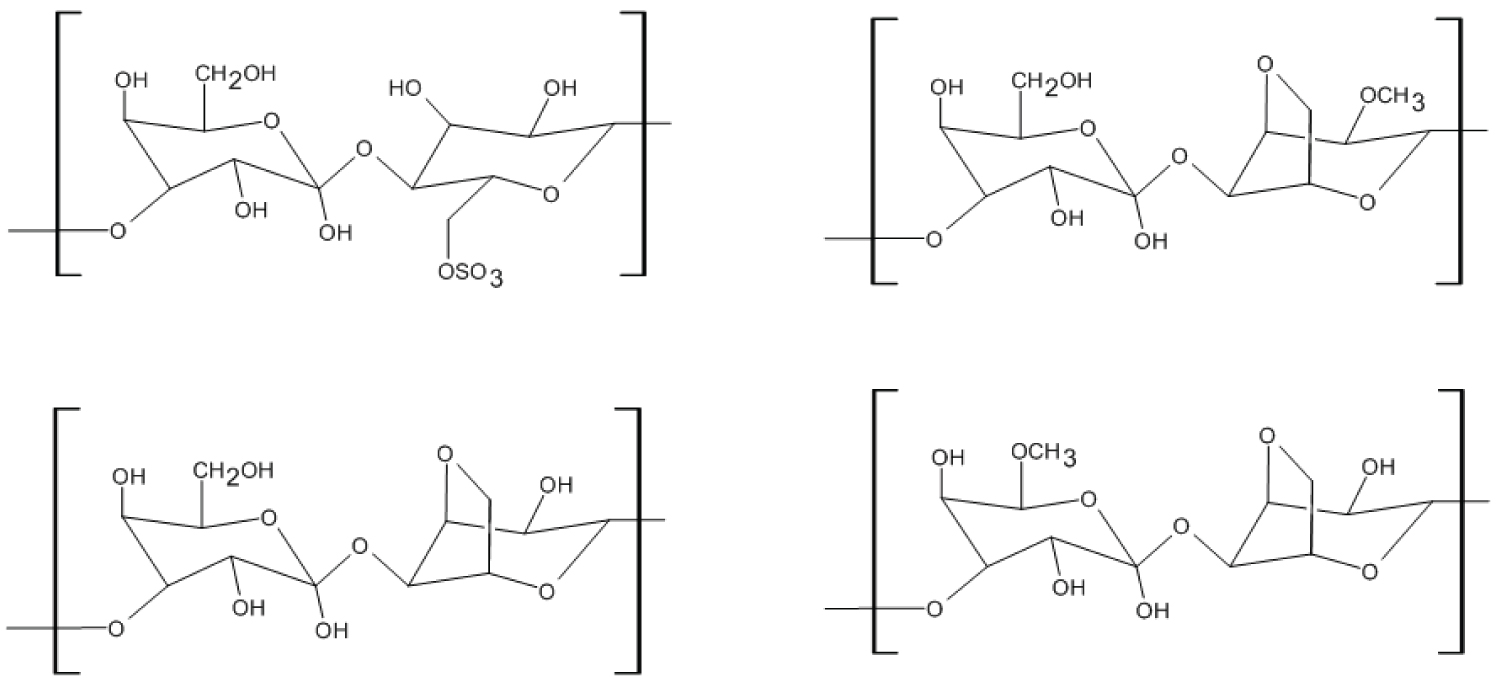
In Porphyra specifically, the water soluble dietary fibre phycocolloid porphyran is gathering attention. Porphyran generally consists of 3-linked β-D-galactose and 4-linked α-L-galactose 6-sulfate, with some partial modification in different subunits, as shown in Figure 3 [118,119]. Porphyran can encompasses more than 40% of the dry weight of Porphyra [14]. Porphyran is being researched for possibly being health beneficial such as its anti-inflammatory and antioxidant capacity [120,121,122]. A research in rats showed that porpyrans are capable of moderate neurological motor function improvement [123].
Induction: Seasonal variation influences the total carbohydrate concentration, varying between respectively 39.4 and 47.2 g/100g DW between December and February for Korean P. yezoensis samples where total dietary fibre ranged between 27.2-34.9 g/100g DW, insoluble dietary fiber concentrations ranged between 18.5-26.9 g/100g DW and soluble dietary fiber ranged 4.9-8.4 g/100g DW [112]. The total carbohydrate content of P. dioica gathered in Galway was found at 57.48% DW in July and at 26.21% DW in December, although no analysis method was described [91]. For P. capensis no clear seasonal influence in total sugar content was found, due to high variability in sugar concentrations [124]. For Bangiophyceae it was suggested that low molecular weight carbohydrate concentrations and compositions are species dependant and might be identified based on patterns in their low molecular carbohydrate content [125].
Analytical methods: An often-used analytical method for soluble carbohydrates is phenolic sulfuric acid colorimetry [78-108], first described by Dubois in 1956 [126]. In this assay, samples are hydrolysed under acidic conditions, often using sulfuric acid. Then, under presence of phenol and sulfuric acid, spectrophotometric analysis at 490 nm is compared to a glucose standard. This method is applicable to free sugars, methylated sugars and oligo-and poly-saccharides. Another often-used technique is indirect calculation of carbohydrate quantification by determining other proximate substituents (protein, ash, fatty acids and residual water) and stating the residual weight as the carbohydrate fraction [112]. However, it has to be mentioned that this indirect approach is prone to inaccuracies based by the analysis of other constituents and the inclusion of unidentified and unquantified constituents in the residual weight. Polysaccharide extraction was optimized for hot water extraction, finding an optimum temperature of 80 ℃, solid:liquid ratio of 1:20 and an extraction time of 2h. This resulted in a yield of 3% for Py. yezoensis [117]. Microwave assisted extraction of polysaccharide from P. haitanensis showed a maximum yield 3.6% [127]. Extraction methods based on water at elevated temperatures show lower yield of soluble polysaccharides. For monomeric sugar composition analysis, either directly extracted or after depolymerization, Gas Liquid Chromatography (GLC) [128] or High-Performance Anion Exchange Chromatography (HPAEC) are often chosen. For oligosaccharides and polysaccharide linkage composition analysis Gas Chromatography Mass Spectrometry (GC-MS). For obtaining detailed oligosaccharide and polysaccharide profiles and compositions, Matrix Assisted Laser-induced Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) and ultra-high-performance liquid chromatography mass spectrometry (UHPLC-MSn) methods can be used. These methods are highly precise, but are also costly and time-consuming. Furthermore, sample preparation is more complicated, exemplified by degradation of sugars during alkaline and/or acid depolymerization influencing the saccharide composition and functional groups. Another analytical method for primary hydrocolloid identification, such as porphyran, is Fourier Transformed Infrared spectroscopy (FTIR). Using FTIR analysis, sun-dried and ground macroalgae amongst others can be qualitatively analysed to identify their primary hydrocolloid [129].
Fatty acids
Presence and bioactivity: Fatty acids from marine environment, most notably omega 3 fatty acids such as C20:5ω3 eicosapentaenoic acid (EPA) and C22:6ω3 docosahexaenoic acid (DHA) as shown in Figure 4, are often cited as health beneficial. Fatty acids are researched for their affectability as anti-inflammatories, antioxidants and cardio-vascular enhancers [131]. Fatty acids are also being researched as alternative lipid source for fuels [132]. The predominant fatty acids in Porhyra are the omega-3 fatty acid C20:5ω3 eicosapentaenoic acid and the saturated fatty acid C16:0 hexadecanoic acid (palmitic acid). The dominant fatty acid for Py. yezoensis is EPA, comprising more than 50% of the total fatty acid content during all seasons [112]. Palmitic acid was reported as the most present fatty acid at 46.5-57.6% of total lipid content in commercially bought dried P. tenera [133]. It should be noted that eicosapentaenoic acid was not identified. Work on the lipidome for Porphyra in different life stages is ongoing, highlighting the potential for Porphyra as possible source of health beneficial fatty acids [134].
The crude lipid content was determined 4.4 g/100g DW in P. vietnamensis [108]. A crude lipid content of 8.88 g./100g DW was found in Porphyra sp, where it must be noted that a 4 hour Soxhlet reflux was used to increase lipid yield, instead of the often used solid-solvent maceration [78]. For Porphyra sp. a total lipid content of 1.03 g/100g DW is reported using chloroform:methanol extraction [109]. An overview of crude lipid content is shown in Table 4.
Induction: The effect of abiotic factors on the lipid composition in Porphyra is not well-researched. Induction trials with abiotic factors to optimize fatty acid production in red seaweeds have not been published to the knowledge of the authors. Within research towards the seasonal variability of Porphyra composition, lipids are more often analysed. P. dioica was sampled in June and Nov 2021 from Galway Bay, western Ireland and showed respectively 0.8 ± 0.2% DW and 1.7 ± 0.5% DW of total fatty acid. The percentage PUFA within the total fatty acid was in both seasons around 45% [135]. Crude lipid content for P. yezoensis was found to be between 0.7 ± 0.2 and 1.1 ± 0.2 during a full growth season from November 2011 to March 2012 in Korea. PUFA compositions were found between 59.6% and 64.6% of the total fatty acid composition [112].
Analytical methods: Lipid extraction is often done using an hydrophobic chloroform:methanol extraction solvent. For quantitative crude lipid analysis, crude lipid quantities are often determined gravimetrically, by evaporation of solvent and weighting. It must be noted that photoactive molecules such as chlorophyll are also extracted efficiently in organic liquids such as chloroform and methanol [136] and can comprise towards 0.5 g/100g DW [137]. Therefore, spectrophotometric analysis for chlorophylls may be used on crude lipid extracts, in order to partly account for additional untargeted compounds [138]. Quantitative fatty acid profiling is usually performed using LC-MS [139] or via GC-MS after transmethylation for FAME analysis (Fatty Acid Methyl Ester) [140,141], identifying and quantifying individual fatty acids.
Pigments and aromatic constituents
Presence: Carotenoids are class of pigment compounds found in photosynthetic bodies of Porphyra, extracted as part of the crude lipid content. The order of Bangiales, including both Porphyra and Pyropia is described as belonging to the Lutein group, as lutein is often the most found abundant carotenoid, although variances between species occur [142]. P. perforate was shown to have a carotenoid signature of 35% lutein, 11.4% zeaxanthin, 4.1% α-carotene and 49.5% β-carotene based on total carotenoid content. This composition did not stroke with the classification as lutein dominant. For P. suborbiculata a carotenoid composition of 65% lutein and 35% zeaxanthin was reported [142]. In Py. yezoensis also lutein, zeaxanthin, α-carotene, and β-carotene were described as the major carotenoids, with Lutein being predominant [143]. After a 45 day laboratory cultivation of three strains of Py. haitanensis, chlorophyll α concentrations between 7.68 ± 0.14 - 9.37 ± 0.20 mg.g-1 DW, phycoerythrin concentrations between 40.80 ± 1.00 - 51.00 ± 2.28 mg.g-1 DW and phycocyanin concentrations between 31.26 ± 0.80 - 48.36 ± 0.81 mg g-1 DW were found, being an example of interstrain variation that can occur under identical growth conditions [144].
Induction: For P. yezoensis it was shown that the chlorophyll α concentration was higher in March than in January for samples harvested in Nantong, China for two of three tested strains. However, it must be noted that interstrain variation was also significant, with the third tested strain showing lower chlorophyll α levels in March [137]. For the phycobiliprotein phycoerythrin in P. dioica it was reported that concentrations in winter were higher than in summer in Brittany, France [10]. For the phycobiliproteins phycoerythrin, allophycocyanin and phycocyanin higher concentrations were found in January in relation to March across 3 strains of P. yezoensis with concentrations in March being between 6-8 mg.g-1 FW for all three phycobiliproteins [137]. For P. linnearis and P. umbililcalis respectively 29 mg g-1 FW and 26 mg g-1 FW of phycoerythrin were reported, with highest concentrations at 100 ℃ and 250 µmoles L-1 ammonium in a laboratory setting [33]. Photosynthetic pigment content is higher in shaded blades in comparison to sun exposed blades for P. umbilicalis with antioxidant capacity increasing most in summer [145]. For Porphyra and Pyropia species it was shown from meta-analysis that xanthophyll concentrations are mostly enhanced under hypersaline conditions and under increased lighting conditions. It was suggested that pre-harvesting 24 hour induction with PAR radiation at 100 µmol photons m-1 s-1 and a 12:12 light:dark cycle would result in optimal xanthophyll levels for Porphyra and Pyropia species, with Pyropia species showing higher total carotenoid content and Porphyra showing higher lutein and zeaxanthin concentrations [146].
Analysis: The analysis of chlorophylls and carotenoids is often performed using photo spectroscopic techniques, such as UV/Vis spectroscophy for rapid scanning and high performance liquid chromatography for component separation, identification and quantification [143,147]. Techniques around the analysis of carotenoids and chlorophylls are mostly based on their light-absorbing property, as they are natural pigments. For identification of breakdown components and elucidation of decay pathways, mass spectroscopy is an often-used technique, with LC-MS-MS being the preferred method and advances are made in the elucidation of carotenoid metabolomics [148].
Inorganic constituents, minerals and moisture content presence
Inorganic material, macro- and micro-minerals and metals are often grouped together under ash content in proximate compositions and can encompass up to 20-30% DW. Macroalgae, including Porphyra are being researched as nutraceuticals and food for their favourable mineral content [141,45,149]. Macroalgae can also take up heavy metals, but concentrations are very species dependent. Heavy metal concentrations from macroalgae, including Porphyra, collected in Norway in October 2014 showed the presence of heavy metals, but concentrations mostly remained below concentrations for EU regulations in food or feed [150].
Porphyra sp. was incinerated at 550 ℃ for 2-3h and showed an ash content of 28.16% DW [78]. For P. vietnamensis an ash content of 25.2 g/100g DW was found after heating for 4h at 550 ℃ [108]. An ash content between 7.25 and 3.76 g/100g DW for Py. yezoensis after heating for 5h at 500 ℃ [151]. Following AOAC guidelines, an ash content of 6.46 g/100g DW was found for P. columbina [152]. For Porphyra sp. an ash concentration of 19.07 g/100g DW was found after heating for 5 h in an electric oven at 525 ℃ [109].
Induction: There is seasonal variation in ash content of Porphyra spp. over a two year time period, ash contents between 12.0 and 18.7 g/100g DW were found for multiple different species [153]. These seasonal differences were also shown for mineral content in Porphyra, collected between January and April on multiple sites of the South Korean coast [151]. Ecological conditions, such as urban proximity, also influence mineral concentrations in macroalgae [74,154].
Analytical methods: Ash content is often determined gravimetrically after incineration of dried material at temperatures of 500 ℃ or higher for multiple hours. For mineral composition determination, samples are often acid digested and analysed via atomic absorption spectroscopy (AAS) [152] or via Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) [151]. Alternatively, ion chromatography coupled to conductivity analysis can be used on aqueous extracts [78]. Macroalgal samples should be rinsed with freshwater before drying, to cleanse samples from adherent seawater.
Moisture content: Moisture content of Porphyra is often gravimetrically determined. Using oven drying at 600C, McDermid reported for P. vietnamensis a water content of 90.3 g/100g FW [108]. Paiva [78] reported a water content of 87.1 g/100g FW for Porphyra sp. Mok [154] reported a moisture content for Py. yezoensis of 89.2 g/100g FW (Table 5 and Table 6).
Mycosporine-Like Amino Acids
Special attention in this review is given to the Mycosporine-like amino acids (MAA's), as this group of cellular constituents may have future broad applications as UV-photoprotectant in cosmetics. Mycosporine-like amino acids are currently a topic of research and application and prototype development using MAA's extracts are starting to be published.
Chemical properties
Mycosporine-like amino acids (MAA's) are founds in a wide variety of different organisms, ranging from marine macroalgae, to freshwater microalgae to terrestrial microalgae [155,156,157,158,159] as well as fungi, marine invertebrates and even fish [160,161,162]. The primary function of MAA's is to protect the organism from the harmful effects caused by UV irradiation which are amongst others photosynthesis inhibition, DNA damage and protein denaturation [65]. Over 30 MAA's and MAA's derivatives are identified and their chemical properties researched [163]. For Porphyra, the most predominantly found MAA's are porphyra-334, shinorine and palythine [164,165], where other MAA's such as asterina are sometimes found [34].
MAA's typically have a molecular weight below 400 Da and consist of cyclohexenone or cycloheximine backbone with a conjugated amino acid [5]. MAA's exhibit molar extinction coefficients up to 50.000 M-1 cm-1 with absorption maxima between 309-362 nm [60], with a reported molar extinction efficient for respectively Porphyra-334 at 42,300 M-1 cm-1 in [166] and for Shinorine at 44,700 M-1cm-1 [163] in aqueous solutions at 334 nm. MAA's are colourless and soluble in both aqueous and hydrophilic organic solvents.
MAA's have shown to be resistant to heat stress. A mycosporine-like amino acid extract of the red seaweed species Gracilaria cornea consisting mostly of porphyra-334 and/or shinorine showed no significant decrease in absorption after heat treatment at 75 ℃ for six hours [191]. Porphyra-334 has been reported to be stable for over 80 days at room temperature. When treated at 60 ℃, porphyra-334 slowly decreased in absorbance in solution at pH 1-11 over a 25 hour timeframe. At a temperature of 80 ℃ this decrease was more rapid. At pH 12-13, porphyra-334 showed rapidly declining absorbance over a 4 hour period, also at room temperature [169].
A selection of MAA's showed antioxidant activity [163]. Mycosporine-glycine had the highest antioxidant activity, followed by asterina-330 and palythine [166], while as Porphyra-334 and shinorine showed scarce scavenging activity [167]. Abiotic environmental characteristics play a major role in bioactivity of MAA's. Porphyra-334, shinorine and palytine were reported to be more active than synthetic phenolic antioxidants in Folin-Ciocalteu assay's, while being less active than these standard compounds in the radical scavenging ABTS bioassay and Ferric Reducing Antioxidant Power (FRAP) assay [168]. This while all of these assays have the same mode of electron transfer. Since Folin-Ciocalteu assay is done at pH around 8 and ABTS and FRAP assay's are done at acidic pH, this would mean that MAA's bioactivity is higher in slightly alkaline conditions compared to acidic conditions [168]. As with heat stability, antioxidant activity is heavily pH reliable, often showing higher bioactivity at slightly alkaline pH [169]. An overview of the most common MAA’s and their structures is shown in Table 7.
Presence and biosynthesis of mycosporine-like amino acids
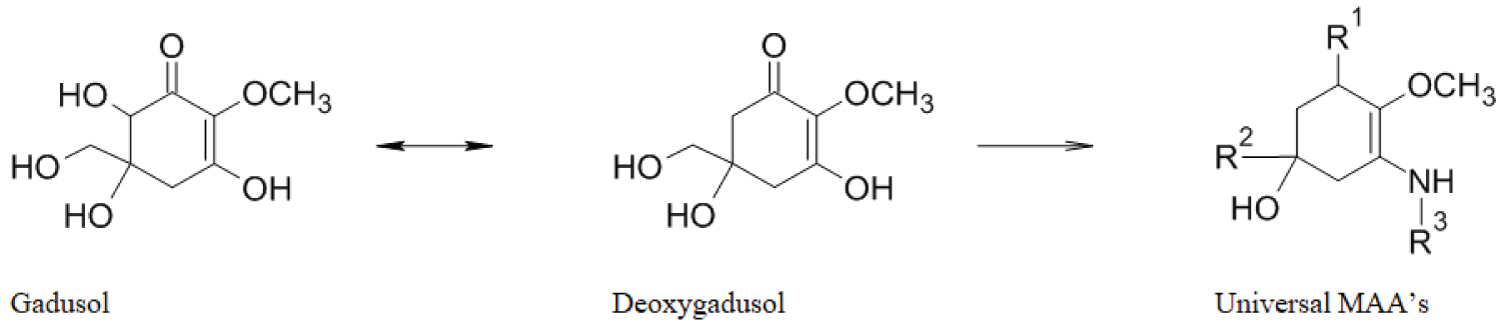
Mycosporine-like amino acids are deemed to be synthesized following the shikimate pathway [5], present in both in eukaryotic as prokaryotic organisms. It is opted that mycosporine-glycine is synthesised from a gadusol precursor, then being chemically converted via deoxygadusol into other mycosporine-like amino acids to cover a wide range of UV-radiation, as shown in Figure 5 [170]. The Shikimate pathway is not found in animals, thus accumulation of MAA ‘s in these organisms is supposedly done by ingestion of MAA's from algal diets [171]. An overview of often found MAA's is given in Table 8, showing variations in presence of MAA's in Porphyra.
Induction of mycosporine-like amino acids
Induction of MAA's biosynthesis may be achieved by induction with PAR radiation and/or UV irradiation [172-176]. This seems to be species dependent, with publications either observing or not observing induction. The fact that species belonging to the genus Porphyra are commonly found in the intertidal area, makes it logical that these organism have (elevated) UV reducing compounds such as MAA's. There are also seasonal variations within MAA's content and concentration in Porphyra, including P. dioica [177], although this was not found for P. dioica sampled in January and July on the coast of Brittany [10]. As discussed by [177], more research is needed to elucidate interactive effects of PAR and UV, depth, salinity, temperature and nutrients on MAA's accumulation. There was no linear combination for the independent variables light and temperature that explains MAA's accumulation in P. dioica. It is to be expected to exposure to UVA or UVB would increase MAA concentrations/composition. It was shown that 300 μM ammonium increased MAA's accumulation in P. leucostica and P. umbilicalis, but 0 and 100 μM ammonium only lowered MAA's content in P. leucostica [34], possibly indicating nitrogen-regulated induction. A division into three physiological algal groups was proposed, based on their MAA's accumulation [5]. This system details the three groups as containing:
• High initial MAA's concentrations that are not further enhanced through light/UV induction.
• Lower initial MAA's concentrations that can be enhanced by light/UV induction.
• Low initial MAA's concentrations that are not enhanced through light/UV induction.
Using this division, P. endiviifolium and P. umbilicalis were classified as having high initial MAA's content with no induction from a treatment with PAR light or UV radiation. P. plocamiestris was classified as having low initial MAA's content with induction during light/radiation treatment. This is in line with results found for P. endiviifolium, but is contradictory with results found for P. plocamiestrisis. MAA's in Porphyra plocamiestrisis were induced under UVA and UVB, while Porphyra endiviifolium shows induction under UVA with no additional benefit of UVB induction [172]. For Porphyra umbilicalis high initial MAA's concentrations were found, not further enhanced with UV induction, in line with the proposed physiological classification system [165]. Although research has been published towards the accumulation of MAA's in Porphyra, consensus has not been reached on the influence of UV-induction and other abiotic factors on the biosynthesis and enhanced accumulation of MAA's in red seaweeds.
Isolation and detection of MAA's
MAA's samples are usually aqueous solutions, often partly purified or crude extracts [10]. An often used method for extraction and purification of MAA's from macroalgal samples is extraction in 20% aqueous methanol at 45 ℃ for 2.5 hours. Then after airdrying, samples are redissolved in 100% methanol to remove methanol insoluble components sugars after which methanol is evaporated and MAA's are redissolved in water ready for analysis [173,178,179]. A second option is extracting in 0.2% aqueous acetic acid with 0.5% methanol at 4 ℃ for 12 hours on a shaker after which samples are centrifuged and filtered [180]. This method is specifically designed as a universal crude method from both prokaryotic and eukaryotic samples.
A third extraction method, often used on microbial biomass [181] is overnight maceration in methanol at 4 ℃. Then the extract is centrifuged and methanol is evaporated to dryness at 45 ℃. afterwards, extracts are redissolved in water, adding a drop of chloroform to remove apolar organic impurities such as chlorophyll before centrifugation and filtration [182].
Other less often-used purification methods are also reported, such as purification by solid-phase extraction [183] or preparative thin layer chromatography [184].
HPLC analysis of MAA's is commonly done using a C18 reversed phase column. The mobile phase is mostly an isocratic aqueous solution with 1-0.02% acetic acid. One research group [185,186] describes an isocratic aqueous liquid phase consisting of 0.1% acetic acid and 25% methanol. Commonly, these analyses have a run time between 15-30 minutes and show a high retention. Detection is done spectrophotometrically in the 280-360 nm range using Diode array detection (DAD). Since pure MAA's standards are not readily available, peak identification is often done by comparison of retention time, retention pattern and absorption maxima of known MAA's published in older publications. Due to the same reason quantification is often done by using approximate molar extinction coefficients, instead of a more accurate methods like an internal standard or pure compounds as external standard. One must be aware that this can become inaccurate, especially in samples with volatile and complex MAA's distribution profiles [172,187].
A quantitative analysis using capillary electrophoresis as separation with diode-array detection is reported [188]. Here, the liquid phase consists of a 30 mM aqueous sodium tetraborate solution at pH 10.3 Separation voltage, temperature and detection wavelength were respectively 25kV, 25 ℃ and 320 nm. The stationary phase consisted of fused silica capillary with 75 µm internal diameter and 80 cm effective length. This method is validated for quantitative determination of MAA's in, amongst others, P. umbilicalis in a run time of approximately 30 minutes.
Commercial usage of MAA's
MAA's are currently being offered as UV-active macroalgal extract for cosmetics such as HelioGuard 365 from Mibelle Biochemistry [189] and Helionori from Gelyma [189]. It must be mentioned that MAA's are currently not allowed as primary active UV-filter in sunscreens, but are only allowed as secondary UV-filter by the European Chemicals Agency (ECHA) and the American Food & Drug Administration (FDA). However, the Environmental Effects Assessment Panel (EEAP) have showed their concerns about the potential hazards caused by common used UV-filters and has mentioned MAA's as possible solution [190]. Research has been done to MAA's, but more focus on quantification would be beneficial as absolute concentrations are often unknown. A rise in availability of analytical standards would promote quantitative analysis. Further research could focus more on induction and applicability of MAA's, rather than comparisons of MAA's between different species.
Conclusion
When reviewing the presence & bioactivity, induction and isolation of Porphyra constituents, the broad possibilities and applications of Porphyra and its constituents are obvious.
Although advances are made in Porphyra taxonomy, identification of samples from the field keeps being problematic until genetic identification is more readily available and affordable. Differences in taxonomic approach does complicate the interpretation of published scientific literature. This complication should be thought of when reviewing published knowledge. However, with the rise of genomic identification, these issues will phase out over time.
Publications about the chemical composition of Porphyra are readily available. Major differences in reported concentrations of constituents have been showed in this review. In order to gain understanding of these variations, abiotic & biotic and seasonal factors should be researched and clarified. For example, individual differences in chemical compositions between Porphyra specimen should be connected to their specific growth conditions. Mapping the influence of external factors on chemical compositions will, over time, favourably impact cultivation and usages of Porphyra. Due to these major differences in chemical composition, research towards local growth and chemical composition is incentivized in order to increase our understanding of external factors on Porphyra constituents.
The reliability, accuracy and preciseness of analytical procedures are widely varying. It is advised to consider the robustness of the analytical technique, in the context of the research. For example, when determining protein content by performing a Kjeldahl-based technique, a Protein Conversion Factor should be determined instead of usage of the universal standard of 6,25 which is often not applicable to Porphyra. The preciseness or robustness of the conclusion that is given, should correspond to the robustness and preciseness by the analytical technique that is being applied. Use of standard reference material should be encouraged.
A prime example of advances in application of Porphyra constituents is the application and research towards Mycosporine-like amino acids as photo-protectant in sun creams and cosmetics. These advances have led to consideration of MAA's as novel UV-blocker. With all effort towards Porphyra constituents, more product applications are to follow in time.
Porphyra is an economic important species, mostly in Asia. Due to global interest in Porphyra, both as food, feed and as nutraceutical, a substantial increase in Porphyra cultivation is expected. However, especially in Europe, there is still a development gap between knowledge of and industrial utilization of Porphyra. The transition from scientific research towards economically and technically feasible industrial applications has yet to be made. Taking into consideration the forecasted growth of Porphyra industry in Asia and the abundancy of wild Porphyra in Europe, opportunities are presenting itself. It is advantageous from an economic point of view to aim for full biomass valorisation. Few process flow diagrams for total Porphyra valorisation are being published [190]. Due to this insight total biorefinery of Porphyra biomass and side streams is starting to become a bigger research point. This can lead to economical gain of Porphyra as prime aquaculture crop, also outside of Asia.
References
- Guillemin ML (2016) The bladed Bangiales (Rhodophyta) of the South Eastern Pacific: Molecular species delimitation reveals extensive diversity. Molecular Phylogenetics and Evolution 94: 814-826.
- Yang LE, Deng YY, Xu GP, et al. (2020) Redefining Pyropia (Bangiales, Rhodophyta): Four New Genera, Resurrection of Porphyrella and Description of Calidia pseudolobata sp. nov. From China. Journal of Phycology 56: 862-879.
- Pimentel FB (2020) Effect of in vitro simulated gastrointestinal digestion on the antioxidant activity of the red seaweed Porphyra dioica. Food Research International 136: 109309.
- Wang X (2020) Economically important red algae resources along the Chinese coast: History, status, and prospects for their utilization. Algal Research 46: 101817.
- Singh SP, Kumar S, Rastogi RP, et al. (2008) Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian Journal of Experimental Biology 46: 7-17.
- Koh YH, Kim MS (2018) DNA barcoding reveals cryptic diversity of economic red algae, Pyropia (Bangiales, Rhodophyta): description of novel species from Korea. Journal of Applied Phycology 30: 3425-3434.
- Meynard A (2019) Genetic and morphological differentiation of Porphyra and Pyropia species (Bangiales, Rhodophyta) coexisting in a rocky intertidal in Central Chile. Journal of Phycology 55: 297-313.
- Patra JK, Lee SW, KwonYS, et al. (2017) Chemical characterization and antioxidant potential of volatile oil from an edible seaweed Porphyra tenera (Kjellman, 1897) Chemistry Central Journal 11: 1-10.
- KhairyHM, El-shafay SM (2013) Seasonal variations in the biochemical composition of some common seaweed species from the coast of abu qir bay alexandria egypt. Oceanologia 2: 435- 452.
- Lalegerie F, Lajili S, Bedoux G, et al. (2019) Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Marine Environmental Research 147: 37-48.
- Green LA, Neefus CD (2016) Effects of temperature, light level, and photoperiod on the physiology of Porphyra umbilicalis Kützing from the Northwest Atlantic, a candidate for aquaculture. Journal of Applied Phycology 28: 1815-1826.
- Sutherland JE, Lindstrom SC, Nelson WA, et al. (2011) A new look at an ancient order: Generic revision of the bangiales. Journal of Phycology 47: 1131-1151.
- Brodie J, Irvine L, Neefus CD, et al. (2008) A molecular and morphological redescription of the species, with a typification update. Taxon 57: 1328-1331.
- Cao J, Wang J, Wang S, et al. (2016) Porphyra species: A mini-review of its pharmacological and nutritional properties. Journal of Medicinal Food 19: 111-119.
- Yang LE, Lu QQ, Brodie J, et al. (2017) A review of the bladed Bangiales (Rhodophyta) in China: history, culture and taxonomy. European Journal of Phycology 52: 251-263.
- Kong F, Sun P, Cao M, et al. (2014) Complete mitochondrial genome of Pyropia yezoensis: Reasserting the revision of genus Porphyra. Mitochondrial DNA 25: 335-336.
- Lee MK, Kim IH, Choi YH, et al. (2015) A peptide from Porphyra yezoensis stimulates the proliferation of IEC 6 cells by activating the Insulin-like growth factor I receptor signaling pathway International Journal of Molecular Medicine 35: 533-538.
- Cho TJ, Rhee MS (2018) Health Functionality and Quality Control of Laver (Porphyra, Pyropia): Current Issues and Future Perspectives as Edible Seaweed. Marine Drugs 18: 14.
- Zhang J, "Seaweed Industry in China," Beijing, 2018.
- Kim JK, Charles Y, Eun Kyoung H, et al. (2017) Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 32: 1-13.
- Gittenberger A, Rensing M, Dekker R, et al. (2015) "Native and non-native species of the Dutch Wadden Sea in 2014," Office for Risk Assessment and Research, The Netherlands Food and Customer Product Safety Authority of the Ministry of Economical Affairs.
- Sánchez N, Vergés A, Peteiro C, et al. (2014) Diversity of bladed Bangiales (Rhodophyta) in Western Mediterranean: Recognition of the genus Themis and descriptions of T. ballesterosii sp. nov., T. iberica sp. nov., and Pyropia parva sp. nov. Journal of Phycology 50: 908-929.
- Gunnarsson K, Egilsdóttir S, Nielsen R, et al. (2016) A collections-based approach to the species and their distribution based on the bladed Bangiales (Rhodophyta) of Iceland. Botanica Marina 59: 223-229.
- Lovatelli Cai J, Aguilar-Manjarrez A, Cornish J, et al. (2021) F A O Fisheries (2021) Seaweeds and microalgae: An overview for unlocking their potential in global aquaculture development 1229.
- Ferdouse F, Holdt SL, Smith R, et al. (2018) The global status of seaweed production, trade and utilization.
- Knoop J, Griffin JN, Barrento S, et al. (2020) Cultivation of early life history stages of Porphyra dioica from the British Isles. Journal of Applied Phycology 32: 459-471.
- Redmond S, Green L, Yarish C, et al. (2014) "New England Seaweed Culture Handbook," New England Seaweed Culture Handbook, 92.
- Silva DM, Silva DM, Luisa Valente MP, et al. (2015) Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. Journal of Applied Phycology 27: 1671-1680.
- Blouin N, Xiugeng F, Peng J, et al. (2007) Seeding nets with neutral spores of the red alga Porphyra umbilicalis (L.) Kützing for use in integrated multi-trophic aquaculture (IMTA). Aquaculture 270: 77-91.
- Park M, Shin SK, Do YH, et al. (2018) Application of open water integrated multi-trophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 497: 174-183.
- Kim JK, Yarish C, Hwang EK, et al. (2017) Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. 32: Korean Society of Phycology 32: 1-13.
- Fortes MD, Lfining K (1980) Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgolander Meeresunterss 34: 15-29.
- Kim JK, Kraemer GP, Neefus CD, et al. (2007) Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. Journal of Applied Phycology 19: 431-440.
- Korbee N, Huovinen AP, Figueroa AFL, et al. (2004) Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Marine Biology 146: 645-654.
- Figueroa FL, Aguilera J, Niell FX, et al. (1995) Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). European Journal of Phycology 30: 11-18.
- Pereira R, Yarish C, Sousa-Pinto I, et al. (2006) The influence of stocking density, light and temperature on the growth, production and nutrient removal capacity of Porphyra dioica Bangiales, Rhodophyta Quimica Nova 252: 66-78.
- Hwang EK, Yotsukura N, Pang SJ, et al. (2019) Seaweed breeding programs and progress in eastern Asian countries. Phycologia 58: 484-495.
- Jiang H, Ding H, Zhang P, et al. (2020) Selection and characterization of an improved strain (A-13) of Pyropia yezoensis (Bangiales, Rhodophyta) Aquatic Botany 163: 103213.
- Blouin NA, Brodie JA, Grossman AC, et al. (2011) Porphyra: A marine crop shaped by stress Trends in Plant Science 16: 29-37.
- Cao Y, Green-gavrielidis LA, Eriksen RL, et al. (2019) A pilot study of genetic structure of Porphyra umbilicalis Kützing in the Gulf of Maine using SNP markers from RNA-Seq 3: 1493-1503.
- Varela-Alvarez E, Paulino C, Serrao EA, et al. (2017) Development and characterization of twelve microsatellite markers for Porphyra linearis Greville 145: 127-130.
- Royer CJ (2017) Advancing Development of Porphyra umbilicalis as a Red Algal Model System and Aquaculture Crop. Dissertation.
- Brawley SH (2017) Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis.
- Saga N, Yukiwro Kitadep Porphyra : A model plant in marine sciences. Fisheries Science 68: 1075-1078.
- Wells ML, Potin P, Craigie JS, et al. (2017) Algae as nutritional and functional food sources: Revisiting our understanding. Journal of Applied Phycology 29: 949-982.
- Fleurence J, Morançais M, Dumay J, et al. (2018) Seaweed proteins in Proteins in Food Processing: Second Edition. Elsevier Inc 245-262.
- Chen X, Wu M, Yang Q, et al. (2017) Preparation, characterization of food grade phycobiliproteins from Porphyra haitanensis and the application in liposome-meat system. LWT - Food Science and Technology 77: 468-474.
- Garcia-Vaquero M, Hayes M (2016) Red and green macroalgae for fish and animal feed and human functional food development. Food Reviews International 32: 15-45.
- Angell AR, Angell SF, Nys R de, et al. (2016) Seaweed as a protein source for mono-gastric livestock. Trends in Food Science and Technology 54: 74-84.
- Malcorps W (2019) The sustainability conundrum of fishmeal substitution by plant ingredients in Shrimp Feeds. Sustainability 11: 1-19.
- Øverland M, Mydland LT, Skrede A, et al. (2019) Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. Journal of the Science of Food and Agriculture 99: 13-24.
- Wan AHL, Davies SJ, Soler-Vila A, et al. (2018) Macroalgae as a sustainable aquafeed ingredient. Reviews in Aquaculture 1-35.
- Walker AB, Fournier HR, Neefus CD, et al. (2009) Partial Replacement of Fish Meal with Laver Porphyra spp. in Diets for Atlantic Cod. North American Journal of Aquaculture 71: 39-45.
- Harnedy PA, FitzGerald RJ (2011) Bioactive proteins, peptides, and amino acids from macroalgae. Journal of Phycology 47: 218-232.
- Bito T, Teng F, Watanabe F, et al. (2017) Bioactive Compounds of Edible Purple Laver Porphyra sp. (Nori)," Journal of Agricultural and Food Chemistry 65: 10685-10692.
- Blouin N, Calder BL, Perkins B, et al. (2006) Sensory and fatty acid analyses of two Atlantic species of Porphyra (Rhodophyta). Journal of Applied Phycology 18: 79-85.
- Venkatraman KL, Mehta A (2019) Health Benefits and Pharmacological Effects of Porphyra Species. Plant Foods for Human Nutrition 74: 10-17.
- Shannon E, Abu-Ghannam N (2019) Seaweeds as nutraceuticals for health and nutrition. Phycologia 58: 563-577.
- Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: Functional food applications and legislation. Journal of Applied Phycology 23: 543-597.
- Carreto JI, Carignan MO (2011) Mycosporine-like amino acids: Relevant secondary metabolites. chemical and ecological aspects. Marine Drugs 9: 387-446.
- Rosic NN (2019) Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens. Marine Drugs 17: 1-17.
- Chrapusta E, Kaminski A, Duchnik K, et al. (2017) Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Marine Drugs 15: 326.
- Downs CA, Kramarsky-Winter E, Segal R, et al. (2016) Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands," Archives of Environmental Contamination and Toxicology 70 : 265-288.
- de la Coba F, Aguilera J, Korbee N, et al. (2019) UVA and UVB Photoprotective capabilities of topical formulations containing mycosporine-like amino acids (maas) through different biological effective protection factors (BEPFs). Marine Drugs 17: 1.
- Pangestuti R, Siahaan EA, Kim SK, et al. (2018) Photoprotective substances derived from marine algae. Marine Drugs 16: 11.
- Choi YH, Yang DJ, Kulkarni A, et al. (2015) Mycosporine-like amino acids promote wound healing through focal adhesion kinase (FAK) and mitogen-activated protein kinases (MAP kinases) signaling pathway in keratinocytes. Marine Drugs 13: 7055-7066.
- Oyamada C, Kaneniwa M, Ebitani K, et al. (2008) Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Marine Biotechnology 10: 141-150.
- Kim S, You DH, Han T, et al. (2015) Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). Journal of Photochemistry and Photobiology B: Biology 141: 301-307.
- Ryu J, Park SJ, Kim IH, et al. (2014) Protective effect of porphyra-334 on UVA-induced photo aging in human skin fibroblasts," International Journal of Molecular Medicine 34: 796-803.
- Bux F, Chisty Y (2016) Algae Biotechnology - products and processes.
- Barbot Y, Al-Ghaili H, Benz R, et al. (2016) A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Marine Drugs 14: 120.
- Roleda MY, Hurd CL (2019) Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58: 552-562.
- Forbord S (2020) Latitudinal seasonal and depth-dependent variation in growth , chemical composition and biofouling of cultivated Saccharina latissima (Phaeophyceae) along the Norwegian coast. 23rd International Seaweed Symposium 2215-2232.
- Wallenstein FM, Couto RP, Amaral AS, et al. (2009) Baseline metal concentrations in marine algae from São Miguel (Azores) under different ecological conditions - Urban proximity and shallow water hydrothermal activity. Marine Pollution Bulletin 58: 438-443.
- Aitken KA, Melton LD, Brown MT, et al. (1991) Seasonal protein variation in the New Zealand seaweeds Porphyra columbina Mont. and Porphyra subtumens J. Ag. (Rhodophyceae)," The Japanese journal of Phycology. 39: 307-319.
- Cian RE, Drago SR, De Medina FS, et al. (2015) Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Marine Drugs 13: 5358-5383.
- Admassu H, Abera T, Abraha B, et al. (2018) Proximate, Mineral and Amino acid Composition of Dried Laver (Porphyra spp.) 6: 149-154.
- Paiva L, Lima E, Patarra RF, et al. (2014) Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chemistry164: 128-135.
- Biancarosa I, Espe M, Bruckner CG, et al. (2016) Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. Journal of Applied Phycology 29: 1001-1009.
- Lourenço SO, Barbarino E, De-Paula JC, et al. (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycological Research 50: 233-241.
- Harrysson H, Hayes M, Eimer F, et al. (2018) Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. Journal of Applied Phycology 30: 3565-3580.
- Lee HA, Kim IH, Nam TJ, et al. (2015) Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. International Journal of Molecular Medicine 36: 1701-1706.
- Cian RE, Martínez-Augustin O, Drago SR, et al. (2012) Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Research International 49: 364-372.
- Lafarga T, Acién-Fernández FG, Garcia-Vaquero M, et al. (2020) Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Research 48.
- Cermeño M, Stack J, Tobin PR, et al. (2019) Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food and Function 10: 3421-3429.
- Indumathi P, Mehta A (2016) A novel anticoagulant peptide from the Nori hydrolysate. Journal of Functional Foods 20: 606-617.
- Fan X, Bai L, Mao X, et al. (2017) Novel peptides with anti-proliferation activity from the Porphyrahaitanesis hydrolysate. Process Biochemistry 60: 98-107.
- Mao X, Bai L, Fan X, et al. (2017) Anti-proliferation peptides from protein hydrolysates of Pyropia haitanensis. Journal of Applied Phycology 29: 1623-1633.
- Admassu H, Gasmalla MAA, Yang R, et al. (2018) Identification of Bioactive Peptides with a-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp). Journal of Agricultural and Food Chemistry 66: 4872-4882.
- Pana C, Ma J, Tao F, et al. (2021) Novel insight into the antioxidant proteins derived from laver (Porphyra haitanensis) by proteomics analysis and protein based bioinformatics. Food Bioscience 101134.
- Stack J, Tobin PR, Gietl A, et al. (2017) Seasonal variation in nitrogenous components and bioactivity of protein hydrolysates from Porphyra dioica. Journal of Applied Phycology 29: 2439-2450.
- Fu S, Xue S, Chen J, et al. (2021) Effects of different short-term uv-b radiation intensities on metabolic characteristics of porphyra haitanensis. International Journal of Molecular Sciences 22: 1-15.
- Mæhre HK, Dalheim L, Edvinsen GK, et al. (2018) Protein Determination - Method Matters. Food 7:5.
- Connor JO, Meaney S, Williams GA, et al. (2020) Extraction of Protein from Four Di ff erent Seaweeds Using Three Different Physical Pre-treatment Strategies. Molecules 25: 2020.
- Kjeldahl J (1883) Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern,. Zeitschrift für analytische Chemie 22: 366-382.
- Nagappan T, Suraiza N, Rahmah N, et al. (2020) Bioprospecting Cultivated Tropical Green Algae, Caulerpa racemosa (Forsskal) J. Agardh: A Perspective on Nutritional Properties, Antioxidative Capacity and Anti-Diabetic Potential. Foods 9: 1313.
- Angell AR, Mata L, Nys R de, et al. (2016) The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. Journal of Applied Phycology 28: 511-524.
- Kambhampati S, Li J, Evans BS, et al. (2019) Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry. Plant Methods 15: 46.
- Escoubeyrou K, Tremblay L (2014) Quantification of free, dissolved combined, particulate, and total amino acid enantiomers using simple sample preparation and more robust chromatographic procedures Limnology and Oceanography. Methods 12: 421-431.
- Smutniak I, Rubaj J, Korol W, et al. (2017) Method validation for determination of amino acids in feed by UPLC. Accreditation and Quality Assurance 152: 247-252.
- Gorissen SHM, Crombag JJ R, Senden JMG, et al. (2018) Protein content and amino acid composition of commercially available plant based protein isolates. Amino Acids 50: 1685-1695.
- Darragh AJ, Garrick DJ, Moughan PJ, et al. (1996) Correction for Amino Acid Loss during Acid Hydrolysis of a Purified Protein 207: 199-207.
- Kiss D, Tóth R, Zurbó Z, et al. (2020) Determination of amino acid composition of foods by photometric methods, Part 2 - Determination of methionine, cystine, lysine and arginine. Journal of Food Investigation.
- Lowry OH, Rosebrough NJ, Farr AL, et al. (1951) Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193: 265-275.
- Bradford MM (1976) A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 254: 248-254.
- Outline C (2019) Quantification and Analysis of Proteins 187-214.
- Martinez-Hernandez GB, Castillejo N, del M Carrion-Monteagudo M, et al. (2018) Nutritional and bioactive compounds of commercialized algae powders used as food supplements. Food Science and Technology international 24: 172-182.
- Mcdermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. Journal of Applied Phycology 15: 513-524
- Sánchez-Machado DI, López-Cervantes J, López-Hernández J, et al. (2004) Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chemistry 85: 439-444.
- Smith JL, Summers G, Wong R, et al. (2010) Nutrient and heavy metal content of edible seaweeds in New Zealand. New Zealand Journal of Crop and Horticultural Science 38: 19-28.
- FAO/WHO/UNU (2002) Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition. Geneva.
- Shin DM, An SR, In SK, et al. (2013) Seasonal Variation in the Dietary Fiber, Amino Acid and Fatty Acid Contents of Porphyra yezoensis. Korean Journal of Fisheries and Aquatic Sciences 46: 337-342.
- Machado M, Machado S, Pimentel FB, et al. (2020) Amino acid profile and protein quality assessment of macroalgae produced in an integrated multi-trophic aquaculture system. Foods 9: 10.
- Percival E (1979) The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. British Phycological Journal 14: 103-117.
- Ahmed ABA, Adel M, Karimi P, et al.(2014) Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. Advances in Food and Nutrition Research 73: 197-220.
- Wu Y, Huo Y, Xu L, et al. (2020) International Journal of Biological Macromolecules Purification, characterization and antioxidant activity of polysaccharides from Porphyra haitanensis. International Journal of Biological Macromolecules 165: 2116-2125.
- Pan Y, Meng Y, Geng Y, et al. (2017) Study on Extraction of Porphyra Polysaccahride and its Antioxidant Properties. Advances in Engineering Research 153: 203-209.
- Geng L, Wang J, Zhang Z, et al. (2019) Structure and Bioactivities of Porphyrans and Oligoporphyrans. Current Pharmaceutical Design 25: 1163-1171.
- Morrice LM, McLean MW, Long WF, et al. (1984) Porphyran primary structure. Hydrobiologia 116: 572-575.
- Zhang Q, Li N, Zhou G, et al. (2003) In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacological Research 48: 151-155.
- Zhang Z, Zhang Q, Wang J, et al. (2010) Chemical modification and influence of function groups on the in vitro-antioxidant activities of porphyran from Porphyra haitanensis. Carbohydrate Polymers 79: 290-295.
- Qiu HM (2020) Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydrate Polymers 246: 116626.
- Sun C, Wu F, Chen D, et al. (2018) Therapeutic effects of polysaccharides extracted from Porphyra yezoensis in rats with cerebral ischemia/reperfusion injury. Archives of Biological Sciences 70: 233-239.
- Zhang Q (2005) Chemical characteristics of a polysaccharide from Porphyra capensis (Rhodophyta) Carbohydrate Research 340: 2447-2450.
- Karsten U, West JA, Zuccarello GC, et al. (1999) Low molecular weight carbohydrate patterns in the Bangiophyceae (Rhodophyta). Journal of Phycology 35: 967-976.
- Dubois M, Gilles KA, Hamilton JK, et al. (1956) Colorimetric Method for Determination of Sugars and Related Substances. analytical chemistry 28: 350-356.
- Yu P, Zhang Y (2017) Separation and purification of Porphyra haitanensis polysaccharide and its preliminary structural characterization. Separation Science and Technology (Philadelphia) 52: 1835-1842.
- Xu SY, Huang X, Cheong XL, et al. (2017) Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Marine Drugs 15: 1-16.
- Gómez-Ordóñez E, Rupérez P (2011) FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids 25: 1514-1520.
- Zhang Q, Yu P, Li Z, et al. (2003) Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis 305-310.
- Larsen R, Eilertsen KE, Elvevoll EO, et al. (2011) Health benefits of marine foods and ingredients. Biotechnology Advances 29: 508-518.
- Sato N, Moriyama T, Mori N, et al. (2017) Lipid metabolism and potentials of biofuel and high added-value oil production in red algae. World Journal of Microbiology and Biotechnology 33: 1-11 .
- Ambrozova JV (2014) Influence of Extractive Solvents on Lipid and Fatty Acids Content of Edible Freshwater Algal and Seaweed Products, the Green Microalga Chlorella kessleri and the Cyanobacterium Spirulina platensis. Molecules 19: 2344-2360.
- da Costa E (2018) High-Resolution Lipidomics of the Early Life Stages of the Red Seaweed Porphyra dioica. Molecules 23: 1.
- Schmid M, Guihéneuf F, Stengel DB, et al. (2014) Fatty acid contents and profiles of 16 macroalgae collected from the Irish Coast at two seasons. Journal of Applied Phycology 26: 451-463.
- Wood LW (1985) Chloroform-Methano Extraction of Chlorophyll a. J Fish Aqunt Sci 42: 2-7.
- Zhang T (2012) Analysis of photosynthetic pigments and chlorophyll fluorescence characteristics of different strains of Porphyra yezoensis. Journal of Applied Phycology 24: 881-886.
- Vissers AM (2017) Leaf phenolics and seaweed tannins, Analysis, enzymatic oxidation and non-covalent protein binding. Food And Agriculture Organization Of The United Nations.
- Kamphorst JJ, Fan j, Lu W, et al. (2011) Liquid chromatography-high resolution mass spectrometry analysis of fatty acid metabolism. Analytical Chemistry 83: 9114-9122.
- Niemi C, Lage S, Gentili FG, et al. (2019) Comparisons of analysis of fatty acid methyl ester (FAME) of microalgae by chromatographic techniques. Algal Research 39: 101449.
- Susanto E, Fahmi AS, Hosokawa M, et al. (2019) Variation in Lipid Components from 15 Species of Tropical and Temperate Seaweeds. Marine Drugs 17: 630.
- Schubert N, García-Mendoza E, Pacheco-Ruiz I, et al. (2006) Carotenoid composition of marine red algae. Journal of Phycology 42: 1208-1216.
- Koizumi J (2018) Carotenoid Profiling of a Red Seaweed Pyropia yezoensis: Insights into Biosynthetic Pathways in the Order Bangiales. Marine drugs 16: 426.
- Ding H, Fei Q, Zhang P, et al. (2020) Isolation and characterization of a heat-resistant strain with high yield of Pyropia haitanensis induced by ultraviolet ray. Aquaculture 521: 735050.
- Sampath-Wiley P, Neefus CD, Jahnke LS, et al. (2008) Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: Fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). Journal of Experimental Marine Biology and Ecology 361: 83-91.
- Piña F, Contreras-Porcia L (2021) Enhancement of Xanthophyll Synthesis in Porphyra/Pyropia Species (Rhodophyta, Bangiales) by Controlled Abiotic Factors: A Systematic Review and Meta-Analysis. Marine Drugs 19: 221.
- Esteban R (2009) Carotenoid composition in Rhodophyta: Insights into xanthophyll regulation in Corallina elongate. European Journal of Phycology 44: 221-230.
- Arathi BP, Sowmya PRR, Vijay K, et al. (2015) Metabolomics of carotenoids: The challenges and prospects - A review. Trends in Food Science and Technology 45: 105-117.
- Burtin P (2003) Nutritional value of seaweeds. Electron. J Environ Agric Food Chem 2: 498-503.
- Biancarosa I (2018) Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. Journal of the Science of Food and Agriculture 98: 2035-2042.
- Jung SM, Kang SG, Son JS, et al. (2016) Temporal and spatial variations in the proximate composition, amino acid, and mineral content of Pyropia yezoensis. Journal of Applied Phycology 28: 3459-3467.
- Cian RE, Fajardo MA, Alaiz M, et al. (2014) Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. International Journal of Food Sciences and Nutrition, 65: 299-305.
- Kusumo HT (1993) Chemical composition of Porphyra spp in British Columbia, Canada.
- Mok JS (2011) Proximate Composition and Mineral Content of Laver Porphyra yezoensis from the Korean Coast. Korean Journal of Fisheries and Aquatic Sciences 44: 554-555.
- Hwang E, Ki K, Chung H, et al. (2013) Proximate Composition , Amino Acid , Mineral , and Heavy Metal Content of Dried Laver. Prev Nutr Food Sci 18: 139-144.
- Xiong F, Kopecky J, Nedbal L, et al. (1999) The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (Chlorophyta). Aquatic Botany 63: 37-49.
- Singh SP, Klisch M, Sinha RP, et al. (2008) Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. Photochemistry and Photobiology 84: 1500-1505.
- Klisch M, Häder D (2008) Mycosporine-Like Amino Acids and Marine Toxins - The Common and the Different. Marine Drugs 6: 147-163.
- De La Coba F, Aguilera J, Figueroa FL, et al. (2009) Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. Journal of Applied Phycology 21: 161-169.
- Shick JM, Dunlap WC (2002) Mycosporine-like amino acids and related gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu Rev Physiol 64: 223-262.
- La Barre S, Roullier C, Boustie J, et al. (2014) Mycosporine-Like Amino Acids (MAAs) in Biological Photosystems. Outstanding Marine Molecules 333-360.
- Sinha RP, Singh SP, Häder DP, et al. (2007) Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. Journal of Photochemistry and Photobiology B: Biology 89: 29-35.
- Wada N, Sakamoto T, Matsugo S, et al. (2015) Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 4: 603-646.
- Karsten U, Escoubeyrou K, Charles F, et al. (2009) The effect of re-dissolution solvents and HPLC columns on the analysis of mycosporine-like amino acids in the eulittoral macroalgae Prasiola crispa and Porphyra umbilicalis 63: 231-238.
- Gröniger A, Hallier C, Häder DP, et al. (1999) Influence of UV radiation and visible light on Porphyraumbilicalis: Photoinhibition and MAA concentration. Journal of Applied Phycology 11: 437.
- Zhaohui Z, Xin G, Tashiro Y, et al. (2005) The isolation of prophyra-334 from marine algae and its UV-absorption behaviour. Chinese Journal of Oceanology and Limnology 23: 400-405.
- De La Coba F, Aguilera J, Figueroa FL, et al. (2009) Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. Journal of Applied Phycology 21: 161-169.
- Torres P, Santos JP, Chow F, et al. (2018) Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Research 34: 57-67.
- Zhang Z, Tashiro Y, Matsukawa S, et al. (2005) Influence of pH and temperature on the ultraviolet-absorbing properties of porphyra-334. Fisheries Science 71: 1382-1384.
- La Barre S, Roullier C, Boustie J, et al. (2014) Mycosporine-Like Amino Acids (MAAs) in Biological Photosystems in Outstanding Marine Molecules: Chemistry, Biology. Analysis.
- Helbling EW, Menchi CF, Villafañe VE, et al. (2002) Bioaccumulation and role of UV-absorbing compounds in two marine crustacean species from Patagonia, Argentina. Photochemical and Photobiological Sciences 1: 820-825.
- Hoyer K, Karsten U, Wiencke C, et al. (2002) Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Marine Biology 141: 619-627.
- Korbee Peinado N, Abdala Díaz RT, Figueroa FL, et al. (2004) Ammonium and uv radiation stimulate the accumulation of mycosporine-like amino acids in porphyra columbina (rhodophyta) from patagonia, argentina 40: 248-259.
- Huovinen P, Ulloa N, Morales V, et al. (2004) Ultraviolet-absorbing mycosporine-like amino acids in red macroalgae from Chile 47: 21-29.
- Lalegerie F, Stiger-Pouvreau V, Connan S, et al. (2020) Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. Journal of Applied Phycology 32: 2641-2656.
- Amy M, Apprill, Michael P, et al. (2003) Effects of ultraviolet radiation on Laminaria saccharina in relation to depth and tidal height in the Gulf of Maine. Marine Ecology Progress Series 256: 75-85.
- Guihéneuf F, Gietl A, Stengel DB, et al. (2018) Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. Journal of Applied Phycology 30: 2573-2586.
- Karsten U, Sawall T, Wiencke C, et al. (1998) A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycological Research 46: 271-279.
- Chaves-Peña P, De La Coba F, Figueroa FL, et al. (2020) Quantitative and qualitative HPLC analysis of mycosporine-like amino acids extracted in distilled water for cosmetical uses in four rhodophyta Marine Drugs. 18: 27.
- Volkmann M, Gorbushina AA (2006) A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiology Letters 255: 286-295.
- Singh SP, Kumari S, Rastogi RP, et al. (2008) Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian Journal of Experimental Biology 46: 7-17.
- Singh SP, Ha SY, Sinha S La, et al. (2014) Photoheterotrophic growth unprecedentedly increases the biosynthesis of mycosporine-like amino acid shinorine in the cyanobacterium Anabaena sp., isolated from hot springs of Rajgir (India). Acta Physiol Plant 36: 389-397.
- Hartmann A, Holzinger A, Ganzera M, et al. (2016) Prasiolin, a new UV-sunscreen compound in the terrestrial green macroalga Prasiola calophylla (Carmichael ex Greville) Kützing (Trebouxiophyceae, Chlorophyta. Planta 243: 161-169.
- Carreto JI, Carignan MO, Daleo G, et al. (1990) Occurrence of mycosporine-like amino acids in the red-tide dinoflagellate Alexandrium excavatum: UV-photoprotective compounds?. Journal of Plankton Research 12: 909-921.
- Rastogi RP, Incharoensakdi A (2013) UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyan bacterium Gloeocapsa sp. CU2556. Journal of Photochemistry and Photobiology B: Biology 130: 287-292.
- Rastogi RP, Incharoensakdi A (2014) Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanbacterium Lyngbya sp. CU2555. FEMS Microbiology Ecology 87: 244-256.
- Sinha RP, Klisch M, Walter Helbling E, et al. (2001) Induction of mycosporine-like amino acids (MAAs) in cyanobacteria by solar ultraviolet-B radiation. Journal of Photochemistry and Photobiology B: Biology 60: 129-135.
- Hartmann A, Murauer A, Ganzera M, et al. (2017) Quantitative analysis of mycosporine-like amino acids in marine algae by capillary electrophoresis with diode-array detection. Journal of Pharmaceutical and Biomedical Analysis 138: 153-157.
- HELIONORI® - GELYMA. (n.d.). Retrieved May 8, 2020.
- EEAP (2018) Environmental Effects Assessment Panel Update Report, 2017 (Issue January).
- Sinha RP, Klisch M, Gröniger A, Häder DP (2000) Mycosporine-like amino acids in the marine red alga Gracilaria cornea - Effects of UV and heat. Environ Exp Bot 43: 33-43.
Corresponding Author
Department of Estuarine and Delta Systems, NIOZ Royal Netherlands Institute for Sea Research, P.O. Box 140, 4401 NT Yerseke, The Netherlands.
Copyright
© 2022 van Groenigen J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.