Introduction of Multidisciplinary Team at a Comprehensive Cancer Center Affects Time to Tissue Diagnosis and Definitive Treatment
Context Summary
Key Objective
Determine whether the introduction of a multidisciplinary team (MDT) affects time to diagnosis and treatment for lung cancer patients.
Knowledge Generated
The introduction of an MDT dramatically shortened the time intervals between when a radiologic abnormality was first identified to date of histological confirmation of disease and to initiation of definitive treatment. It did not significantly decrease the time interval from histological confirmation of disease to initiation of definitive treatment.
Abstract
Purpose: Increasing complexity in cancer treatment prompts the adoption of multidisciplinary care, which has become a standard of quality care in oncology. This study assessed whether the introduction of a multidisciplinary team (MDT) in a cancer center affected patient retention, time to tissue diagnosis, and commencement of definitive treatment. This study also assessed the effects of treatment modality, age, race, ethnicity, and insurance on these outcomes.
Patients and methods : Patients diagnosed with any stage of lung cancer that presented to a tertiary care hospital between April 2019 and December 2022 were included in the study population. The date the radiological abnormality was first identified was used to divide patients into Pre-MDT and MDT groups. By which, 251 patients were identified as Pre-MDT and 207 patients identified as MDT. Welch's t-test and non-paired Wilcoxon test were performed for each time interval, i.e., radiologic abnormality to treatment, diagnosis to treatment, radiologic abnormality to treatment intervals, the two groups. Python was used to conduct these statistical analyses.
Results : There was a mean 204.1-day reduction in time from first abnormal imaging to diagnosis (p<0.001) and 184.0-day reduction in time from first abnormal imaging to treatment (p<0.001) after the introduction of the MDT. The 3.3-day reduction in time from diagnosis to treatment after the introduction of the MDT was not significant. Patient retention was not significantly changed by the MDT.
Conclusion : The introduction of a multidisciplinary team can drastically decrease the time from when a potential lung malignancy is first identified on imaging to receiving a cancer diagnosis and treatment. Race and treatment modality are the strongest correlation factors for these intervals. However, it did not significantly decrease the time interval from histological confirmation of disease to initiation of definitive treatment.
Introduction
According to the American Cancer Society, lung cancer is the third most common cancer in the United States [1]. More people in the United States die from lung cancer than any other type of cancer. This is true for both men and women [1]. Primary lung cancer generally has a poor prognosis if not diagnosed at an early stage [2]. Missed opportunities for early diagnosis of lung cancer can occur due to failure to recognize potential diagnostic clues or failure to complete the diagnostic work-up in a timely manner [3]. Lung cancer patients experience substantial delays from development of symptoms to first initiating treatment. This may contribute to an advanced stage at diagnosis and poor long-term survival [4]. Shortening the diagnostic and treatment delay times might be possible with little extra cost by a multidisciplinary team approach [5].
A multidisciplinary team (MDT) in oncology is defined as the cooperation between different specialized professionals involved in cancer care with the overarching goal of improving treatment efficiency and patient care [6]. MDT working is increasingly recognized as a standard for high‐quality cancer care [7]. There is an increasing trend to use MDT in the investigation and management of patients with suspected or proven lung cancer [8]. Lung cancer‐specific MDTs therefore require insight from a diverse group of specialists, including pulmonologists, thoracic surgeons, radiologists, medical oncologists, pathologists, radiation oncologists, palliative care specialists, and specialist nurses [7].
According to the Centers for Disease Control (CDC) the two main types of lung cancer are small cell lung cancer and non-small cell lung cancer [9]. People with non-small cell lung cancer can be treated with surgery, chemotherapy, radiation therapy (RT), targeted therapy, or a combination of these treatments [9]. People with small cell lung cancer are usually treated with RT and chemotherapy [9]. Lung cancer management is now increasingly complex, especially with novel modalities such as targeted therapies, immune checkpoint inhibitors and stereotactic body radiotherapy delivery [10]. This increasing complexity underscores the importance of taking a multidisciplinary approach to management that extends throughout the continuum of care from diagnosis to supportive and end-of-life care [11].
A multidisciplinary team can decrease the time to both diagnosis and treatment, as well as increase accuracy of diagnosis [12]. A retrospective review of 376 patients presented at a weekly multidisciplinary thoracic oncology conference (MTOC) found that patients who received concordant care between MTOC recommendation and actual clinical care had had significantly shorter delays to the initiation of definitive therapy, as well as longer overall survival and progression-free survival.” [13]. A prospective study of 431 patients referred to a lung cancer multidisciplinary clinic for the management of known or suspected thoracic malignancy found that there was a high rate of adherence to international guideline recommendations concerning timely lung cancer diagnosis, staging and treatment implementation [14]. Another study comparing 535 patients treated prior to initiation of the multidisciplinary conference and 687 patients within the multidisciplinary conference found that the multidisciplinary thoracic malignancy conference increased the percentage of patients receiving complete staging, a multidisciplinary evaluation and adherence to nationally accepted care guidelines while decreasing the interval from diagnosis to treatment significantly [15].
The natural evolution of this approach was the development of oncological functional units: disease-site specific cancers focused on the management and provision of services for cancer patients. These units integrate a multidisciplinary committee and include all the departments involved in a patient's care with the aim of facilitating the intervals and interactions between the different professionals, hence reducing time to diagnosis and/or commencement of treatment [6]. Introduction of a multidisciplinary team in a comprehensive cancer center is an opportunity to improve patient care. It is widely stated that when all providers are present under the same roof simultaneously then the interval between initial presentation to diagnosis, staging and commencement of treatment decreases thereby expediting care. Whether introduction of multidisciplinary care impacts efficiency of treatment and diagnostic timeline needs to be studied.
This study assessed if the introduction of an MDT in a cancer center decreases time to tissue diagnosis and commencement of definitive treatment for lung cancer patients. This study also assessed the effects of insurance, sex, race, and age on these outcomes. Patient retention as defined by being in follow-up with the oncology team/ cancer center for >6 months from initial consultation was also assessed.
Methods
Study population
All patients with radiographic abnormalities that presented to the tertiary care hospital between April 2019 to December 2022 who were diagnosed with any stage of lung cancer and received definitive treatment at the facility have been included in the study population. These patients were identified via tumor registry and cross-referenced with the electronic medical record (EMR). These patients range from 25 to 95 years old, consist of all genders, all racial and ethnic backgrounds, and a combined last status of alive or dead.
All patients were reassigned a study number different from their medical record number (MRN). Biopsy date, date of histological result, and initial treatment date were recorded. Patient age of diagnosis, sex, race, ethnicity, initial treatment status, primary insurance provider between points A and C, and whether the patient was in contact with the facility’s MDT beyond 6 months from date of first contact were also recorded from the EMR. First line treatment modality and date were cross referenced with the EMR and the tumor registry. All data was recorded on a secure spreadsheet.
Human subject’s MRN number was kept on a password protected database. All data will be entered into a computer that is password protected. The data including the data sheet and patient identifiers will be stored in an excel spreadsheet on google drive. Data will be stored in a locked office of the investigators and maintained for a minimum of three years after the completion of the study.
This study only presents the risk of data confidentiality to the participants and does not present any direct benefit to the participants. Consent was waived by the IRB as this is a retrospective chart review.
Study design
This is a retrospective chart review of patients diagnosed with and treated for lung cancer with surgery, radiation, or chemotherapy at the tertiary care hospital. Data was compiled from the facility’s cancer registry database including first date of contact at the facility, date of diagnosis, place of diagnosis, age of diagnosis, and combined last status. The start date of chemotherapy, RT, and surgery is entered into the cancer registry database for each patient, so registry software was used to calculate the number of days from date of diagnosis to when the first treatment was started.
Multidisciplinary team was introduced in the cancer center in March 2021. Point A is the time when the radiographic abnormality is first identified. Point B is when tissue diagnosis is established. Point C is when definitive treatment is started. The duration between Point A and Point B and between Point B and Point C will be compared before and after the introduction of MDT.
A total of 507 patient cases from our institution’s electronic medical recording system were selected for review based on the diagnosis of lung cancer at any stage between the dates of April 2019 through December 2022.
Sample size
507 patient cases from the EMR that fulfilled the inclusion criteria were reviewed. Overall, 49 patients were subsequently omitted from analysis in this study due to insufficient data. Point A - date radiological abnormality was first identified - was used to divide patients into Pre-MDT and MDT categories. A total of 251 and 207 patients were identified as Pre-MDT and MDT, respectively.
4 diagnoses were duplicates of already recorded patients and were therefore omitted. 7 cases were listed as medical record numbers that did not align with a patient chart and were therefore omitted; these patients were likely trauma cases that were not later reassigned an MRN. 38 cases were omitted due to insufficient data; 31 of which either opted to not have a biopsy or expired prior to scheduled biopsy date.
For the remaining 458 patients, Point C of 19 cases were missing, and Point B of 69 cases were missing. Hence, 19 A-B intervals, 88 B-C intervals, and 69 A-C intervals were replaced with null value due to lack of sufficient data available in our EMR. 95 cases have histological diagnosis using tissue obtained during their surgical resection, i.e. their first-line therapy, thus Point C is earlier than Point B. B-C intervals for these patients were not omitted from statistical analysis. Histological diagnosis date was based on the date of the initial report confirming the presence of cancer. The diagnosis date was not delayed reflecting amendments for histologic subtypes.
Surgical intervention includes surgical lung biopsy and curative resection. Medical oncology intervention includes any antineoplastic therapy including cytotoxic chemotherapy, targeted treatments, check point inhibitors (CPI). Radiation was included in the setting of curative or palliative intent.
MDT model
Our multidisciplinary team consists of interventional pulmonologists, thoracic oncologists, radiation oncologists, palliative medicine, pulmonologists, oncology rehabilitation, onco-cardiologists, thoracic surgeons, chest radiologists. Our physicians work in both inpatient and outpatient environments. Patients are treated by providers and staff outside of the Cancer Care Team while in the inpatient setting.
Statistical analysis
We analyzed 458 and collected data including timeline of initial consultation/referral to cancer center, tissue diagnosis, recommended treatments and follow up visits. For the dataset, the categorical features are first encoded into binary variables with a one-hot encoding scheme. Then, the encoded dataset is split into two groups: before and after the introduction of the MDT. Summary statistics of each group are obtained, including the count, mean, minimum, maximum, and 20%, 50%, 75% percentile. Finally, for each time interval, i.e., A-B, B-C, A-C intervals, Welch's t-test and non-paired Wilcoxon test have been performed for the two groups, Pre-MDT Vs MDT. Python was used to conduct these statistical analyses.
Results
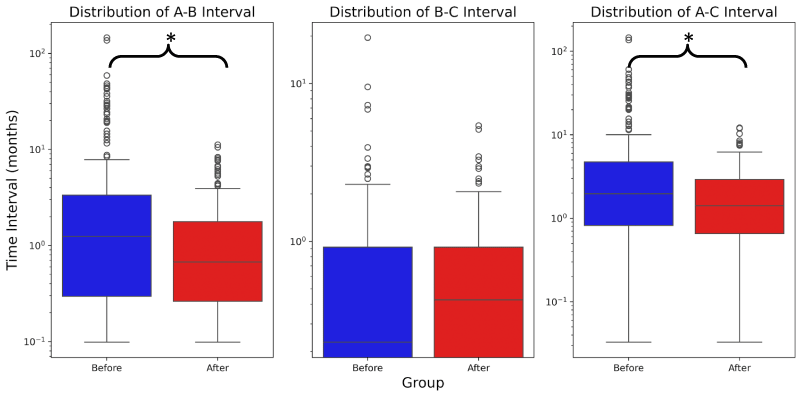
Results of Welch’s t-test and non-paired Wilcoxon t-test show that the mean 204.1-day reduction of the A-B interval after the introduction of the MDT was significant (Pre-MDT: 247.2 days, MDT: 43.1 days, p<0.001). The average B-C interval was not significantly changed after the introduction of the MDT (Pre-MDT: 21.5 days, MDT: 18.2 days, Welch’s T-test p>0.488, non-paired Wilcoxon p>0.288). The average A-C interval was decreased by 184.0 days (Pre-MDT: 253.0 days, MDT: 69.0 days, p<0.001) (Figure 1). Pathology took an average of 5-days to provide biopsy results both before and after the introduction of the MDT.
The top five correlated features for each time interval before and after MDT are presented in Table 1. Age at diagnosis and primary insurance were the top correlated features for A-B and A-C intervals for Pre-MDT patients. These features plus treatment type highly correlate with Pre-MDT B-C intervals. Under the MDT, surgical intervention, race, and living status are the top correlated features for all intervals. These features also show stronger correlation compared to the Pre-MDT intervals’ top correlated features. For example, Pre-MDT A-B interval’s top feature is primary insurance as Medicare (r = 0.138329) compared with the MDT where the top feature is surgery as first line treatment (r = 0.230753). Race is not present as a correlative feature for the Pre-MDT A-B or A-C interval, but non-Hispanic Black patients saw positive correlation for A-B (r = 0.212675) and A-C (r = 0.261471) intervals. First line treatment outside of the facility is also positively correlated with increased B-C and A-C intervals. Additionally, patients who were deceased at or prior to the 6-month retention date show negative correlation for A-B (r = -0.126006), B-C (r = -.0.159619), and A-C (r = 0.159569) intervals after the introduction of the MDT. Living status was not correlated with Pre-MDT B-C or A-C intervals and negligibly correlated with Pre-MDT A-B interval (r = 0.068096).
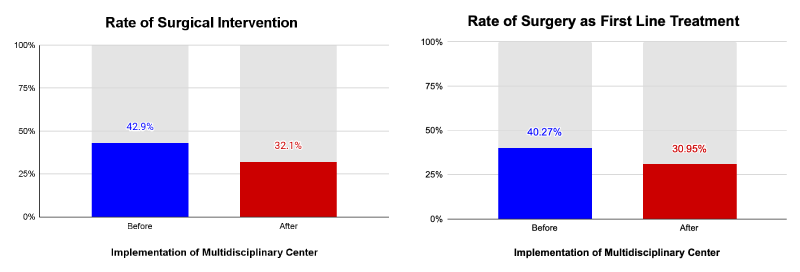
After the introduction of the MDT, patients are less likely to have surgery as their first line treatment and less likely to have any surgical intervention throughout their care (Figure 2). Including those who did not receive any treatment, the utilization of surgical services decreased from 36.25% to 25.12% with the introduction of the MDT. For those who received any anti-cancer treatment, surgery was still less likely to be used under the MDT. Pre-MDT patients underwent surgery 42.9% of the time compared to 32.1% of the time under the MDT. The rate of surgery as first line treatment decreased by 9.3%. Pre-MDT patients underwent surgery as first line treatment 40.3% of the time compared to 30.1% of the time under the MDT. For patients whose first line treatment was surgery, B-C interval was negatively correlated with the in-house surgery department (r = -0.243417), but positively correlated for patients whose surgery was outside of the facility (r = 0.266607; Table 1).
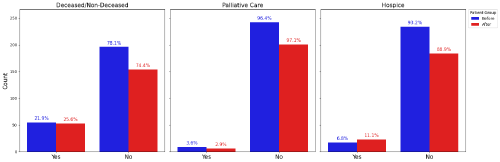
Patient retention was not significantly affected by the introduction of the MDT (Figure 3). By the end of the 6-month retention period, 21.9% Pre-MDT patients were deceased compared to 25.6% with the MDT (Figure 4). 10.4% Pre-MDT patients were lost to follow-up after being placed on hospice or palliative care compared to 14.0% under the MDT (Figure 4). Neither the increase in patient deaths nor the rise in hospice/palliative transitions after MDT implementation were statistically significant.
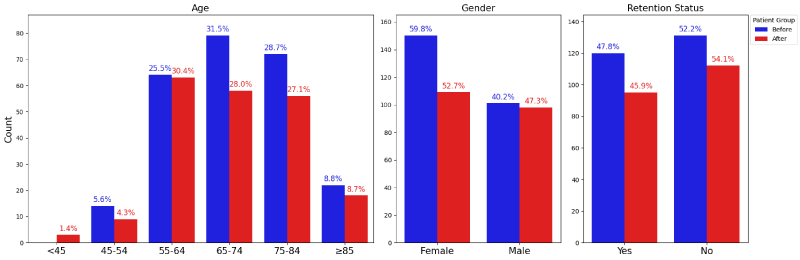
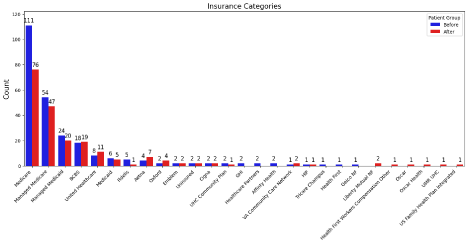
There was a higher percentage of female patients prior to the MDT (60.0% Vs. 48.8%), but other demographic markers were not significantly different between the two groups. The majority of the patients are White, Non-Hispanic or Latino (Figure 3). The most common primary insurance providers are Medicare and Managed Medicare throughout the study, accounting for over half of all patients (Figure 5). Patients aged 65 and older comprise approximately two-thirds of all patients (Figure 3).
Discussion
Implementation of a multidisciplinary center is not a new approach to patient care in oncology. Multidisciplinary meetings are utilized at increasing rates [16] and are seen as a standard of high quality care [17]. It is a well-established model in the management of breast cancers [18,19]. However, we have yet to see sufficient data on the effects of an MDT on lung cancer.
The current study shows that the time from the first radiologic presence of disease to histologic confirmation of cancer decreased after the introduction of the MDT. Although the overall change in time from diagnosis to definitive treatment was not significantly decreased with the MDT, patients who received treatment outside of the facility experienced longer delays in treatment. The patient’s primary insurance provider showed the strongest or second strongest correlation prior to the MDT and played less of a role in delays to diagnosis and treatment once the MDT was introduced. With the MDT present, we see that surgical patients experience prolonged intervals from initial imaging to diagnosis and initial imaging to treatment.
The effects of race, especially for non-Hispanic Black or African American patients, became more prominent as the effects of insurance subsided with the introduction of the MDT. Time to diagnosis and treatment after an abnormality was discovered on imaging was longer for Black patients compared to their non-Black counterparts. Previous studies support that insurance and race are associated with discordant care and patients who receive cordant care experience shorter delays in treatment [13]. However, these time intervals in the current study are comparisons within the facility and not compared to the national average or guidelines. Patient sex did not have a significant effect on time to diagnosis which is as expected [11,20].
We did not see a significant decrease in time from the date of histological confirmation to the date of initial treatment. However, the timeframe from initial imaging to initial treatment was significantly decreased after the introduction of the MDT. Patients who expired within 6 months of initial contact were correlated with shorter time to diagnosis. Sufficient time has not passed to properly determine the relationship between this MDT and patient outcomes such as overall survival, progression-free survival, and treatment adherence. However, we hope future investigation will find that shorter time to diagnosis contributes to improved survival in patients with advanced-stage lung cancer [21].
We expect to see a continued downward trend in surgical intervention among lung cancer patients managed by the MDT. With the introduction of the MDT came the addition of an interventional pulmonologist who can perform minimally invasive biopsies. Alongside this, newer technologies such as robotic-assisted bronchoscopy, ablative devices, and advanced laser-based techniques were adopted, enabling more precise and less invasive diagnostic and therapeutic procedures. Interventional pulmonology techniques such as endobronchial ultrasound-guided biopsies offer lower complication rates, and equal or improved diagnostic yields compared to traditional mediastinoscopy [22,23].
However, the employment of one highly specialized physician is not enough to improve patient care, rather, it is the MDT around one or more physicians that leads to improved outcomes [24,25]. Previous studies have shown that multidisciplinary meetings can improve patient outcomes. Patients treated by MDTs have decreased anesthesia inductions, endotracheal intubations, and clinic visits [25]. We saw that patients were less likely to undergo surgical intervention after the formation of the MDT, which may be due to the introduction of an interventional pulmonologist to the team.
MDT structure
Much of the literature regarding multidisciplinary centers and teams involves organ-specific disease such as an MDT dedicated to bone primary malignancies and bone metastasis [20,26]. The current study’s MDT is not organ-specific and covers both inpatient and outpatient care. However, this study aims to fill the gap in data regarding the relationship between lung cancer and MDTs, thus only lung malignancies were included. The Lung Cancer Evaluation Center (LCEC) at another local cancer center that differs from the study’s MDT in that the LCEC is outpatient based and dedicated to lung cancers [20]. The LCEC showed improved 10-year survival of stage-4 lung cancer patients [20]. Due to the limited time span of our study, we are unable to properly evaluate long term survival. Patients treated by the LCEC were more likely to have surgical intervention compared to non-LCEC patients, but the authors propose that this may be due to the differences in stage at presentation between the two groups [20]. This trend in surgical rates is the reversed relationship of that shown by the facility, which further prompts the need to investigate the effects of MDTs on lung cancer patients.
The entirety of the MDT is employed by the facility, which differentiates our approach from previous studies which tend to include physicians and surgeons from multiple practices [7,25] More data needs to be collected to determine the effect of an MDT employed under a common employer such as in our study compared to multidisciplinary meetings across multiple specialties. The benefits of a multidisciplinary center may not be limited to larger, hospital-based systems. The American Society of Clinical Oncology (ASCO) currently has a pilot program for the Oncology Medical Home for community practices. This model explicitly expands beyond a team of physicians by including nurses, medical technicians, care coordinators, pharmacists, and more [27]. Additionally, incorporating IT and EMR staff into the MDT can improve employee satisfaction with documentation, improve National Comprehensive Cancer Network (NCCN) guideline adherence, and reduce emergency department visits [15,28,29].
Patient outcomes
Although data varies between studies, MDT presentations can improve survival rates in lung cancer patients [11,20]. This may be of particular importance to elderly patients and patients with advanced diseases. A Swedish study showed that esophageal cancer patients with advanced age, disease, or comorbidities had higher 3-year survival rates when discussed at MDMs [16]. Patients are also more likely to be treated with curative intent when discussed at MDMs and for those not treated with curative intent, they are twice as likely to have a proactive palliative care plan at time of diagnosis if discussed at an MDM [16].
Healthcare access in the US is heavily influenced by patient demographics. When MDM participation is optional as described in the study, elderly patients and patients with a lower education level were less likely to be presented at MDMs [16]. Utilization of an MDT under the same institution may help reduce biases for cancer patients compared to a case-by-case decision to utilize a multidisciplinary approach. MDT’s may be of particular importance to lung cancer patients as it is a disease that overwhelmingly affects elderly patients [30]. With this age range comes a high percentage of patients on Medicare. A retrospective review of the US SEER-Medicare database found that the average time from imaging to diagnosis was 20 days and that patients with a shorter imaging-to-diagnosis interval had lower overall survival [21]. When stratified by stage, this trend only held true for stage IV patients [21]. However, the imaging date in this study was the date of radiologic suspicion of lung cancer rather than our definition of first radiological abnormality. Further investigation is warranted to determine if our A-B and A-C intervals influence overall survival.
With the US's difficulties in healthcare access dependent on financial access to care, the results of our study are particularly important as they demonstrate that adoption of an MDT decreases the effect of primary insurance providers on time to diagnosis and treatment. However, race and ethnicity became the main correlative factor after the introduction of the MDT (Table 1). Race and insurance status have been shown to correlate with discordant care under MDTs.13 Black men are 12% more likely to develop lung cancer compared to their white male counterparts [31].
As previously described, MDTs are increasingly more common and even seen as standard of care in some instances and improve NCCN guideline compliance. However, patients need to be able to afford their cancer treatment and we continue to see projected increases in the cost of cancer care [32,33]. An MDT-based electronic pathway to support physicians’ treatment decisions was found to decrease cost of care without compromising survival [28]. Antineoplastic are stated to be the largest contribution to cost savings, but surgical costs were not described [28]. Since our study showed a trend away from surgery with the MDT, it would be interesting to see how this affected patients’ overall treatment costs. Patient satisfaction with MDT-driven care is quite high with one study finding that 98% of patients being satisfied or very satisfied with their care [26].
Although not statistically significant, patient retention showed a slight trend towards improvement following the implementation of the MDT. However, this change was modest, and several factors may explain why a more substantial increase in retention was not observed. First, a higher proportion of patients died within six months post MDT, indicating that the MDT may have managed a greater number of patients with advanced or rapidly progressing disease (Figure 4). Second, although MDTs are designed to improve care coordination, patients may have been appropriately transitioned to external palliative or hospice care, which would remove them from the institution’s follow-up database. Third, the COVID-19 pandemic likely disrupted follow-up during the early MDT implementation period due to limited in-person visits, patient hesitancy, and overall system strain. Fourth, the presence of multiple tertiary care centers in close geographic proximity may have led patients to transfer care elsewhere, lowering retention due to increased healthcare system competition. Lastly, our retention metric defined as follow-up over six months may not fully reflect successful care completion or transitions in cases where shorter-term care was appropriate.
Conclusion
The introduction of a multidisciplinary team can drastically decrease the time from when a potential lung malignancy is first identified on imaging to receiving a cancer diagnosis and treatment. The MDT in this study did not significantly decrease the time from diagnosis to initiation of treatment. The main correlative factors for these time intervals are treatment modality and race.
Limitations
The cases used for this study were not refined to require all time points (Point A, Point B, and Point C) to fall between April 2019 to December 2022. For example, one lung nodule was followed for 20 years, and a biopsy was not indicated until after the MDT was introduced. Definitive treatment was not documented for all patients due to death, treatment refusal, or lack of sufficient data. Performance status and stage at diagnosis was not analyzed but would likely affect patient outcomes [16]. The short 2-year window on either side of the MDT-introduction time point does not currently allow for adequate survival assessment. Additionally, a 6-month period may be too short to properly determine patient retention. Adherence to imaging surveillance recommendations set forth by NCCN guidelines would be more appropriate. Patient retention may be an effect geographic convenience as patients tend to seek care based on proximity and ability to coordinate clinic visits [25,33].
It is worth further investigation to control for histological type as Small cell lung cancer (SCLC) treatment recommendations and survival rates differ between Non-small cell lung cancer (NSCLC) and SCLC [1,9]. However, whether or not a patient is treated under an MDT may not affect survival differently for SCLC when compared to NSCLC [11]. The current study also did not control for malignancy as a recurrence or primary lesion. The study timeframe includes the COVID-19 pandemic. There was a lower cancer incidence than anticipated during 2020 and 2021 due to delays in care and reduced cancer screenings [1].
With the retrospective nature of this study, many patient cases lacked sufficient data which further reduced the small sample size. Some patients partially sought care externally and/or sought out a second opinion which may influence the measured time intervals. Data was collected directly from the EMR thus patient cases are not blinded. There were a few patients who expired, were lost to follow-up, or went to hospice prior to a potential Point B and/or Point C.
Acknowledgements
Conflict of interest
Authors declare that they have no conflicts of interest. No funding was received for the completion of this project.
Data safety monitoring
Monitoring is an ongoing review of the study throughout its duration. Any action resulting in a temporary or permanent suspension of the study should be reported to the IRB and to the Office of Clinical Research. The PI is responsible for reporting any reasons outside the planned study design such as in compliance with the protocol or if there is any delay in the initiation of the study due to administrative reasons.
Abbreviations
COVID-19 - Coronavirus disease-2019; EMR - Electronic Medical Record; MDT - Multidisciplinary Team; RT - Radiation Therapy; MTOC - Multidisciplinary Thoracic Oncology Conference; MDM - Multidisciplinary meeting; MRN - Medical Record Number; NSCLC - Non-small cell lung cancer; SCLC - Small cell lung cancer; NCCN - National Comprehensive Cancer Network; US - United States of America; IRB - International Review Board; PI - Primary Investigator; SEER - Surveillance Epidemiology and End Results; LCEC - Lung Cancer Evaluation Center; BCBS - Blue Cross Blue Shield; GHI - Group Health Incorporated; NF - No-Fault Insurance.
References
- (2025) Cancer Facts & Figures 2025. American Cancer Society. Atlanta: American Cancer Society.
- Yoshimoto A, Tsuji H, Takazakura E, et al. (2002) Reasons for the delays in the definitive diagnosis of lung cancer for more than one year from the recognition of abnormal chest shadows. Intern Med 41: 95-102.
- Singh H, Hirani K, Kadiyala H, et al. (2010) Characteristics and predictors of missed opportunities in lung cancer diagnosis: An electronic health record-based study. J Clin Oncol 28: 3307-3315.
- Ellis PM, Vandermeer R (2011) Delays in the diagnosis of lung cancer. J Thorac Dis 3: 183-188.
- Salomaa ER, Sällinen S, Hiekkanen H, et al. (2005) Delays in the diagnosis and treatment of lung cancer. Chest 128: 2282-2288.
- Taberna M, Gil Moncayo F, Jané-Salas E, et al. (2020) The Multidisciplinary Team (MDT) approach and quality of care. Front Oncol 10: 85.
- Popat S, Navani N, Kerr KM, et al. (2021) Navigating diagnostic and treatment decisions in non-small cell lung cancer: Expert commentary on the multidisciplinary team approach. Oncologist 26: e306-e315.
- Prabhakar CN, Fong KM, Peake MD, et al. (2015) The effectiveness of lung cancer MDT and the role of respiratory physicians. Respirology 20: 884-888.
- (2024) Treatment of Lung Cancer. Centers for Disease Control and Prevention.
- Al Zaidi M, Wright GM (2020) Locally advanced non-small cell lung cancer: the place of specialist thoracic surgery in the multidisciplinary team. Transl Lung Cancer Res 9: 1680-1689.
- Stone E, Rankin N, Kerr S, et al. (2018) Does presentation at multidisciplinary team meetings improve lung cancer survival? Findings from a consecutive cohort study. Lung Cancer 124: 199-204.
- Horvath LE, Yordan E, Malhotra D, et al. (2010) Multidisciplinary care in the oncology setting: Historical perspective and data from lung and gynecology multidisciplinary clinics. J oncol pract 6: e21-e26.
- Osarogiagbon RU, Phelps G, McFarlane J, et al. (2011) Causes and consequences of deviation from multidisciplinary care in thoracic oncology. J Thorac Oncol 6: 510-516.
- Conron M, Phuah S, Steinfort D, et al. (2007) Analysis of multidisciplinary lung cancer practice. Intern Med J 37: 18-25.
- Freeman RK, Van Woerkom JM, Vyverberg A, et al. (2010) The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with lung cancer. Eur J Cardiothorac Surg 38: 1-5.
- Lindblad M, Jestin C, Johansson J, et al. (2024) Multidisciplinary team meetings improve survival in patients with esophageal cancer. Dis Esophagus 37: doae061.
- Selby P, Popescu R, Lawler M, et al. (2019) The value and future developments of multidisciplinary team cancer care. Am Soc Clin Oncol Educ Book 39: 332-340.
- Harness JK, Bartlett RH, Saran PA, et al. (1987) Developing a comprehensive breast center. Am Surg 53: 419-423.
- Kanbour-Shakir A, Harris KM, Johnson RR et al. (1997) Breast care consultation center: Role of the pathologist in a multidisciplinary center. Diagn Cytopathol 17: 191-196.
- Bilfinger TV, Albano D, Perwaiz M, et al. (2018) Survival outcomes among lung cancer patients treated using a multidisciplinary team approach. Clin Lung Cancer 19: 346-351.
- Romine PE, Sun Q, Fedorenko C, et al. (2022) Impact of diagnostic delays on lung cancer survival outcomes: A population study of the US SEER-Medicare database. JCO Oncol Pract 18: e877-e885.
- Ernst A, Anantham D, Eberhardt R, et al. (2008) Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 3: 577-582.
- Yasufuku K, Pierre A, Darling G, et al. (2011) A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 142: 1393-1400.
- Marshall CL, Balentine CJ, Robinson CN, et al. (2011) A multidisciplinary cancer center maximizes surgeons' impact. J Surg Res 171: 15-22.
- Vilanova-Sánchez A, Reck CA, Wood RJ, et al. (2018) Impact on patient care of a multidisciplinary center specializing in colorectal and pelvic reconstruction. Front Surg 5: 68.
- Ibrahim T, Flamini E, Fabbri L, et al. (2009) Multidisciplinary approach to the treatment of bone metastases: Osteo-Oncology Center, a new organizational model. Tumori 95: 291-297.
- The seven domains of the OMH standards. Community Oncology Alliance.
- Jackman DM, Zhang Y, Dalby C, et al. (2017) Cost and survival analysis before and after implementation of dana-farber clinical pathways for patients with stage IV non-small-cell lung cancer. J Oncol Pract 13: e346-e352.
- Sprandio JD, Flounders BP, Lowry M, et al. (2013) Data-driven transformation to an oncology patient-centered medical home. J Oncol Pract 9: 130-132.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016. National Cancer Institute.
- Mariotto AB, Enewold L, Zhao J, et al. (2020) Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev 29: 1304-1312.
- Mariotto AB, Yabroff KR, Shao Y, et al. (2011) Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 103: 117-128.
- Aggarwal A, Han L, Lewis D, et al. (2024) Association of travel time, patient characteristics, and hospital quality with patient mobility for breast cancer surgery: A national population-based study. Cancer 130: 1221-1233.
Corresponding Author
Thomas Joseph Ciccolella, New York Institute of Technology College of Osteopathic Medicine, Northern Boulevard, P.O. Box 8000, Old Westbury, NY 11568.
Copyright
© 2025 Ciccolella TJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.