Pseudoprogression in Non-Small Cell Lung Cancer Brain Metastases Attributable to the Anti-PD-1 Antibody Nivolumab
Abstract
Pseudoprogression is a challenging obstacle in the interpretation of therapeutic response to radiation and chemotherapies for metastatic brain lesions. Combination of stereotactic radio surgery post-treatment effects and anti-PD-1 antibody therapy may cause benign edematous enhancements in treated brain metastases which mimic tumor progression. Herein we report a case of a patient who experienced pseudoprogression in previously irradiated brain metastases after systemic therapy with immune checkpoint inhibitors targeting PD-1. The patient presented with severe headache after starting nivolumab therapy and subsequent MRI showed interval enhancement of lesions along with worsened edema. Therapy was not discontinued; however, vitamin E and Pentoxifylline were provided and follow-up MRI showed resolution of edema and shrinkage of prior brain metastases. With this we show pseudoprogression as a rare phenomenon associated with anti-PD-1 therapy which practitioners should remain cognizant of when evaluating enhancement of previously inactive brain lesions. For mild to moderate cases, a corticosteroid-sparing approach using vitamin E and pentoxifylline can avoid antagonizing the effector function of T-cell dependent therapy.
Keywords
Stereotactic radiosurgery, Programmed death protein 1 (PD-1), Brain metastases, Immune checkpoint inhibitor, Tumor-infiltrating lymphocytes, Pentoxifylline
Abbreviations
Cgy: Centigray; CT: Computed Tomography; FLAIR: Fluid-Attenuated Inversion Recovery; ICAM-1: Intercellular Adhesion Molecule 1; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homolog; LUL: Left Upper Lobe; MRI: Magnetic Resonance Imaging; MHC: Major Histo Compatibility Complex; Nivo: Nivolumab; NSCLC: Non-Small Cell Lung Carcinoma; PD-1: Programmed Death Protein 1; PD-L1: Programmed Death-Ligand 1; RECIST: Response Evaluation Criteria In Solid Tumors; SRS: Stereotactic Radio Surgery
Introduction
Nivolumab is a fully human immunoglobulin G4 Programmed Death Protein 1 (PD-1) antibody which binds to the PD-1 receptor of activated human T-cells with high affinity, thereby blocking interaction with its shared ligands. In functional lymphocyte assays, nivolumab promotes T-cell proliferation and interferon-gamma release, including antigen-specific recall by memory T-cells. Immune checkpoint inhibitors such as nivolumab may induce apparent increases in the sizes of metastatic lesions prior to regression [1,2]. This phenomenon may be partly due to T-cell infiltration into established tumors, resulting in inflammatory infiltrates misinterpreted as tumor growth [3]. Pseudoprogression is also commonly observed following radiotherapy for brain metastases, although it is often characterized by a specified time course [4,5]. Herein we report a case of a patient who experienced pseudoprogression in a previously irradiated brain metastasis after systemic therapy with nivolumab.
Case Presentation
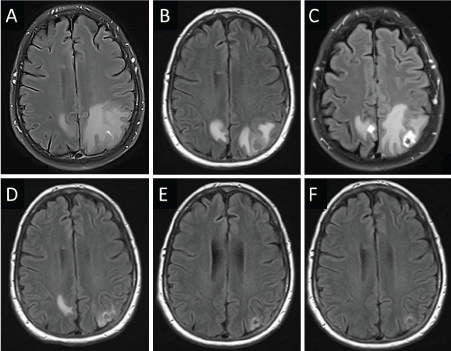
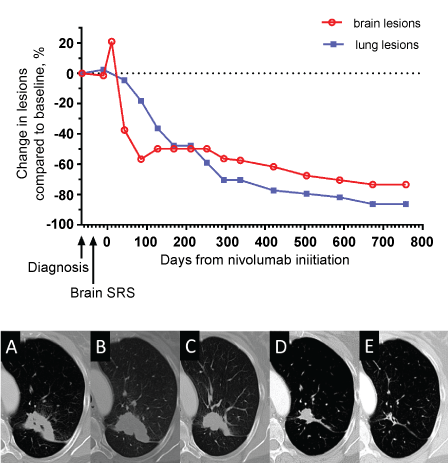
A 54-year-old female 35 pack-year former smoker presented with a severe headache. Magnetic Resonance Imaging (MRI) of the brain revealed a left cerebellar mass with compression of the 4th ventricle, a right cerebellar, a left parietal, and a small right mesial parietal lesion (Figure 1A). Computed Tomography (CT) of the thorax showed a Left Upper Lobe (LUL) cavitary mass. Craniotomy with gross total resection of the left cerebellar metastasis revealed poorly differentiated lung adenocarcinoma with a KRASG12C mutation, TTF1 +, Napsin A +, CK7 +, CK20 - and was strongly positive for membranous PD-L1 expression in tumor cells by immunohistochemistry (Dako, Clone 28-8). She then received Stereotactic Radiosurgery (SRS) using the Brainlab Novalis® TX (Heimstetten, Germany), consisting of single fractions of 2,200 Centigray (cGy) to each of the three remaining brain lesions. Interval necrosis was seen on MRI (Figure 1B). Forty-two days later, she began nivolumab mono therapy (3 mg/kg) intravenous infusion every 2 weeks on a clinical trial. She experienced severe headache 7 days after her first infusion, and imaging revealed worsened edema with interval enlargement of enhancing brain metastases (Figure 1C). In an effort to spare corticosteroids, she began vitamin E (1000 units daily) and pentoxifylline (400 mg twice daily), and her headache resolved within five days. Nivolumab therapy was continued as scheduled. Follow-up brain MRI showed resolution of previous edema and shrinkage of prior brain metastases (Figure 1D). Thus, the previously observed lesion enhancement and edema was diagnosed as pseudoprogression. Continued follow-up showed reduction in LUL mass via thorax CT scan and brain MRI showed no signs of brain metastases with continued reduction in parietal and cerebellar lesions (Figure 2). More than 2 years later, she continues to receive nivolumab every 2 weeks, without any further toxicity.
Discussion
Pseudoprogression remains a rare event with PD-1 blockade. In the seminal phase I trial of nivolumab, only 4.6% of NSCLC patients had unconventional responses; mostly emergence of new lesions coupled with persistent reduction in target lesions [6]. Pseudoprogression is particularly challenging to the assessment of tumor response in clinical practice [7]. Nonetheless, the occurrence of pseudoprogression in previously treated intracranial metastases remains an important clinical scenario for clinicians to be aware of.
In this case, one cannot entirely rule out that pseudoprogression was due to prior radiation alone. However, radiation-induced pseudoprogression in brain tumors usually appears at least eight weeks after therapy and uncommonly involves mass enhancements [5]. This longer timeline may be because radiation pseudoprogression has been attributed to disrupted myelin synthesis from oligodendrocyte injury [8]. In a pilot study of stereotactic radiation of small brain metastases, radiation effects required a median of 8 months to become clinically apparent and imaging changes persisted for months, even after initiation of vitamin E/pentoxifylline [9]. Similarly, SRS-related radiation necrosis has been reported to occur at a median of 15 months after treatment [10]. In contrast, symptoms were notable in this index case within 7 days post-nivolumab, and symptom resolution was rapid. This short interval is consistent with other reports of intracranial pseudoprogression with PD-1 antibody [11,12]. Overall, the timeline of events in the case seem to support a causal role for nivolumab.
The underlying mechanism of the pseudoprogression observed with immune checkpoint inhibitors in the setting of prior radiation remains unclear. Radiation stimulates antigen processing, presentation, and diversity. For brain tumors in particular, external beam radiation induces Major Histocompatibility Complex (MHC) class I expression in a dose-dependent manner [13]. Radiation enhances the Intercellular Adhesion Molecule 1 (ICAM-1) and Fas expression on tumor cells, thereby stimulating T-cell infiltration into tumor [14]. This may present similarly to a radiation recall reaction which is an acute inflammatory reaction confined to previously irradiated areas associated with cytotoxic chemotherapies. However, radiation recall appears to be more associated with cutaneous manifestations [15]. Pseudoprogression and accompanying edema is reported to occur with brain lesions [16]. Combining SRS with anti-PD-1 in murine C57 BL/6J glioma models increased the intratumoral cytotoxic to regulatory T-cell ratio, thereby promoting a pro-inflammatory tumor microenvironment [17]. This combination increased animal survival compared to mono therapy. Along these lines, an orthotopic intracranial murine C57 BL/6 glioma model testing SRS combined with immune checkpoint inhibitors found synergistic activity compared to either modality alone, and higher density of CD4+ and CD8+ tumor infiltrating lymphocytes noted in combination treatment [18]. Complete objective responses correlated with formation of antigen-specific protective memory.
The durability of response in both extra-cranial and intra-cranial tumor for our subject attests to the importance of antigen-specific memory cells. Without a post-treatment brain biopsy, we could not identify the cell types responsible for the inflammatory phenomena observed in this case. The peripheral enhancement with accompanying edema we observed at day +10 (Figure 1) may have been attributable to immune cell infiltration. Murine BALB/c nu/nu models have demonstrated immune cell infiltration of NSCLC brain metastatic foci by microglia forming cavities to attenuate tumor proliferation [19]. PD-1 attenuates T-cell activity late in the immune cell activation process, and PD-1 blockade may produce an enhancing necrotic or edematous appearance in T-cell targeted tumor tissue [20]. Infiltrates of activated microglial cells and scattered CD8+ cells were identified in a resected intracranial melanoma metastasis following treatment with pembrolizumab [11]. For a similar case of a NSCLC brain metastasis enlarging 3 months after nivolumab, resection of the lesion revealed large areas of coagulative necrosis and reactive gliosis [12]. In a trial of nivolumab for relapsed/refractory glioblastoma multiforme, some cases of intracranial pseudoprogression were observed. In an asymptomatic subject, after five nivolumab doses, routine MRI revealed new periventricular contrast enhancement [21]. Resection of the involved lesion showed an inflammatory CD45+ infiltrate, with pronounced intratumoral macrophages. Therapy was continued, no corticosteroids were required, and the edema resolved. In our case, we used pentoxifylline with Vitamin E for the headache. Numerous case reports demonstrate clinical regression of radiation induced fibrosis with improvement of clinical symptoms following combined Pentoxifylline and Vitamin E therapy [22]. Pentoxifylline is a methylxanthine derivative with reports of anti-TNF α effect and inhibitory effect on inflammatory reaction while Vitamin E appears to scavenge reactive oxygen species and reduce free radical induced damage associated with increased rate of damaged DNA removal. Combined, these agents show significant anti fibrotic effect in cases of radiation induced fibrosis in randomized trials [23]. We used this approach to attenuate the inflammatory environment of the lesions without antagonizing the effect of immune therapy on the targeted lesions. Together, these cases indicate that systemic corticosteroids can sometimes be avoided in favor of observation, or supportive measures for mildly symptomatic cases.
Conclusion
Pseudoprogression of previously irradiated brain metastases is a rare but notable phenomenon associated with anti-PD-1 therapy. We suggest that clinicians and radiologists remain cognizant of this possibility in patients who experience apparent enlargement of previously inactive brain metastases. Moreover, a corticosteroid-sparing approach for symptoms can be considered in select circumstances.
Acknowledgements
This research was supported by an award from the Office of Research, Innovation & Scholarly Endeavors (RISE) at USF Health, Morsani College of Medicine.
Funding
This work was supported by the National Institutes of Health [P30 CA076292/CA/NCI NIH HHS].
Ethics Approval and Consent to Participate
Yes, all participants provided written informed consent. Approved by Liberty IRB; IRB00003411.
References
- Lipson EJ, Sharfman WH, Drake CG, et al. (2013) Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res 19: 462-468.
- Lipson EJ (2013) Re-orienting the immune system: Durable tumor regression and successful re-induction therapy using anti-PD1 antibodies. Oncoimmunology 2: e23661.
- Wolchok JD, Hoos A, O'Day S, et al. (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15: 7412-7420.
- Wiggenraad R, Bos P, Verbeek-de Kanter A, et al. (2014) Pseudo-progression after stereotactic radiotherapy of brain metastases: lesion analysis using MRI cine-loops. J Neurooncol 119: 437-443.
- Parvez K, Parvez A, Zadeh G (2014) The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci 15: 11832-11846.
- Gettinger SN, Horn L, Gandhi L, et al. (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33: 2004-2012.
- Clarke JL, Chang S (2009) Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep 9: 241-246.
- Brandsma D, Stalpers L, Taal W, et al. (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9: 453-461.
- Williamson R, Kondziolka D, Kanaan H, et al. (2008) Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg 86: 359-366.
- Telera S, Fabi A, Pace A, et al. (2013) Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol 113: 313-325.
- Cohen JV, Alomari AK, Vortmeyer AO, et al. (2016) Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res 4: 179-182.
- Doherty MK, Jao K, Shepherd FA, et al. (2015) Central Nervous System Pseudoprogression in a Patient Treated with PD-1 Checkpoint Inhibitor. J Thorac Oncol 10: 100-101.
- Klein B, Loven D, Lurie H, et al. (1994) The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg 80: 1074-1077.
- Garnett CT, Palena C, Chakraborty M, et al. (2004) Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 64: 7985-7994.
- Burris HA 3rd, Hurtig J (2010) Radiation recall with anticancer agents. Oncologist 15: 1227-1237.
- Hygino da Cruz LC Jr, Rodriguez I, Domingues RC, et al. (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 32: 1978-1985.
- Zeng J, See AP, Phallen J, et al. (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 86: 343-349.
- Belcaid Z, Phallen JA, Zeng J, et al. (2014) Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One 9: e101764.
- Noda M, Seike T, Fujita K, et al. (2009) The role of immune cells in brain metastasis of lung cancer cells and neuron-tumor cell interaction. Ross Fiziol Zh Im I M Sechenova 95: 1386-1396.
- Shih K, Arkenau HT, Infante JR (2014) Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 74: 1993-2013.
- Sampson J, Vlahovic G, Sahebjam S, et al. (2015) Preliminary safety and activity of nivolumab and its combination with ipilimumab in recurrent glioblastoma (GBM): CHECKMATE-143. Journal of Clinical Oncology.
- Chiao TB, Lee AJ (2005) Role of pentoxifylline and vitamin E in attenuation of radiation-induced fibrosis. Ann Pharmacother 39: 516-522.
- Delanian S, Lefaix JL (2004) The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol 73: 119-131.
Corresponding Author
Ben C Creelan, MD, MS, Department of Thoracic Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive FOB1, Tampa, FL 33612, USA, Tel: 813-745-3050, Fax: 813-745-3027.
Copyright
© 2017 John S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.