A Quantitative Assessment of Lung Tumor Motion under Abdominal Compression using 4D-CT Imaging
Abstract
Stereotactic Body Radiation Therapy (SBRT) has rapidly emerged as a curative procedure for early stage non-small cell lung cancer (NSCLC). The ablative hypo-fractionated radiation doses used with SBRT requires very small tumor margins, making tumor motion management very important to reduce the risk of long term morbidity. However, in conventional free-breathing situations, lung tumors can reportedly move up to 2.5 cm requiring a large treatment margin. In this clinical study, we analyzed the efficacy of using simple abdominal compression to dampen and suppress tumor motion. Sixty-five patients treated with SBRT between 2009 and 2013 were investigated. Patients were anatomically categorized based on lung lobe location as follows: 17 had lesions appearing in the right upper lobe (RUL), 7 in right middle lobe (RML), and 18 in right lower lobe (RLL), 14 in left upper lobe (LUL) and 9 in left lower lobe (LLL). 4D-CT data sets were acquired using a GE RT16 CT scanner with a 1.25 mm slice width in conjunction with a Varian's Real-time Position Management (RPM) system. On the GE Advantage 4D workstation, images were binned in 10 phases, T00 being the maximum inspiration phase & T50, the maximum expiration phase. The tumor volume was segmented at its centroid position using the CT-lung window. Tumor displacement was then measured from phase to phase in all three directions superior-inferior, anterior-posterior & medial-lateral. The mean tumor movement in each lobe was as follows: RUL = 3.8 ± 2.0 mm (mean ITV: 9.1 cm3), RML = 4.7 ± 2.8 mm (mean ITV: 9.2 cm3), RLL = 6.6 ± 2.6 mm (mean ITV: 12.2 cm3), LUL = 3.8 ± 2.4 mm (mean ITV: 18.5 cm3), & LLL = 4.7 ± 2.5 mm (mean ITV: 11.9 cm3). These results show tumor motion is anatomic (lobe) location dependent, specifically the magnitude of motion increases as the target nears the diaphragm. Furthermore, we found tumor amplitude to be respiratory cycle dependent as well, generally decreasing for shorter cycle duration. For RUL lesions, a strong correlation between the ranges of tumor movement with respect to respiratory cycle was noticed. Besides, for RML & RLL tumors, a linear correlation between the ratio of GTV50 volume with ipsilateral lung volume vs. tumor motion has been found [R2 > 0.9]. No tumor motion dependence on the tumor size was seen. The present study has demonstrated the usefulness of a simple abdominal compression in the management of NSCLC using radiotherapy.
Keywords
Four dimensional computed tomography (4D-CT), Stereotactic body radiation therapy (SBRT), Non-small cell lung cancer (NSCLC)
Introduction
The ultimate goal of stereotactic body radiation therapy (SBRT) is to achieve complete local tumor control while minimizing damage to surrounding tissue. Unlike conventional external beam radiation therapy, SBRT delivers a very high dose of radiation in a relatively short overall treatment time, resulting in greater local tumor eradication [1,2]. The clinical advantage of SBRT can be improved by reducing dose to normal tissue and critical structures, maximizing tumor coverage via conformal beam delivery and accurate target localization [3], and patient immobilization [4]. In most SBRT cases, the entire tumor excursion in a breathing cycle is used to define an internal target volume (ITV) to ensure an adequate dose distribution is delivered relative to a moving target [5]. Since lung SBRT is reserved for small tumor volumes, it is generally well tolerated unlike standard lung radiotherapy where excessive tumor margins limit our ability to prescribe higher radiation dose as well as increase the risk of pulmonary toxicity [6]. Since long term morbidity data is scarce for this new extreme form of hypo-fractionation regimen, it is of paramount importance that the ITV is constrained to a minimal target volume in order to diminish the levels of damage to nearby healthy tissue [7].
Lung SBRT is particularly challenging for two major reasons. First, lung tissue is a relatively low density medium. Irradiation of normal lung tissue of nominal density 0.25-0.37 g/cm3 surrounding the tumor [8] gives rise to a greater range of secondary Compton scatter which increases the risk of healthy tissue damage [9]. Second, tumors within the lungs can exhibit a high degree of dynamic displacement. In free breathing scenarios, lung tumor amplitudes as much as 2.5 cm have been reported [10]. Therefore, motion management is very important in the treatment of lung tumors, particularly with NSCLC patients being treated with SBRT because of the ablative nature of dose being delivered [11-13].
Even though effectiveness of abdominal compression in reducing lung tumor has been studied by various authors [14-18], this study is the first to publish the largest population of such patients with their tumors further subdivided in individual lobes. Further 4D-CT has been used to evaluate the overall range of tumor movement with respect to both volume and respiratory cycle duration to substantiate whether or not there is correlation. We will also study if tumor volume and lung volumes have any relationship with the tumor motion. Fundamentally, understanding tumor trajectory in terms of location, volume and patient respiratory cycle would lead to custom designed margins to potentially reduce the long-term toxicity of the critical structures.
Materials and Methods
Patient population
Sixty-five patients diagnosed with early stage Type I/II medically inoperable NSCLC between January 2009 and July 2013 were included in this retrospective study. All patients were treated for lung SBRT under controlled breathing conditions using an external Stereotactic Body Frame System. The sample had 38 females and 27 males with a median age of 82 years (range 54-89 yr). Tumors were categorized by lung lobe location: Right Upper Lobe (RUL), Right Middle Lobe (RML), Right Lower Lobe (RLL), Left Upper Lobe (LUL), and Left Lower Lobe (LLL). Prior to treatment, these patients underwent 4D-CT scanning under controlled breathing conditions for motion assessment (i.e. using abdominal compression) and subsequent treatment planning. Gross tumor volumes (GTV) were contoured by a radiation oncologist in each of the 10 binned phases. Internal target volumes (ITV) for patient planning was determined by using a BOOLEAN "OR" operation on the GTVs drawn in each of the 10 phases. Table 1 summarizes the patient information and treatment volume statistics according to tumor location.
Image acquisition
Patients were immobilized in the supine position with arms above their head using a Medical Intelligence BodyFIX® immobilization system (Elekta, Sweden) along with an abdominal compression diaphragm (model # P10102-119). The abdominal compression system was then used to dampen the tumor motion. The abdominal compression system involved a rectangular bar which was firmly attached to the body frame (BodyFix), a triangular rigid plate and a graduated screw. The rectangular bar had the necessary hole through with the graduated screw can be moved to exert pressure on the underlying rigid plate which was placed approximately 3-4 cm below the costal margin of the ribs and inferior to the xiphoid process. The abdominal compression screw was then tightened until the patient was able to tolerate it and did not feel any undue discomfort. The position of the screw was recorded during Simulation and reproduced during each treatment session. 4D-CT images were performed using a GE RT16 CT scanner (General Electric Medical Systems, Waukesha, WI) along with a Varian Real-time Position Management (RPM) respiratory gating system (Varian Medical Systems, Palo Alto, CA) version 1.7.5. The RPM system utilizes an infrared emitter, block reflector, and camera to measure the patients' respiratory pattern typically displayed as an asymmetric harmonic waveform. In all the cases, these 4D-CT scans included only the tumor along with 3-5 cm superior-inferior margins and were scanned using 1.25 mm slice width. On the Advantage 4D Workstation, images were binned in 10 phases where T00 signifies the patient maximum inhalation phase and T50 represents maximum exhalation. It is also noted that the Advantage 4D system displays values of minimum, maximum and average respiratory cycle data of the 4D-CT images, information which we used later in this study.
Measurement technique
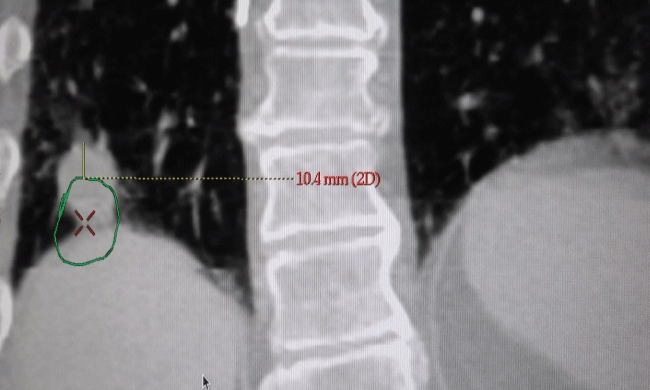
Following respiratory binning, on the Advantage 4D work station 4D-CT scan images were analyzed using a CT-"lung window". In this window, the lesion was segmented in axial, coronal and sagittal views approximately at the centroid of the tumor in phase T50 (exhale phase). Motion amplitudes were systematically determined for all the phases by studying the maximum 3D displacement of the tumor along each axis. Actual range of tumor displacement was performed by using the measurement tool feature of the Advantage 4.1 software. To minimize error in location, the tumor was visually maximized as much as possible on screen, insuring we were observing the tumor relatively close to its centroid cross-section. The process was repeated in all the cut-sections [transverse, sagittal and coronal] resulting in 2 pairs of motion values along each axis [superior-inferior, lateral-medial and anterior-posterior]. Maximum value along each axis was then recorded for each patient. The methodology of this technique is illustrated in figure 1.
3D tumor magnitude, mobility and statistical considerations
The overall magnitude (ΔM) of tumor movement was calculated by taking the square of the amplitude along each direction such that:
Where Δx represents tumor displacement along the medial-lateral (ML), Δy along the anterior-posterior (AP), and Δz along the superior-inferior (SI) directions. It follows that the tumor mobility rate can be defined as
Where Δt represents the duration of the patient's respirator cycle.
We used Spearman's correlation to determine the relationship between tumor motion (ΔM) with respect to both volume (GTV50) and respiratory cycle duration (Δt), respectively, with criteria for significance being p < 0.05. Spearman rho is given by:
Where di is the difference in rank from two data sets (ΔM and GTV50 for the first analysis and ΔM and Δt for the second analysis), and n is the sample number (number of patients in that lobe). The strength of the Spearman correlation is constrained by the criteria such that -1 ≤ rs ≤ 1. Prism 6 (GraphPad Software, Inc., La Jolla, California) statistical software was used for all analysis.
Results
Anatomical dependence
Table 2 summarizes the data gathered in this study, listing the average respiratory cycle in comparison to the amplitude of tumor movement in all three directions by tumor location. It shows that greatest range of tumor motion occurred in the RLL with a mean magnitude of 6.6 ± 2.6 mm, while a slightly less magnitude of 4.7 ± 2.5 mm was observed for the LLL. In the upper regions of the lungs, both the RUL and LUL exhibited the least range of movement of 3.8 ± 2.0 and 3.8 ± 2.4 mm, respectively. As compared to ML (Δx) and AP (Δy) amplitudes, we found the average range of motion in the SI (Δz) direction to progressively increase from 2.2 ± 1.3 to 5.8 ± 2.2 mm as we move from the superior to inferior directions of the lungs, suggesting the predominate driving mechanism was caused by the movement of the diaphragm. In all cases, the ML direction had the least range of movement whereas in the LLL cases, we recorded the greatest mean motion of 2.0 ± 0.8 mm.
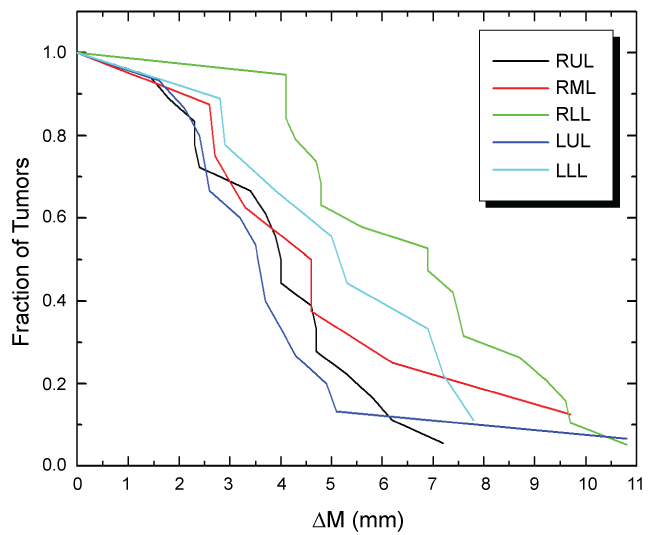
Following similar presentation by Lui, et al. [11] from a familiar histogram perspective figure 2 illustrates the overall range of tumor motion versus the fraction of tumors with respect to lobe location. Once again, what we are seeing is greater overall movement is happening in lower lobes, with an exceptional outlier found in the LUL. From this graph, it is readily evident that none of tumors in this cohort exceeded more than 10.8 mm of overall movement.
Tumor volume dependence
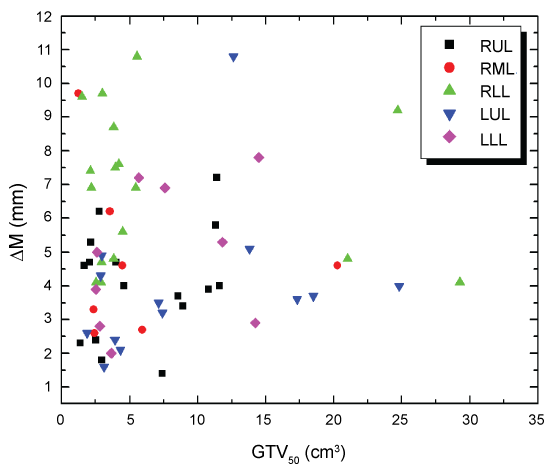
Out of all cases analyzed, the mean GTV volume was 7.90 ± 8.15 cm3. The breakdown by lobe location for GTV is as follows: RML = 5.77 ± 6.55, RLL = 7.28 ± 8.66, RUL = 5.90 ± 3.97, LUL = 13.28 ± 16.52 and LLL = 7.28 ± 5.01 cm3. Figure 3 is a scatter plot showing the overall tumor motion as function of the GTV50 for each patient delineated at the end of the expiration phase (T50). Even when grouping by lobe location, what we are not seeing is the anticipated motion-volume relationship where an increasing GTV volume leads to a reduced range of tumor motion when abdominal compression is used. It should be noted, however, that all our patients are early type I/II stage NSCLC, so as compared to other studies, our tumor volumes are relatively small and below a certain threshold to indicate any kind of trend. Using Spearman's rho test, we found no statistical correlation between the tumor magnitude and GTV50 in any of the lobes. This is in contrast to the general belief that small tumors generally have larger tumor motion and vice-versa. Even so, figure 3 does reveal the general trend for which anatomic location is playing a major role in governing the overall range of tumor displacement.
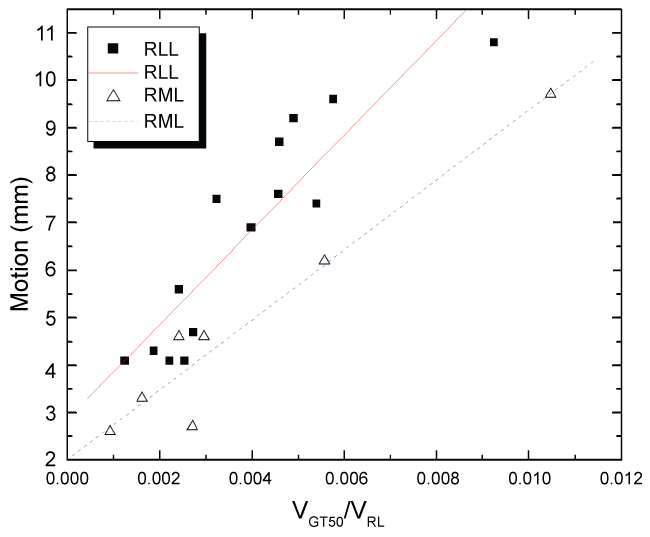
While no relation was noticed between the tumor motion and ipsilateral lung volume, however, when GTV50 Volume/Ipsilateral lung volume was plotted against tumor motion for different lobes, a strong linear correlation was noticed for RLL & RML patients [R2 > 0.9 and higher] (Figure 4). As expected, RLL tumors demonstrated a higher slope then RML patients.
Respiratory cycle dependence
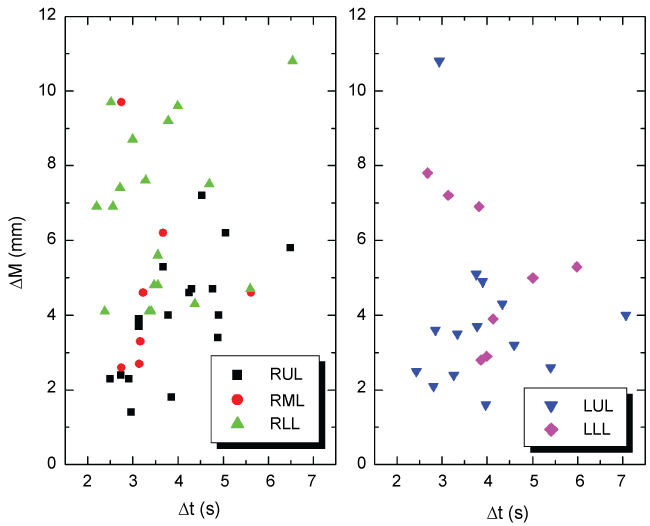
The median respiratory cycle for all patients was found to be 3.81 ± 1.08 seconds, ranging from a minimum cycle of 2.50 seconds to maximum of 7.07 seconds. Figure 5 shows the magnitude of tumor movement for each individual case plotted as a function of respiratory cycle duration (Δt) for the right and left lungs.
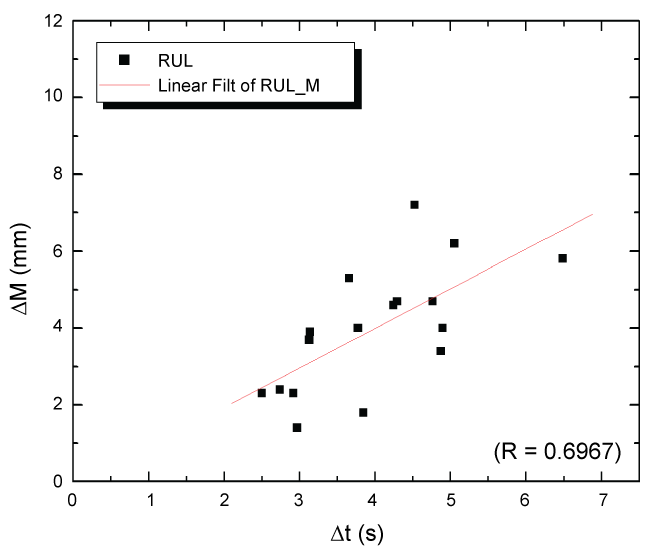
Using two-tailed Spearman statistical correlation analysis for RUL tumors, we did find a strong, positive monotonic correlation between overall tumor motion and respiratory cycle (rs = 0.682, n = 17, p < 0.003). As shown in figure 6, the data represented in RUL is tightly grouped for which a linear fit projects an intercept close to an origin intercept. In the remainder lobes, we found no Spearman correlation, for which our results are as follows: RML (rs = 0.291, n = 7, p = 0.520), RLL (rs = 0.176, n = 18, p = 0.484), LUL (rs = 0.164, n = 14, p = 0.584), and LLL (rs = 0.524, n = 9, p = 0.197).
From the data listed in table 2, we calculated the tumor mobility rate by dividing the mean motion magnitude by cycle duration. From our results, the average tumor mobility increases as the tumor's distance from diaphragm decreases and is given as for RUL = 0.95 ± 0.49 mm/s, RML = 1.35 ± 0.62 mm/s, RLL = 1.83 ± 0.71 mm/s, LUL = 0.98 ± 0.50 mm/s, and LLL = 1.15 ± 0.53 mm/s. Figure 7 is plot of tumor magnitude and mobility with respect to relative location for the right and left lungs. As shown in figure 7b and figure 7d, if we were to assume an arbitrary unit spacing between the right lung lobes, then the best fit to these mean data values would be a linear function with positive slope. In any case, this graph illustrates inferiorly located (closer to diaphragm) tumors will generally move at faster rates over superiorly located tumors (near apex of the lung).
Discussion
Pulmonary tumor movement is primarily driven by diaphragm motion, expansion of the chest wall cavity, and internal organ actions such as cardiac rhythms generated by the heart. To restrict lung tumor movement, the implementation of respiratory management techniques [19] such as deep inspiration breath hold (DIBH) [20], end-exhale phase respiratory gating [21] and use of abdominal compression devices [14,22] have proven to be considerably effective. DIBH technique depends on active patient participation and compliance limiting the number of patients who can benefit from it to be less than 50% [21].
In the end-exhale respiratory gating technique, the patient's respiratory trace is traced and recorded during simulation. Then an appropriate gate is defined when the linear accelerator will be ON. This pattern is then used during treatment which could be up to 30-40% of the total beam ON time. This technique results in a longer treatment time and the need for additional staff's time and effort [21,23]. It is important to note that respiratory gating technique relies on the assumption that the external surrogate truly represents the tumor motion in 3D. This, however, may not be reliable because of variations in the correlation and phase shifts between the surrogate and internal structure motion [24,25]. Moreover, patients need either an audio or video assistance during treatment to ensure a reproducible breathing pattern. Some centers ignore the tumor motion during the chosen gate and do tumor segmentation only at the mid-level or at 50% phase. As a result, the benefit of respiratory-gated radiation therapy in the management of thoracic malignancy is controversial and many authors claiming that it offers only minimal benefit [26,27]. In comparison, while the end-exhale respiratory gating technique is better tolerated by patients over DIBH, it doesn't provide the physical separation of the tumor from critical structures. In both techniques, patient's audio coaching and/or visual feedback play an important role [21].
Abdominal compression, on the other hand, requires less treatment time and doesn't depend as much on patient compliance. Controlled/Forced shallow breathing is a method using abdominal compression to dampen the patients breathing and tumor motion. In previous studies, Negoro, et al. reported that abdominal pressure using the Stereotactic Body Frame System reduced respiratory tumor motion significantly, from a range of 8 to 20 mm to a range of 2 to 11 mm [14]. Liu, et al. reported that the average tumor motion left-right, anterior-posterior and caudal-cranial direction were 1.2, 1.6, and 14.7 mm for lower lung tumors using abdominal compression plate, respectively. [12]. Recently, Timmerman, et al. studied the tumor motion for lung and liver SBRT patients using abdominal compression using medium and high compression [15]. They found that the mean total motion was 13.6, 8.3 & 7.2 mm with no compression, medium compression and high compression, respectively, though these values are not exclusive of lung tumors. In comparing tumor displacements in free breathing patients, abdominal compression can clearly restrict motion by as much as a factor of 2, making it our choice modus of operandi. Besides, unlike external surrogates used in respiratory gating [24,25], there should not be any concern about the phase shift between the external surrogate and the tumor motion which improves the confidence in the dose delivery accuracy.
In this retrospective study, we found median lung tumor movement along SI direction at the base of lungs to be 4.7 mm for patients under abdominal compression, less than a factor of 2 of what has been reported in free breathing studies [11-13]. The primary mechanism behind this decrease in tumor motion is the abdominal compression control by limiting diaphragmatic movement and reducing breathing volume. Tumors located in the apex of the lung regions (RUL and LUL) exhibit the least range of motion, whereas tumors residing towards the base of the lungs had the greatest range motion. Our observations confirm that tumor movement is primarily in the SI direction, and are in consistent agreement with results previously reported by Seppenwoodle [11], Liu [12] and Yu [13]. In the upper regions of the lung, we measured comparable ML and AP motion amplitudes indicating movement may be caused by cardiac motion and/or chest expansion-compression, where Hanley, et al. reported the amplitude of chest wall movement can range from 2-2.5 mm [28].
In our study, the mean respiratory tumor motion for all patients irrespective of tumor location was 1.9 ± 0.8 mm (range, 1.7-4.6 mm) in the medial-lateral direction, 2.4 ± 1.3 mm (range, 2.3-8.7 mm) in the anterior-posterior direction, 3.6 ± 2.3 mm (range, 3.0-9.3 mm) in the superior-inferior direction, and 4.7 mm (range, 1.4-10.8 mm). A 2-tailed paired t-test was carried out to test the significance of motion along two mutually perpendicular axis (Anterior-posterior vs. medial-lateral), (Superior-Inferior vs. medial-lateral) and (superior-inferior vs. anterior-posterior) which did not reveal any correlation. Abdominal compression levels were set by a single clinician with continuous feed-back from the patient and hence were more reproducible. None of the patients complained of any discomfort either during a CT simulation or actual treatment. It is also important to note that abdominal compression not only minimizes the intrafraction tumor motion, but also reduces the need of respiratory gating during treatment. The later increases the treatment time by reducing the duty-cycle to 40-60%, depending upon the gate selected. Moreover, the non-coplanar nature of the treatment beams commonly used in SBRT treatments, also requires frequent re-tracking of the RPM software, thereby, further increasing the treatment time. Shorter overall treatment time using abdominal compression improved patients comfort and was well tolerated.
As illustrated in figure 3, the variation in the range of tumor movement is significant and can be caused by a number of factors. First, it is well known that "unfixed" tumors exhibit a greater range of motion. In an in-depth investigation performed by Seppenwoolde, et al. [11] lung tumors tagged with gold markers confirmed that unfixed tumors exhibited greater range of motion as compared to those attached to a rigid surface such as the chest wall. It is also noted the lungs are not entirely homogeneous, where brachial tubal structures would be stiffer as compared to surrounding elastic lung tissue. Likewise, we could anticipate patients with inelastic lung tissue characteristics, such as those suffering from emphysema, would have dampening effect leading to a decrease in tumor mobility as well.
Second, in general smaller tumors will tend to experience greater overall movement. As compared with results reported by Plathow, et al. [29] and Maxim, et al. [30] where the observed magnitude of tumor motion decreases as GTV increases, we were unable to verify this behavior due to our much smaller tumor volume sample size. It is important to note that in our study we were only looking patients diagnosed with early stage type I/II NSCLC where our maximum GTV measured no greater than 5 cm in size. In other words, all our tumors could be considered small. Conversely, considering the data presented by Lui, et al. [12] where from their 156 patient cohort analyzed tumor volumes up to 800 cc, it becomes more apparent when a certain threshold tumor volume is reached which causes the overall movement to decrease. On a similar note, Lui also measured the average diaphragm displacement to be 1.65 cm, which in some cases is less than tumor movement itself [12]. They attribute the sponginess of the lung tissue giving rise to a linear "rubber band" effect, and, in association with diaphragm movement, to account for the greater SI directed tumor displacement.
Finally, pulmonary function dependence governing tumor motion remains to be determined [13]. Although we did find a strong correlation in the RUL between tumor movement and patient's respiratory cycle as shown in figure 5, the remainder lobes did not show any similar correlation. We have come to realize small sample size may be a difficult problem to overcome, particularly when trying to find patients diagnosed with lesions in the RML. In retrospect, however, Seppenwoolde, et al. reported tumor motion was found to be more asymmetrical for patients with relatively long breathing cycles, giving rise to more pronounced tumor hysteresis [11]. Although we expect shallow breathers to experience less tumor movement, in two anomalous cases as seen in figure 5 the RML and LUL data shows the greatest range tumor movement occurred for patients with relatively short respiratory cycle (< 3 s, approximately 25% less than average). In both these cases, the measured tumor motion along the AP direction was substantial (RML Δy = 4.7 mm and LUL Δy = 8.7 mm) leading to an overall greater tumor magnitude. Considering the frequency of a nominal cardiac rhythm is approximately 3-4 times that of a normal breathing cycle [11], we believe in these cases where AP displacement is relatively large, may represent a certain moment when both the respiratory and cardiac maximum displacement was captured simultaneously in the same phase bin. Unfortunately, due to the limited scope of this study, we have no cardiac data to support this hypothesis. It is for this reason we did not project a linear fit of our tumor movement versus respiratory cycle data through zero, which would neglect any contribution of residual tumor motion caused by other internal organ movements.
It is interesting to note both tumor movement and mobility were consistently found to be greater in right versus the left lung. More specifically, tumors in RLL had a greater range of displacement than those in LLL which may be due to the fact that the heart will generally occupy about half the surface area of the diaphragm when looking from an axial midsection perspective. As compared to the left lung, the right lung experiences approximately twice as much surface area of the diaphragm which comes into contact with the outer pleurae membrane at the base of the lung, thus resulting in a greater direct force driving movement. Accordingly, we think of the base of the heart acting as a dampening mechanism, yielding a comparatively lesser range of motion in the left lung than that in right lung.
The error bars are large because of the variation of tumor sizes in each of the lobes, the limited number of SBRT patients, and patient comfort variations. Patient comfort is a major factor because breathing compression level for each patient varies. Although controlled breathing patients have an abdominal pressure device to reduce diaphragmatic motion, each patient's breathing pattern differs. Moreover, each patient may have different level of tolerance to bear the amount of abdominal pressure.
In summary, lung tumor movement is complex [31] and can be influenced by a number of factors. Although tumor motion is more driven in the SI direction as we approach the base of the lungs, the correlation with diaphragm motion is not entirely clear due to tissue composition, delay in tumor movement, whether or not the tumor is fixed, lung in homogeneity's, and overall non-isotropic movement [11,13]. In future work, it would be advisable to simultaneously record both respiratory motion and cardiac cycles to account for anomalous cases where AP motion may be playing a major role. Improvement of dose distribution conformality will ultimately be dictated by the accuracy of tumor trajectory.
One of the biggest short comings of the present work is the absence of the tumor motion data without any abdominal compression. This is due to the retrospective nature of this study and we have to rely on the free breathing lung tumor motion amplitude from the literature. However, this does not invalidate our results and the conclusions. Moreover, there was no quantitative standard for the application of abdominal compression to this group of patients. This was strictly the clinical evaluation of each individual case and the expertise acquired by the same Radiation Oncologist on all cases treated on this report.
Understanding of lung tumor motion plays an important role in the treatment of NSCLC with SBRT. Reducing tumor motion allows for more precise delivery of the prescription dose to smaller targets, therefore, improving the therapeutic ratio. Judicious use of the abdominal compression device may also be helpful in lesser toxicity due to reduced margin to the tumor and hence small volumes of normal structures.
Conclusion
This clinical study has shown that the range of lung tumor mobility to be lobe location dependent increasing as we are near the lower lobes of the lungs though tumors in right lower lobe showed more motion than of left lower lobe. No tumor motion dependence on the tumor size was seen though this could be due to the fact that the cohorts of patients in this group all had volumes which are much smaller than that in a general lung tumors. We also found a strong correlation for the RUL lesions between the ranges of tumor movement with respect to respiratory cycle. In general, but not always the case, the range of tumor motion was found to increase with the breathing period. Besides, for RML & RLL tumors, a linear correlation between the ratio of GTV50 volume with ipsilateral lung volume and tumor motion was found [R2 > 0.9]. A simple abdominal compression ensures comparatively shorter treatment times for patients, thereby, improving their comfort and compliance during treatment and was well tolerated by our patients.
Acknowldgements
The authors would like to thank Ms. Haritha Jupudy for her assistance in determining the 3D lung tumor motion and Mrs. Jennet Hardwick help in the retrieval of archived 4DCT data.
References
- Benedict SH, Yenice KM, Followill D, et al. (2010) Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys 37: 4078-4101.
- Robert D Timmerman, Hak Choy, James M Galvin, et al. (2004) RTOG 0236 A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I/II Non-Small Cell Lung Cancer. Radiation therapy oncology group.
- Byhardt RW, Martin L, Pajak TF, et al. (1993) The influence of field size and other treatment factors on pulmonary toxicity following hyperfractionated irradiation for inoperable non-small cell lung cancer (NSCLC)-analysis of a Radiation Therapy Oncology Group (RTOG) protocol. Int J Radiat Oncol Biol Phys 27: 537-544.
- Lax I, Blomgren H, Naslund I, et al. (1994) Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta oncol 33: 677-683.
- Wambersie A (1998) Prescribing, recording, and reporting photon beam therapy. Journal of the ICRU 4: 2.
- Allen AM, Siracuse KM, Hayman JA, (2004) Evaluation of the influence of breathing on the movement and modeling of lung tumors. Int J Radiat Oncol Biol Phys 58: 1251-1257.
- Timmerman R, McGarry R, Yiannoutsos C, et al. (2006) Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 24: 4833-4839.
- Garnett ES, Webber CE, Coates G, et al. (1977) Lung density: clinical method for quantitation of pulmonary congestion and edema. Can Med Assoc J 116: 153-154.
- Ekstrand KE, Barnes WH (1990) Pitfalls in the use of high energy X rays to treat tumors in the lung. Int J Radiat Oncol Biol Phys 18: 249-252.
- Mageras GS, Pevsner A, Yorke ED, et al. (2004) Measurement of lung tumor motion using respiration-correlated CT. Int J Radiat Oncol Biol Phys 60: 933-941.
- Seppenwoolde Y, Shirato H, Kitamura K, et al. (2002) Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys 53: 822-834.
- Liu HH, Balter P, Tutt T, et al. (2007) Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 68: 531-540.
- Yu ZH, Lin SH, Balter P, et al. (2012) A comparison of tumor motion characteristics between early stage and locally advanced stage lung cancers. Radiother Oncol 104: 33-38.
- Negoro Y, Nagata Y, Aoki T, et al. (2001) The effectiveness of an immobilization device in conformal radiotherapy for lung tumor: reduction of respiratory tumor movement and evaluation of the daily setup accuracy. Int J Radiat Oncol Biol Phys 50: 889-898.
- Heinzerling JH, Anderson JF, Papiez L, et al. (2008) Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys 70: 1571-1578.
- Han K, Cheung P, Basran PS, et al. (2010) A comparison of two immobilization systems for stereotactic body radiation therapy of lung tumors. Radiother Oncol 95: 103-108.
- Bengua G, Ishikawa M, Sutherland K, et al. (2010) Evaluation of the effectiveness of the stereotactic body frame in reducing respiratory intrafractional organ motion using the real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys 77: 630-636.
- Bouilhol G, Ayadi M, Rit S, et al. (2013) Is abdominal compression useful in lung stereotactic body radiation therapy? A 4DCT and dosimetric lobe-dependent study. Phys Med 29: 333-340.
- Keall P, Vedam S, George R, et al. (2006) The clinical implementation of respiratory-gated intensity-modulated radiotherapy. Med Dosim 31: 152-162.
- Onishi H, Kuriyama K, Komiyama T, et al. (2003) CT evaluation of patient deep inspiration self-breath-holding: how precisely can patients reproduce the tumor position in the absence of respiratory monitoring devices? Med Phys 30: 1183-1187.
- Mageras GS, Yorke E (2004) Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol 14: 65-75.
- Mampuya WA, Nakamura M, Matsuo Y, et al. (2013) Interfraction variation in lung tumor position with abdominal compression during stereotactic body radiotherapy. Med Phys 40: 091718.
- Fox T, Simon EL, Elder E, et al. (2007) Free breathing gated delivery (FBGD) of lung radiation therapy: analysis of factors affecting clinical patient throughput. Lung cancer 56: 69-75.
- Koch N, Liu HH, Starkschall G, et al. (2004) Evaluation of internal lung motion for respiratory-gated radiotherapy using MRI: Part I-correlating internal lung motion with skin fiducial motion. Int J Radiat Oncol Biol Phys 60: 1459-1472.
- Gierga DP, Brewer J, Sharp GC, et al. (2005) The correlation between internal and external markers for abdominal tumors: implications for respiratory gating. Int J Radiat Oncol Biol Phys 61: 1551-1558.
- Hau E, Rains M, Browne L, et al. (2013) Minimal benefit of respiratory-gated radiation therapy in the management of thoracic malignancy. J Med Imaging Radiat Oncol 57: 704-712.
- Ruben JD (2013) Respiratory-gated thoracic radiotherapy: much complexity for how much gain? J Med Imaging Radiat Oncol 57: 701-703.
- Hanley J, Debois MM, Mah D, et al. (1999) Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys 45: 603-611.
- Plathow C, Fink C, Ley S, et al. (2004) Measurement of tumor diameter-dependent mobility of lung tumors by dynamic MRI. Radiother Oncol 73: 349-354.
- Maxim PG, Loo BW, Shirazi H, et al. (2007) Quantification of motion of different thoracic locations using four-dimensional computed tomography: implications for radiotherapy planning. Int J Radiat Oncol Biol Phys 69: 1395-1401.
- Stevens CW, Munden RF, Forster KM, et al. (2001) Respiratory-driven lung tumor motion is independent of tumor size, tumor location, and pulmonary function. Int J Radiat Oncol Biol Phys 51: 62-68.
Corresponding Author
Harish K Malhotra, PhD, DABR, Department of Radiation Medicine, Roswell Park Cancer Institute, Buffalo, NY14263, USA.
Copyright
© 2017 Mohatt D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.