High Doses of Vitamin C and Leukaemia: In Vitro Update
Summary
Vitamin C (ascorbic acid) is an essential nutrient with a number of beneficial effects on the human body. Although the majority of mammals can synthesize their own Vitamin C, humans and a few other species, do not produce it and depend on dietary sources for their Vitamin C supply. Among its many effects on cell function and metabolism, Vitamin C has shown, in vitro, a powerful anticancer effect against a number of human tumour cell lines, including myeloid leukaemia. There are many different mechanistic explanations for the anticancer/anti leukemic effects of Vitamin C and the aim of the present review is to illustrate these mechanisms, showing the results of some preliminary in vitro investigations, and outlining their potential clinical relevance.
Introduction
Vitamin C is an essential nutrient with a number of beneficial functions, for the organism [1], such as
1. Helping the metabolism of tyrosine, folic acid and tryptophan,
2. Increasing the elimination of cholesterol,
3. Contributing to the synthesis of catecholamine's,
4. Helping the body to absorb and breakdown histamine,
5. Enhancing the absorption of non-heme iron,
6. Promoting the synthesis of collagen (its most widely known physiological function),
7. Neutralizing free radicals (it is a reducing agent, "scavenger" of free radicals, and a founder among the natural antioxidants),
8. Protecting DNA from damage due to free radicals and mutagens,
9. Reducing the risk of premature death,
10. Fighting off widespread environmental pollutants,
11. Preventing the development of nitrosamines.
Though ubiquitous, ascorbate is not produced by humans, guinea pigs, some primates, a particular type of fruit eating bat, the majority of fishes and birds [2], who depend on diet for the assumption and use of this fundamental nutrient.
Vitamin C and Leukaemia: Historical Background
The first mention of the therapeutic potentialities of Vitamin C in leukaemia, can be found in the book "The healing Factor: Vitamin C against disease", written by the biochemist Irwin Stone, in 1974 [3]. In his book, Stone refers to a study, performed in 1936 by Stephen and Hawley [4], demonstrating, for the first time, that when the blood is separated into plasma, red blood cells, and white blood cells, there is a 20- to 30-fold concentration of Vitamin C in the white blood cells, as compared to plasma.
Following this report, Barkhan and Howard, by studying a few cases of chronic myelogenous and lymphatic leukaemia, added the evidence that leukemic patients have substantially lower than normal plasma levels of Vitamin C [5].
As noted by Stone, although this knowledge could suggest the use of Vitamin C as a therapeutic agent, in leukaemia, the first clinical trials showed contrasting results, due to the inappropriately low doses administered.
Later on, Vogt, in a literature review [6], confirmed that there are high deficits of Vitamin C in leukemic patients, as also confirmed by the reports of Kyhos and Coll [7] in 1945, and Waldo and Zipf, in 1955 [8].
Since Vitamin C is a natural component of human nutrition, endowed with innumerable beneficial effects on human body [9-11], it is, to say the least, astonishing, to realize how little scientists have done, in eight decades, to verify the role and potentialities of this nutrient, in the prevention and treatment of human leukaemia.
According to Stone, leukaemia reduces the body stores of Vitamin C to very low levels, and any residual Vitamin C circulating in the blood is scavenged and locked in the excessive numbers of leukocytes usually contained in the blood of these patients. As a direct consequence, the plasma levels of Vitamin C are reduced to zero or close thereto, and tissues are no longer being supplied with this most important metabolite, since it is accumulated in leukocytes.
Stone defined "biochemical scurvy" the condition of insufficient Vitamin C supply to body tissues, and proposed that its correction required the administration of Vitamin C at a rate of 25 grams or more per day.
In 2012, seventy-six years after the first observations on the distribution of Vitamin C in leukocytes, an investigation on 131 patients affected by different types of leukaemia, definitively confirmed that leukemic patients have significant lower plasma Vitamin C than normal controls. The reduction of plasma Vitamin C levels in leukaemia, as predicted by Stone, is due to an increased uptake and utilization by the active proliferating leukocytes, leading to tissue biochemical scurvy and consequent increased tendency to bleeding and infections, which are the hallmark of this pathological condition [12,13].
With the above data at hand, it is clear that leukaemia can be viewed as a condition of functional Vitamin C deficiency, associated with biochemical scurvy, and therefore, all leukemic patients are suitable candidates for the treatment with this nutrient.
How Much Vitamin C to Treat Leukaemia? The Concept of "Mega-Doses"
In 1949, Frederik Klenner first reported the successful treatment of bulbar poliomyelitis, with high doses of Vitamin C administered by intramuscular, intravenous and oral route [14].
The same Author had established clinical protocols using massive doses of Vitamin C, to treat a number of different viral conditions [15,16], but it was Stone who formerly defined the concept and the rationale for the use of "mega-doses" (also known as "pharmacologic" doses) of Vitamin C in leukaemia and other cancers.
In particular, Stone observed that man and only a few other species do not produce their own Vitamin C, while the great majority of mammals do, according to their physiologic requirements [17].
This observation led Stone to hypothesize that, due to either insufficient intake or increased consumption of the nutrient, or both, man could easily undergo a condition that he defined "hypoascorbemia". Hypoascorbemia is a reduced amount of circulating Vitamin C (also called "ascorbic acid"), due to the lack of the enzyme L-Gulonolactone Oxidase (GLO), as a consequence of an "inborn error of carbohydrate metabolism" [18-20].
This defect, now very well acknowledged and characterized [21], led Stone to hypothesize that to be in good health, man needs mega-doses (several grams a day), rather than doses in the order of milligrams, as stated by the Recommended Daily Allowances (RDAs) [22-24].
The rationale behind the use of mega-doses of Vitamin C was further refined by the chemist and twofold Nobel Prize, Linus Pauling, who soon became an enthusiastic supporter of the use of this nutrient, in high doses, not only to prevent disease [25-31], but also to treat a number of pathologic conditions, ranging from common cold [32-37], to cancer [38-53], and AIDS [54].
Intravenous Vitamin C and Cancer
Studies on dose-concentration relationship in humans, performed by Levine and co-workers [55], revealed that at oral doses exceeding 250 mg/day, the plasma levels of Vitamin C reach a plateau, and any further increase in the amount administered by mouth, does not determine significant increase in plasma concentration. This is due to multiple "control" mechanisms, including, among others, intestinal absorption, tissue accumulation, renal reabsorption and excretion, and utilization. On the contrary, the intravenous administration of high doses of Vitamin C, bypassing the above control mechanisms, allows plasma concentrations that are 100-fold or higher than maximally tolerated oral doses, and the peak could last for hours within the millimolar (mM) range [56,57].
More importantly, at plasma concentrations easily achievable by intravenous administration (5-10 mM for 1-2 hours), Vitamin C induced death in 75% of 48 cancer cell lines tested in vitro [58], but had no toxic effect on human peripheral white blood cells, fibroblasts, or epithelial cells. This selective cytotoxic effect would be achieved since at high doses, parenteral ascorbate is a peroxide delivery system for the generation of sustainable ascorbate radical and H2O2 in the extracellular space, with consequent oxidative damage to cancer cells [59,60].
Therefore, high doses of Vitamin C would be cytotoxic to cancer cells because of their pro-oxidant, rather than anti-oxidant effects [61,62], even though, some Authors remark that the pro-oxidant activity of Vitamin C, may not be relevant, in vivo [63-65].
It has been suggested that the selective cytotoxic activity of high doses of Vitamin C against cancer cells, as compared to the normal ones, is due to their reduced levels of antioxidant enzymes, catalase, superoxide dismutase, and glutathione peroxidase. The reduced level of antioxidant enzymes leads to cellular damage by accumulation of hydrogen peroxide [66-69], with subsequent intracellular redox imbalance and oxidative damage to different cellular structures [70-72]. However, the proposed mechanistic explanation of the selective cytotoxic action of Vitamin C on cancer cells has always remained elusive and generic.
More recently, Yun and co-workers [73], by investigating the effects of high doses of Vitamin C on KRAS and BRAF mutants cells derived from Colorectal Cancer (CRC), have further refined the mechanist explanation of the anticancer properties of Vitamin C. In particular, according to the Authors, the death of KRAS and BRAF cell mutants of CRC is not caused by the Vitamin C itself, but rather, by its oxidized form, Dehydroascorbic Acid (DHA). While Vitamin C enter cells though specific receptors, called Sodium-Vitamin C Co-Transporters (SVCTs) [74], DHA competes with glucose, for intracellular uptake by Glucose Transporters (GLUT), mainly 1 and 4 subtype receptors [75,76].
Interestingly, both KRAS and BRAF activating mutations are responsible of the upregulation of GLUT1 expression in different types of cancer, including CRC [77,78]. However, as reported by Yun and coll [73], the upregulation of GLUT-1 expression, is not always associated with increased sensitivity of tumour cell lines to the cytotoxic effects of DHA.
Further investigation into the metabolic makeup of KRAS and BRAF mutations CRC-derived cell lines, showed an accumulation of glycolytic intermediates upstream Glyceraldehyde-3-Phosphate-Dehydrogenase (GAPDH) and a contemporary depletion of the metabolites downstream GAPDH, indicating an inhibition or severe reduction of its enzymatic activity, which appears to be the key of the cytotoxic effect of DHA.
In summary, the data reported by Yun and coll. on the effect of DHA on CRC cell lines, indicate that in glycolysis-addicted KRAS and BRAF mutated cell lines, high amounts of DHA are transported into the cancer cells, through the overexpressed GLUT-1 receptors, and then reduced again to Vitamin C inside the cells. The reduction of DHA to Vitamin C scavenges Glutathione (GSH), thus inducing redox imbalance and oxidative stress. Oxidative stress, in turn, leads to inactivation of GAPDH, inhibition of glycolysis, and energetic crisis, which leads to cell death [79].
More precisely, beyond being inactivated directly by ROS (including H2O2), GAPDH function is also hindered by the depletion of Nicotinamide Adenine Dinucleotide (NAD+), caused by the activation of the DNA repairing enzyme, Poly ADP-Ribose Polymerase (PARP), induced by the DNA. In fact, the increased production of ROS, in cancer cells, due to the high doses of Vitamin C, produces increased DNA damage and consequent activation of PARP. PARP, in turn, consumes NAD+ and NAD+ depletion (together with the consequent ATP depletion) determines the energetic crisis leading cancer cells to death [80].
Vitamin C and H2O2
The view that Vitamin C in high concentrations, administered by intravenous infusion, acts as a pro-oxidant, leading to the formation of H2O2, thus inducing oxidative damage to cancer cells, is not new. In 1969, when man first walked on the moon, Benade and co-workers had already demonstrated that Vitamin C could selectively kill cancer cells, without harming normal cells. The Authors suggested that the cytotoxic effect of ascorbate could be due ("in major part") to the intracellular (not extracellular!) generation of toxic hydrogen peroxide produced upon oxidation of the ascorbate, by the cells (and not by the conversion of DHA back to Vitamin C!). This view, was corroborated by the fact that the toxicity of ascorbate was greatly enhanced by the concomitant administration of 3-Amino-1, 2, 4-Triazole (ATA) that inhibit the enzyme catalase, "thus decreasing or destroying the ability of the cancer cells to detoxify H2O2 effectively" [81].
On the other hand, a number of scientific reports demonstrate that human cancer cells show low levels of antioxidant enzymes (including, among others, catalase and glutathione peroxidase), and therefore cannot detoxify hydrogen peroxide [82-84].
Moreover, accumulating evidence suggests that cancer cells produce high amounts of hydrogen peroxide [85], and hydrogen peroxide itself is considered a powerful carcinogen, associated with mutagenic potential [86].
Therefore, the assumption that ascorbate may act as a pro-drug of hydrogen peroxide, to kill cancer cells, is not only in disagreement with the experimental evidence, but is also challenged by the following considerations:
• During the late forties and early fifties, Frederick Klenner reported the successful treatment of 60 cases of bulbar poliomyelitis [14], with high doses of Vitamin C administered continuously by mouth, vein, and intramuscular injection, during 72-96 hours. He also established protocols for the treatment of a number of other viral diseases, based on high doses of Vitamin C [15,16];
• Although he could only figure out the mechanisms involved in this antiviral activity of Vitamin C, he definitely used high doses of the nutrient, to treat dreadful viral diseases successfully. Were these doses pro-oxidant or anti-oxidant? To the best of our current knowledge, we can conclude that several different mechanisms can explain the benefits of Vitamin C in the treatment of viral diseases, but none of them seems to be based on its presumed pro-oxidant properties [10,87-93];
• In no cases, the antiviral properties of Vitamin C have been ascribed to its presumed pro-oxidant activity, when administered in high concentrations, for the treatment of viral infections;
• The toxic effects of hydrogen peroxide on cancer cells were known since 1957, when Reginald Holman published a paper on "Nature", showing that rat implanted with Walker 256 adenocarcinoma, and treated by simply replacing their drinking water with 0.45% hydrogen peroxide, showed a rate of cure of 50-60%. The time taken for complete disappearance of the tumour was 15-50 day depending on the tumour size when the treatment was started [94]. Holman's work was based on the assumption (later confirmed by studies on Vitamin C) that malignant cells are deficient in catalase, and, as such, sensitive to oxidizing agents;
• In 1974, Irwin Stone had already reported on the treatment of leukaemia with high doses of Vitamin C, given by mouth [22], and in the same year, Cameron and Campbell published the results of a clinical trial, showing that, in "untreatable" cancer patients, ascorbate in high doses can bring about some significant improvement in morbidity and mortality [95];
• Two years later (1976), Cameron and Pauling showed that the survival of untreatable cancer patients increased by a factor of about 3 in the majority of cases, and about 20 in 10% of them, under treatment with 10 grams of ascorbate per day, starting with the intravenous route, followed by oral administration [48];
• Later on, the same authors confirmed, in another article, that 10 or more grams of ascorbate per day, significantly prolong the survival and improve the quality of life of untreatable cancer patients [51];
• The ten grams proposed as a standard treatment, in these cases, can certainly be considered "high" doses, if compared with the RDAs, but are still much lower that the doses used in the most recent anticancer clinical trials with intravenous ascorbate [96];
• The results of the two clinical trials published by Cameron & Pauling, were confirmed by Murata and co-workers, in 1982 [97], and therefore, they cannot be considered isolated reports;
• The clinical studies performed at the Mayo Clinic to repeat the experience reported by Cameron & Pauling [98,99], did not follow the method suggested by the two scientists, and therefore failed in its (presumed) intent, even though the results of this work were taken as the "definitive proof" that Vitamin C had no anticancer properties;
• The Mayo Clinic work showed, among others, that Vitamin C is ineffective in Colorectal Cancer (CRC), a conclusion definitively debunked by recent evidences reported by Yun and co-workers [72,78]. The Mayo Clinic, on the other hand, always refused to provide Pauling and other Authors with their data;
• Abram Hoffer, who worked with Linus Pauling, and treated a number of patients with high doses of oral Vitamin C, obtaining essentially the same results as Pauling and Cameron, wrote "Dr. Pauling risked his enormous scientific credibility by his views on using large doses of vitamins. His work on Vitamin C and the common cold and the flu, and later on the role it plays in controlling the ravages of cancer are well known and, in the opinion of Orthomolecular scientists, are correct. We have come to the same conclusions by observing what Vitamin C has done for tens of thousands of patients. It is the most remarkable anti-stress antioxidant and is finding its place in the treatment of more and more difficult chronic diseases including AIDS, cancer, infections, toxemias, schizophrenia and more" [100,101];
• Art Robinson, who, in 1994, published an important article showing that mice with cancer that were given high dose vitamin C in the diet, or fed a diet of raw vegetables, lived up to 20 times longer than controls [102], and all this, happened a long time before the NIH mouse experiment reported by Chen [58-60];
• Hugh Riordan and his research team repeated and extended the early work demonstrating that Vitamin C can selectively kill cancer cells [103-107].
Oral vs. Intravenous Vitamin C
The pharmacokinetic studies of Levine and Padayatty, on Vitamin C, indicate that after oral administration of 200 mg of the nutrient, the maximum plasma concentrations obtained, are not superior to 70-80 μM. This is due to a "tight control", operated by several different mechanisms, including, among others: bioavailability, intestinal absorption, tissue accumulation, renal reabsorption and excretion, and utilization rate as a function of homeostasis. On the contrary, when Vitamin C is administered intravenously, "tight control" is bypassed, until renal excretion restores equilibrium, depending on the dose administered [55-57].
Therefore, according to these data, the intravenous administration of Vitamin C is the only way to achieve plasma concentrations in the order of millimoles, necessary to kill cancer cells.
However, this view is in disagreement with the following evidences:
a) The results obtained by intravenous administration of Vitamin C, do not show the same large effects reported by Robinson, feeding squamous cell carcinoma implanted mice, with large doses of the nutrient [102];
b) Abram Hoffer [100] used oral high doses of Vitamin C in cancer patients and obtained essentially the same significant results as Cameron & Pauling [48-51], Cameron & Campbell [95], and Murata [97];
c) Although Mark Levine maintains that "… only injected ascorbate (Vitamin C) might deliver the concentrations needed to see an anti-tumour effect" [108], neither the legendary scientist Linus Pauling, nor the consultant surgeon, Ewan Cameron, seemed to know the difference between oral and intravenous administration. In fact, in their clinical trial, the protocol started with a few days of 10 grams of intravenous Vitamin C, followed by 10 grams of oral Vitamin C for the whole life [48,51]. Cameron & Campbell, who had already reported on the successful treatment of cancer with oral Vitamin C, two years before Cameron & Pauling [48,51], regarding the administration route, had already observed "… with increasing experience, we tend now to believe that the intravenous regime is probably unnecessary as a routine measure, and need only to be employed in situations where vomiting, anorexia, or other complications of malignancy, preclude oral administration" [95];
d) Plasma concentrations above the 400 μM have been reported, after the administration of a single dose of oral liposomal Vitamin C [109];
e) At times of stress or illnesses (including cancer), the body may absorb extra Vitamin C, as demonstrated by the principle of "bowel tolerance" to the nutrient administered by mouth. According to this principle, when the body is saturated with Vitamin C, a slight diarrhoea may appear, due to intestinal elimination of the nutrient; however, during stress or disease, the amount of oral Vitamin C a patient can tolerate, before the appearance of diarrhoea, increases in proportion with the severity of the condition [110];
This means that the "tight control" hypothesized by Levine and Padayatty, over the plasma concentration of Vitamin C, is either inexistent or relative to disease conditions or stress. To achieve the maximum plasma levels, a typical person may need 20 grams of oral Vitamin C spread throughout the day (3-4 grams every four hours); but cancer patients may require far more [111], and such massive intake may result in plasma concentrations that the tumour may absorb, generation hydrogen peroxide that kills cancer cells.
f) More recently, the paradigm according to which antioxidants inhibit tumorigenesis predominantly by decreasing ROS-mediated DNA damage and mutations [112,113], has been challenged by experimental data, showing that antioxidants such as N-Acetylcysteine (NAC) and Vitamin C exerts their anti-tumorigenic activity by downregulating HIF-1α [114].
Interestingly, these data were obtained not by injecting, but simply feeding mice with large amounts of NAC and Vitamin C, thus once more validating the role of oral administration of Vitamin C (and other antioxidants), in fighting cancer.
Vitamin C and Leukaemia: An In Vitro Update
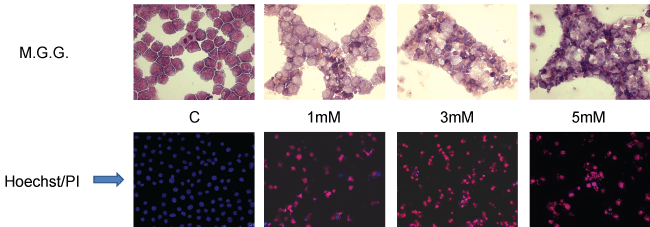
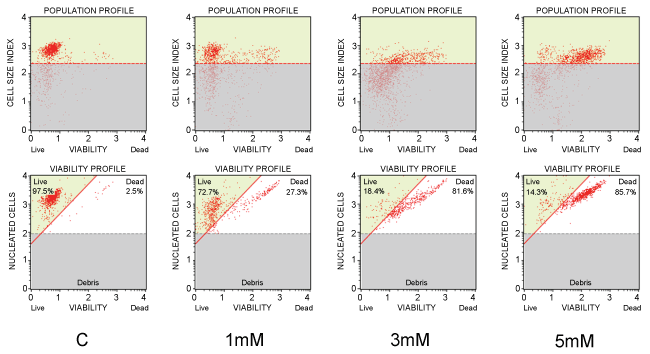
As we have previously demonstrated, high ("pharmacologic") concentrations of Vitamin C (in the form of the sodium salt of ascorbic acid) are capable of eliciting a clear-cut pro-apoptotic/cytotoxic effect on human Promyelocytic Leukaemia derived cell lines (HL60), in vitro [115] (Figure 1 and Figure 2).
This effect is already evident at concentrations of Vitamin C of 1 mM in the culture medium, and it is proportional to the amount of Vitamin C.
Since clinical investigations using high doses of Vitamin C to treat cancer, have reported plasma levels of more than 30 [116], and up to 49 mM [117], it seems reasonable to conclude that protocols using high amounts of Vitamin C, administered by intravenous injection, are not strictly necessary to kill cancer cells in APL.
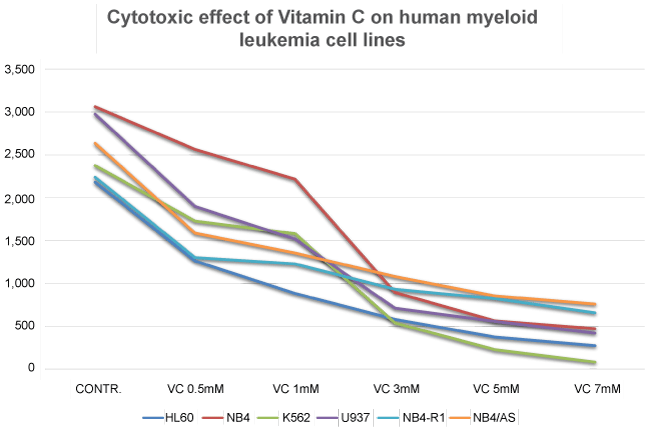
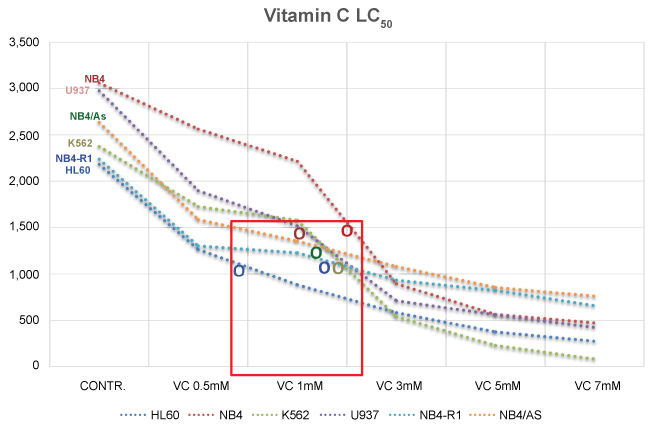
Further investigations, performed by our research group, showed that a plasma concentration of 3 mM of Vitamin C in the culture medium, is sufficient to kill more than a half of the cells in culture (LC50), in a number of different human myeloid leukaemia cell lines, including HL60, K562, U937, NB4, Nb4-R1, and NB4/As [118] (Figure 3 and Figure 4) (Table 1).
It is of interest to consider that in our procedure, the leukemic cells were exposed to increasing concentrations of Vitamin C for no more than two hours, then accurately "washed", re-suspended in fresh culture medium, which did not contain Vitamin C, and further incubated for additional 18-24 hours, before the evaluation of cell survival and apoptosis. Given the results obtained, it is reasonable to conclude that the Vitamin C added to the culture medium (in the form of sodium ascorbate), is rapidly internalized by the leukemic cells, and its "toxic" effects continue for hours (days), even when the nutrient has been removed from the culture medium. This is in agreement with the notion that both normal and leukemic white blood cells tend to accumulate/concentrate Vitamin C [119-122] to levels that are 10-100 fold higher than plasma [123,124], and it is also in disagreement with the view that hydrogen peroxide forms outside the tumour cells [58-60].
Neutrophils, in particular, accumulate vitamin C via the Sodium-Dependent Vitamin C Co-Transporter 2 (SVCT2) [125], and have intracellular levels of 1-2 mM [126]. Therefore, while there is agreement on the fact that, in solid tumours, Vitamin C, initially oxidized to Dehydroascorbic Acid (DHA), is internalized by the cell, via GLUT 1 and 4, and finally reduced again to ascorbic acid, with consumption of glutathione, this does not seem to apply to the case of acute myeloid leukaemia.
More importantly, the parallel exposure of normal hematopoietic precursors (CD34+), isolated from cord blood, to Vitamin C, at the concentrations that are cytotoxic for leukemic cells did not affect their survival, or impair their capacity to proliferate and differentiate in response to myeloid growth factors. These data confirm that Vitamin C is harmless for normal hematopoietic precursors.
Hypoxia Inducible Factor (HIF): The Forgotten Pathway
Hypoxia and induction of Hypoxia-Inducible Factors (HIF) is a hallmark of many tumours [127,128]. HIF-1 is a heterodimeric transcription factor discovered in 1991 [129], composed of two subunits, α and β. The HIF-1α subunit is oxygen sensitive and it is induced by hypoxic conditions, which are very common in cancer. Direct transcriptional targets of HIF-1 include genes regulating, among others, growth and apoptosis, cell migration, energy metabolism, angiogenesis, vasomotor regulation, matrix and barrier functions, and transport of metal ions and glucose [130].
In normoxic conditions, the HIF-1α unit is downregulated by Vitamin C dependent hydroxylases, while in hypoxic conditions (such as those existing in many different types of cancer) HIF-1α hydroxylation is repressed, with consequent increase in HIF-dependent gene transcription, neo-angiogenesis and tumour growth and progression [131].
More importantly, since Vitamin C stimulates HIF-1α prolyl hydroxylases, low levels of Vitamin C promote tumour growth and progression, by reducing HIF-1α hydroxylation [132], thereby stabilizing HIF1-α, while high levels of HIF renders cancer cells more sensitive to Vitamin C-induced toxicity [75]. To confirm this view, Kuiper and coll [133] have recently found an inverse relationship between intra-tumour levels of Vitamin C and HIF activity in both endometrial cancer [134], and Colorectal Carcinoma (CRC) [135].
In 1925, Otto Warburg observed that cancer cells manifest increased rates of lactate production under aerobic conditions ("Warburg Effect") or, in other words, they preferentially utilize glycolysis, instead of oxidative phosphorylation, for metabolism even in the presence of oxygen [136,137].
"Hypoxia" (low oxygen concentration) is a hallmark of solid tumours, usually occurring at the centre of the tumour mass, where blood vessels are abnormal or insufficient to supply adequate amounts of oxygen [138].
In response to the reduced oxygen tension, the HIF is activated to mediate the primary transcriptional adaption to hypoxic stress in cancer cells [139,140].
As previously mentioned, HIFs regulate angiogenesis, cell survival, proliferation, apoptosis, adhesion, and metabolism by transcriptionally activating downstream targets such as vascular endothelial growth factor and erythropoietin. Therefore, HIF (HIF1, in particular) plays a major role in tumour growth, and clinical data suggest that the upregulation of HIF, as determined by the low oxygen tension, is usually associated with increased mortality in a number of different cancers [141-143], and may represent a relevant target for new therapeutic approaches to the disease [144-146].
The HIF Pathway in Leukaemia
The role of HIF-1α in leukaemia, and in particular in Acute Myeloid Leukaemia (AML), has only recently emerged and it is still somewhat controversial. One possible explanation for this delayed interest in the role of hypoxia in leukemia could be the fact that leukaemia is not considered a "solid" tumour, and therefore, the role of oxygen, in its pathogenesis, has been considered inconsequential for long time.
This erroneous view, has been recently reviewed, as data have emerged, demonstrating that leukemic cells are sensitive to the oxygen tension, and hypoxia influences leukemic cell proliferation, differentiation, and resistance to chemotherapy [147].
Migliavacca & coll ha recently demonstrated n oncogenic function of HIF-1α, in the M5 Fab subtype of AML [148]. In particular, the authors have demonstrated that, in M5 AML, HIF-1α mediates the ability of leukemic cells to migrate and invade extramedullary sites.
The same group, has demonstrated that PML-RARα and other fusion proteins involved in the pathogenesis of Acute Promyelocytic Leukemia (APL) behave as transcriptional coactivators of HIFs, and both HIFs and PML-RARα, enhances the progression of APL, by promoting cell migration, homing to bone marrow, and bone marrow neo-angiogenesis [149,150].
Further investigations [151] have demonstrated that HIF-1α plays critical and pleiotropic roles in in the pathogenesis of Chronic Lymphocytic Leukaemia (CLL).
Globally, elevated levels of HIF-1α have been reported in AML [152-155], APL [150], Acute Lymphoblastic Leukemia (ALL) [153,156], and Chronic Myelogenous Leukemia (CML) [157,158]. Furthermore, HIF-1α overexpression conditions disease severity and outcome in both AML and Myelodysplastic Syndrome (MDS) [153,159-161].
Overall, the available data show that hypoxia and HIF-mediated signalling, play a crucial role in leukaemia, and targeting HIF with specific drugs or natural inhibitors, such as Vitamin C, represents a potentially useful approach to its treatment [154,162].
What to Do Next?
The anticancer properties of Vitamin C are known, since at least six decades, even though its use in clinical practice, has only recently re-emerged, after the demonstration that, in relatively high concentrations, it can selectively kill a number of different human tumour cells, both in vitro and in vivo [58-61,73,102,114,116].
The proof of the anticancer efficacy of Vitamin C in high doses, administered by mouth, has been reported four decades ago, by the legendary scientist, Linus Pauling [48,51], and further confirmed, very recently, by experimental, in vitro and in vivo data [73,79,102,114].
Vitamin C is a natural compound, an antioxidant and a life-saving nutrient with multiple beneficial effects on the human body, that man, some primates and a few other mammals do no longer produce. Beyond being a natural and essential nutrient, Vitamin C shows, in vitro, an outstanding efficacy in killing a number of different cancer cells, with an efficiency that no other anticancer drug, presently available on the market, has ever shown.
Vitamin C is extremely selective in its anticancer effect, being able to kill only cancer cells, by sparing, at the same time, all the other cells of the organism, thus being totally harmless for normal cells, and, as such, very well tolerated and without significant side effects. In fact, the only (relative) contraindication to its use, is the lack of the enzyme Glucose-6-Phosphate-Dehydrogenase (G6PDH), a rare genetic condition also known as "favism". More importantly, within an expensive and often artificially inflated market, such as that of the anticancer drugs [163,164], Vitamin C, with its low cost, represents an outstanding opportunity, for both the patients and the healthcare system.
Unfortunately, in spite of all the above characteristics (or just simply "because" of them!), Vitamin C, has never been easily or favourably accepted as an anticancer drug, by the western, business-oriented Medicine. This also explains why, although the data on its anticancer efficacy are outstanding and straightforward, many scientists still prefer to consider "controversial" its role in the treatment of cancer.
As we have seen, the idea that the oral administration of Vitamin C, in high doses, is not effective against cancer is a conceptual artefact born from questionable interpretations of pharmacokinetics data, after oral and/or intravenous administration.
On the other hand, the idea that Vitamin C, administered in high doses by intravenous infusion, behaves as a pro-drug of H2O2, thus inducing pro-oxidant damage to cancer cells, beyond being experimentally questionable, has not led to clinically significant results or outcomes [165-169], even though, encouraging results (to say the least!) emerge from unbiased interpretation of the available data [170]. In particular, as it has been shown up to 110 g/m2/day are very well tolerated by the majority of patients [117], and even in the absence of any significant clinical remission, intravenous Vitamin C is almost invariably associate with a clear-cut improvement in patient's quality of life [96].
As a result, history repeat itself! … and just as Vitamin C was dismissed as ineffective, against cancer, more than thirty years ago, on the ground of flawed clinical trials [98,99], nowadays it runs again the risk of being definitively buried, in spite of the large amount of scientific evidence, demonstrating its extraordinary efficacy in fighting cancer!
It is clear that much remains to be understood about the cytotoxic effects of Vitamin C against cancer, and much more can (and must!) be done, to both improve the intravenous therapy, and further investigate the oral administration route of the high doses of the nutrient.
Improving the intravenous treatment, can (should!) be achieved, by considering:
a) The type of pharmaceutical preparation, the sodium salt of the ascorbic acid to be preferred, when administered by the intravenous route [171];
b) The time and schedule of administration (slow infusion to be preferred) [172,173];
c) The level of tissue oxygenation (cell cultures are better oxygenated than tumour tissues, and this may explain the differences in the outcomes between in vitro and in vivo treatment of cancer) [174]. In clinical settings, an improved tumour tissue oxygenation could be obtained with either ozone or hyperbaric oxygen;
d) The level of blood glucose (glucose may interfere with the uptake of ascorbate by cancer cells) [175,176], and the possibility of associating an adequate dietetic regimen to the treatment with high doses of oral or intravenous Vitamin C.
On the other hand, regarding the oral administration of high doses of Vitamin C to treat cancer, it will be important to consider:
a) The successful results of the oral administration of Vitamin C in different experimental settings [102,114];
b) The results of the clinical studies performed by Cameron & Campbell [95], Cameron & Pauling [48,51], and Murata [97];
c) The rationale behind the use of Vitamin C as an antioxidant (rather than a pro-oxidant) and inhibitor of the HIF [114];
d) The possibility of using "alternative" formulations (such as liposomes), with increased absorption and improved bioavailability [109,173].
Concluding Remarks
The rationale behind the use of high doses of Vitamin C in the treatment of acute leukaemia is strong and very well grounded. In summary:
I. Leukemic patients, almost invariably show a severe deficiency of this nutrient;
II. While it is currently supposed to kill cancer cells by inducing pro-oxidant damage, Vitamin C, is also very effective as an antioxidant, by inhibiting the Hypoxia Inducible Factor (HIF);
III. The mechanistic explanation of the pathogenesis of myeloid leukaemia, includes the possibility that a Vitamin deficiency may induce the neoplastic transformation of myeloid precursors, through an upregulation of the HIF, and the consequent cascade of HIF-dependent cancer genes;
IV. Although administered by intravenous infusion, in the majority of clinical trials performed so far, Vitamin C appears to be effective, in fighting cancer, even when administered by mouth;
V. Vitamin C is very well tolerated, and has no side or undesired effects;
VI. Experimental in vitro data, unequivocally show the cytotoxic effect of Vitamin C against leukaemia as shown in our study on leukemic and normal cell lines, Vitamin C can kill almost every type of Acute and Chronic Myeloid Leukaemia derived cell, without doing any harm to their normal counterpart (CD34+) cells;
VII. Vitamin C is a natural compound, and it is very cheap;
VIII. Do we need more information or evidence, to start clinical trials on Vitamin C, in the treatment of acute and chronic myeloid leukaemia?
Acknowledgement
This chapter has been realized, in part, thanks to the financial support of the "Pescara Cell Factory Foundation".
Conflict of Interests
None to declare.
References
- Iqbal K, Khan A, Ali Khan Khattak MM (2004) Biological Significance of Ascorbic Acid (Vitamin C) in Human Health - A Review. Pakistan Journal of Nutrition 3: 5-13.
- Guy Drouin, Jean-Rémi Godin, Benoît Pagé (2011) The Genetics of Vitamin C Loss in Vertebrates. Curr Genomics 12: 371-378.
- Stone I (1974) The healing Factor: Vitamin C against disease. National Health Federation Bulletin.
- Stephen DJ, Hawley EE (1936) The partition of reduced ascorbic acid in blood. Journal of Biological Chemistry 115: 653-658.
- Barkhan P, Howard AN (1958) Distribution of ascorbic acid in normal and leukaemic human blood. Biochem J 70: 163-168.
- Vogt A (1939) The utilization of Vitamin C in tumor patients and in lymphogranulomatosis. Strahlentherapie 65: 616-623.
- Kyhos FD, Sevringhaus FL, Hagendorn DR (1945) Large Doses of Ascorbic Acid in Treatment of Vitamin C Deficiencies. Arch Intern Med (Chic) 75: 407-412.
- Waldo AL, Zipf RE (1955) Ascorbic acid level in leukemic patients. Cancer 8: 187-190.
- Kuo MS (2013) The Multifaceted Biological Roles of Vitamin C. J Nutr Food Sci 3: 231.
- Mandl J, Szarka A, Bánhegyi G (2009) Vitamin C: update on physiology and pharmacology. Br J Pharmacol 157: 1097-1110.
- Figueroa-Mèndez R, Rivas-Arancibia S (2015) Vitamin C in Health and Disease: Its Role in the Metabolism of Cells and Redox State in the Brain. Front Physiol 6: 1-11.
- Pujari KN, Jadkar SP, Mashal SN, et al. (2012) Variations in vitamin C levels in leukemias. Biomedical Research 23: 307-311.
- Liua M, Ohtania H, Zhoua W, et al. (2016) Vitamin C increases viral mimicry induced by 5-aza-2′-deoxycytidine. Proc Natl Acad Sci U S A 113: 10238-10244.
- Klenner FR (1949) The treatment of poliomyelitis and other virus diseases with vitamin C. South Med Surg 111: 209-214.
- Klenner FR (1951) Massive doses of vitamin C and the virus diseases. South Med Surg 113: 101-107.
- Klenner FR (1948) Virus pneumonia and its treatment with vitamin C. South Med Surg 110: 36-38.
- Stone I (1966) On the Genetic Etiology of Scurvy. Acta Genet Med Gemellol (Roma) 15: 345-350.
- Stone I (1966) Hypoascorbemia, the genetic disease causing the human requirement for exogenous ascorbic acid. Perspect Biol Med 10: 133-134.
- Stone I (1967) The Genetic Disease, Hypoascorbemia: A Fresh Approach to an Ancient Disease and Some of its Medical Implications. Acta Geneticae Medicae et Gemellologiae 16: 52-62.
- Stone I (1972) Hypoascorbemia, Our Most Widespread Disease. Bull Natl Health Fed 18: 6-9.
- http://www.omim.org/entry/240400
- Stone I (1974) Megascorbic Therapy of the Disease Called Leukemia. Cancer Control 2: 1-4.
- Stone I (1977) My Daily Megascorbic Regime for Full Health and Long Life. Better Nutrition.
- Panel on Dietary Antioxidants and Related Compounds; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Uses of DRIs; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board; Institute of Medicine (2000) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, Carotenoids, 529.
- Pauling L (1986) How to Live Longer and Feel Better. W. H. Freeman and Company.
- Pauling L (1983) Vitamin C and longevity. Agressologie 24: 317-319.
- Pauling L (1976) For the best of health--how much vitamin C do you need? Pa Dent J (Harrisb) 43: 53-56.
- Pauling L (1975) Letter: Megavitamin therapy. JAMA 234: 149.
- Pauling L (1974) Are recommended daily allowances for vitamin C adequate? Proc Natl Acad Sci U S A 71: 4442-4446.
- Pauling L (1972) Vitamin C. Science 177: 1152.
- Pauling L (1970) Evolution and the Need for Ascorbic Acid. Proc Natl Acad Sci U S A 67: 1643-1648.
- Pauling L (1976) Vitamin C, the common cold, and the flu. W. H. Freeman, San Francisco.
- Pauling L (1970) Vitamin C and the common cold. Freeman, San Francisco.
- Pauling L (1973) Ascorbic acid and the common cold. Scott Med J 18: 1-2.
- Pauling L (1971) San Francisco: The Significance of the Evidence about Ascorbic Acid and the Common Cold. Proc Natl Acad Sci U S A 68: 2678-2681.
- Pauling L (1971) Ascorbic acid and the common cold. The American Journal of Clinical Nutrition 24: 1294-1299.
- Pauling L (1971) Vitamin C and common cold. JAMA 216: 332.
- Pauling L, Herman ZS (1989) Criteria for the validity of clinical trials of treatments of cohorts of cancer patients based on the Hardin Jones principle. Proc Natl Acad Sci U S A 86: 6835-6837.
- Pauling L (1989) Biostatistical analysis of mortality data for cohorts of cancer patients. Proc Natl Acad Sci U S A 86: 3466-3468.
- Pauling L, Moertel C (1986) A proposition: megadoses of vitamin C are valuable in the treatment of cancer. Nutr Rev 44: 28-32.
- Pauling L, Nixon JC, Stitt F, et al. (1985) Effect of dietary ascorbic acid on the incidence of spontaneous mammary tumors in RIII mice. Proc Natl Acad Sci U S A 82: 5185-5189.
- Kimoto E, Tanaka H, Gyotoku J, et al. (1983) Enhancement of antitumor activity of ascorbate against Ehrlich ascites tumor cells by the copper: glycylglycylhistidine complex. Cancer Res 43: 824-828.
- Pauling L, Willoughby R, Reynolds R, et al. (1982) Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl 23: 53-82.
- Pauling L, Anderson R, Banic S, et al. (1982) Workshop on vitamin C in immunology and cancer. Int J Vitam Nutr Res Suppl 23: 209-219.
- Pauling L (1980) Vitamin C therapy of advanced cancer. N Engl J Med 302: 694-695.
- Pauling L (1979) Ascorbic acid. Lancet 1: 615.
- Cameron E, Pauling L, Leibovitz B (1979) Ascorbic acid and cancer: a review. Cancer Res 39: 663-681.
- Cameron E, Pauling L (1978) Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 75: 4538-4542.
- Pauling L (1977) Vitamin homeostasis in the brain and megavitamin therapy. N Engl J Med 297: 790-791.
- Pauling L (1977) Diet, nutrition, and cancer. The American Journal of Clinical Nutrition 30: 661-663.
- Cameron E, Pauling L (1976) Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 73: 3685-3689.
- Cameron E, Pauling L (1974) The orthomolecular treatment of cancer. I. The role of ascorbic acid in host resistance. Chem Biol Interact 9: 273-283.
- Cameron E, Pauling L (1973) Ascorbic acid and the glycosaminoglycans. An orthomolecular approach to cancer and other diseases. Oncology 27: 181-192.
- Harakeh S, Jariwalla RJ, Pauling L (1990) Suppression of human immunodeficiency virus replication by ascorbate in chronically and acutely infected cells. Proc Natl Acad Sci U S A 87: 7245-7249.
- Levine M, Conry-Cantilena C, Wang Y, et al. (1996) Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A 93: 3704-3709.
- Padayatty SJ, Levine M (2000) Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am Coll Nutr 19: 423-425.
- Padayatty SJ, Sun H, Wang Y, et al. (2004) Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 140: 533-537.
- Chen Q, Espey MG, Sun AY, et al. (2008) Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A 105: 11105-11109.
- Chen Q, Espey MG, Sun AY, et al. (2007) Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A 104: 8749-8754.
- Chen Q, Espey MG, Krishna MC, et al. (2005) Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A 102: 13604-13609.
- Espey MG, Chen P, Chalmers B, et al. (2011) Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med 50: 1610-1619.
- Levine M, Padayatty S, Espey M (2011) Vitamin C: A Concentration-Function Approach Yields Pharmacology and Therapeutic Discoveries. Adv Nutr 2: 78-88.
- Halliwell B (1999) Vitamin C: poison, prophylactic or panacea? Trends Biochem Sci 24: 255-259.
- Carr AC, Frei B (1999) Does vitamin C act as pro-oxidant under physiological conditions? FASEB J 13: 1007-1024.
- Hacisevki A (2009) An overview of ascorbic acid biochemistry. J Fac Pharm Ankara 38: 233-255.
- Riordan NH, Riordan HD, Meng XL, et al. (1995) Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses 44: 207-213.
- Iyanagi T, Yamazaki I, Anan KF (1985) One electron oxidation-reduction properties of ascorbic acid. Biochimica et Biophysica Acta 806: 255-261.
- Sun Y, Oberley LW, Oberley TD, et al. (1933) Lowered antioxidant enzymes in spontaneously transformed embryonic mouse liver cells in culture. Carcinogenesis 14: 1437-1446.
- Jaruga P, Olinste R (1994) Activity of antioxidant enzymes in cancer diseases. Postepy Hig Med Dosw 48: 443-455.
- Park S (2013) The Effects of High Concentrations of Vitamin C on Cancer Cells. Nutrients 5: 3496-3505.
- Frei B, Lawson S (2008) Vitamin C and cancer revisited. Proc Natl Acad Sci U S A 105: 11037-11038.
- Venturelli S, Sinnberg TW, Niessner H, et al. (2015) Molecular mechanisms of pharmacological doses of ascorbate on cancer cells. Wien Med Wochenschr 165: 251-257.
- Yun J, Mullarky E, Lu C, et al. (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350: 1391-1396.
- Savini I, Rossi A, Pierro C, et al. (2008) SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34: 347-355.
- Vera JC, Rivas CI, Fischbarg J, et al. (1993) Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 364: 79-82.
- Tian W, Wang Y, Xu Y, et al. (2014) The hypoxia-inducible factor renders cancer cells more sensitive to vitamin C-induced toxicity. J Biol Chem 289: 3339-3351.
- Yun J, Rago C, Cheong I, et al. (2009) Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325: 1555-1559.
- Sheu JJ, Guan B, Tsai FJ, et al. (2012) Mutant BRAF induces DNA strand breaks, activates DNA damage response pathway, and up-regulates glucose transporter-1 in nontransformed epithelial cells. Am J Pathol 180: 1179-1188.
- Van der Reest J, Gottlieb E (2016) Anti-cancer effects of vitamin C revisited. Cell Res 26: 269-270.
- Uetaki M, Tabata S, Nakasuka F, et al. (2015) Metabolomic alterations in human cells by vitamin C-induced oxidative stress. Scientific Reports.
- Benade L, Howard T, Burk D (1969) Synergistic killing of Ehrlich ascites carcinoma cells by ascorbate and 3-amino-1, 2, 4,-triazole. Oncology 23: 33-43.
- Oberley TD, Oberley LW (1997) Antioxidant enzyme levels in cancer. Histol Histopathol 12: 525-535.
- Cavanagh EM, Honegger AE, Hofer E, et al. (2002) Higher oxidation and lower antioxidant levels in peripheral blood plasma and bone marrow plasma from advanced cancer patients. Cancer 94: 3247-3251.
- Birben E, Sahiner UM, Sackesen C, et al. (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5: 9-19.
- Lòpez-Làzaro M (2007) Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett 252: 1-8.
- Lisanti MP, Martinez-Outschoorn UE, Lin Z, et al. (2011) Hydrogen peroxide fuels aging, inflammation, cancer metabolism and metastasis. Cell Cycle 10: 2440-2449.
- Wintergerst ES, Maggini S, Hornig DH (2006) Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab 50: 85-94.
- Gerber WF, Lefkowitz SS, Hung CY (1975) Effect of ascorbic acid, sodium salicylate, and caffeine on the serum interferon level in response to viral infection. Pharmacology 13: 228-233.
- Karpinska T, Kawecki Z, Kandefer-Szerszen M (1982) The influence of ultraviolet irradiation, L-ascorbic acid and calcium chloride on the induction of interferon in human embryo fibroblasts. Arch Immunol Ther Exp (Warsz) 30: 33-37.
- Furuya A, Uozaki M, Yamasaki H, et al. (2008) Antiviral effects of ascorbic acid and dehydroascorbic acids in vitro. Int J Mol Med 22: 541-545.
- White LA, Freeman CY, Forrester BD, et al. (1986) In vitro effect of ascorbic acid on infectivity of herpeviruses and paramyxoviruses. J Clin Microbiol 24: 527-531.
- Bissell MJ, Hatie C, Farson DA, et al. (1980) Ascorbic acid inhibits replication and infectivity of avian RNA tumor virus. Proc Natl Acad Sci U S A 77: 2711-2715.
- Mikirova NA, Hunninghake R (2014) Effect of high dose vitamin C on Epstein-Barr viral infection. Med Sci Monit 20: 725-732.
- Holman RA (1975) A method of destroying a malignant rat tumour in vivo. Nature 179: 1033.
- Cameron E, Campbell A (1974) The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact 9: 285-315.
- Carr AC, Vissers MCM, Cook JS (2014) The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front Oncol 4: 283.
- Murata A, Morishige F, Yamaguchi H (1982) Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res Suppl 23: 101-113.
- Creagan ET, Moertel CG, O'Fallon JR, et al. (1979) Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 301: 687-690.
- Moertel CG, Fleming TR, Creagan ET, et al. (1985) High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med 312: 137-141.
- Hoffer A (1989) The discovery of Vitamin C. Journal of Orthomolecular Medicine 4: 24-26.
- Hoffer A, Pauling L (2004) Healing Cancer: Complementary Vitamin and Drug Treatments. In: Bob Hilderley.
- Robinson AR, Hunsberger A, Westall FC (1994) Suppression of squamous cell carcinoma in hairless mice by dietary nutrient variation. Mech Ageing Dev 76: 201-214.
- Mikirova N, Hunnunghake R, Scimeca RC, et al. (2016) High-Dose Intravenous Vitamin C Treatment of a Child with Neurofibromatosis Type 1 and Optic Pathway Glioma: A Case Report. Am J Case Rep 17: 774-781.
- Mikirova N, Riordan N, Casciari J (2016) Modulation of Cytokines in Cancer Patients by Intravenous Ascorbate Therapy. Med Sci Monit 22: 14-25.
- Mikirova N, Casciari J, Riordan N, et al. (2013) Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. J Transl Med 11: 191.
- Mikirova N, Casciari J, Rogers A, et al. (2012) Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med 10: 189.
- Riordan HD, Hunninghake RB, Riordan NH, et al. (2003) Intravenous ascorbic acid: protocol for its application and use. P R Health Sci J 22: 287-290.
- (2008) Vitamin C Injections Slow Tumor Growth in Mice. NIH.
- Hickey S, Roberts H, Miller NJ (2008) Pharmacokinetics of oral vitamin C. Journal of Nutritional & Environmental Medicine.
- Cathcart RF (1981) The Method of Determining Proper Doses of Vitamin C for the Treatment of Disease by Titrating to Bowel Tolerance. Orthomolecular Psychiatry 10: 125-132.
- Hickey S, Roberts H (2008) The Real Story of Vitamin C and Cancer. JOM 23: 133-138.
- Poirier MC (2004) Chemical-induced DNA damage and human cancer risk. Nat Rev Cancer 4: 630-637.
- Sharma RA, Farmer PB (2004) Biological relevance of adduct detection to the chemoprevention of cancer. Clin Cancer Res 10: 4901-4912.
- Gao P, Zhang H, Dinavahi R, et al. (2007) HIF-Dependent Antitumorigenic Effect of Antioxidants In Vivo. Cancer Cell 12: 230-238.
- Mastrangelo D, Massai L, Fioritoni G, et al. (2013) Megadoses of Sodium Ascorbate Efficiently Kill HL60 Cells in Vitro: Comparison with Arsenic Trioxide. Journal of Cancer Therapy 4: 1366-1372.
- Monti DA, Mitchell E, Bazzan AJ, et al. (2012) Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One 7: e29794.
- Stephenson CM, Levin RD, Spector T, et al. (2013) Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol 72: 139-146.
- Mastrangelo D, Massai L, Lo Coco F, et al. (2015) Cytotoxic effects of high concentrations of sodium ascorbate on human myeloid cell lines. Ann Hematol 94: 1807-1816.
- Lowry OH, Bessey OA, Brock MJ, et al. (1946) The interrelationship of dietary, serum, white blood cell, and total body ascorbic acid. J Biol Chem 166: 111-119.
- Wilson C (1975) Clinical pharmacological aspects of ascorbic acid. Ann NY Acad Sci 258: 354-376.
- Lykkesfeldt J (2000) Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: reliable reduction with tris[2-carboxyethyl] phosphine hydrochloride. Anal Biochem 282: 89-93.
- Emadi-Konjin P, Verjeea Z, Levinb AV, et al. (2005) Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC). Clin Biochem 38: 450-456.
- Michels AJ, Hagen TM, Frei B (2013) Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Ann Rev Nutr 33: 45-70.
- Bozonet SM, Carr AC, Pullar JM, et al. (2015) Enhanced Human Neutrophil Vitamin C Status, Chemotaxis and Oxidant Generation Following Dietary Supplementation with Vitamin C-Rich SunGold Kiwifruit. Nutrients 7: 2574-2588.
- Corpe CP, Lee JH, Kwon O, et al. (2005) 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem 280: 5211-5220.
- Washko PW, Wang Y, Levine M (1993) Ascorbic acid recycling in human neutrophils. J Biol Chem 268: 15531-15535.
- Aprelikova O, Chandramouli GV, Wood M, et al. (2004) Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem 92: 491-501.
- Nagy MA (2011) HIF-1 is the Commander of Gateways to Cancer. J Cancer Sci Ther 3: 35-40.
- Semenza GL, Nejfelt MK, Chi SM, et al. (1991) Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A 88: 5680-5684.
- Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343-354.
- Traber MG, Stevens JF (2011) Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic Biol Med 51: 1000-1013.
- Knowles HJ, Raval RR, Harris AL, et al. (2003) Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res 63: 1764-1768.
- Kuiper C, Vissers MCM (2014) Ascorbate as a Co-Factor for Fe- and 2-Oxoglutarate Dependent Dioxygenases: Physiological Activity in Tumor Growth and Progression. Front Oncol 4: 359.
- Kuiper C, Molenaar IG, Dachs GU, et al. (2010) Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res 70: 5749-5758.
- Kuiper C, Dachs GU, Munn D, et al. (2014) Increased Tumor Ascorbate is Associated with Extended Disease-Free Survival and Decreased Hypoxia-Inducible Factor-1 Activation in Human Colorectal Cancer. Front Oncol 4: 10.
- Warburg O, Wind F, Negelein E (1927) The Metabolism of Tumors in the Body. J Gen Physiol 8: 519-530.
- Justus CR, Sanderlin EJ, Yang LV (2015) Molecular Connections between Cancer Cell Metabolism and the Tumor Microenvironment. Int J Mol Sci 16: 11055-11086.
- Huang D, Li C, Zhang H (2014) Hypoxia and cancer cell metabolism. Acta Biochim Biophys Sin (Shanghai) 46: 214-219.
- Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447-5454.
- Wang GL, Jiang BH, Rue EA, et al. (1995) Hypoxia-inducible factor-1 is a basic-helix-loop-helix-Pas heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92: 5510-5514.
- Schindl M, Schoppmann SF, Samonigg H, et al. (2002) Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res 8: 1831-1837.
- Mouriaux F, Sanschagrin F, Diorio C, et al. (2014) Increased HIF-1α expression correlates with cell proliferation and vascular markers CD31 and VEGF-A in uveal melanoma. Invest Ophthalmol Vis Sci 55: 1277-1283.
- 143. Kim BW, Cho H, Chung JY, et al. (2013) Prognostic assessment of hypoxia and metabolic markers in cervical cancer using automated digital image analysis of immunohistochemistry. J Transl Med 11: 185.
- Masoud GN, Li W (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5: 378-389.
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721-732.
- Poon E, Harris AL, Ashcroft M (2009) Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev Mol Med 11: e26.
- Muz B, de la Puente P, Azab F, et al. (2014) The role of hypoxia and exploitation of the hypoxic environment in hematologic malignancies. Mol Cancer Res 12: 1347-1354.
- Migliavacca J, Percio S, Valsecchi R, et al. (2016) Hypoxia inducible factor-1a regulates a pro-invasive phenotype in acute monocytic leukemia. Oncotarget 7: 53540-53557.
- Coltella N, Percio S, Valsecchi R, et al. (2014) HIF factors cooperate with PML-RARα to promote acute promyelocytic leukemia progression and relapse. EMBO Mol Med 6: 640-650.
- Percio S, Coltella N, Grisanti S, et al. (2014) A HIF-1 network reveals characteristics of epithelial-mesenchymal transition in acute promyelocytic leukemia. Genome Med 6: 84.
- Valsecchi R, Coltella N, Belloni D, et al. (2016) HIF-1α regulates the interaction of chronic lymphocytic leukemia cells with the tumor microenvironment. Blood 127: 1987-1997.
- Gao XN, Yan F, Lin J, et al. (2015) AML1/ETO cooperates with HIF1α to promote leukemogenesis through DNMT3a transactivation. Leukemia 29: 1730-1740.
- Forristal CE, Brown AL, Helwani FM, et al. (2015) Hypoxia inducible factor (HIF)-2α accelerates disease progression in mouse models of leukemia and lymphoma but is not a poor prognosis factor in human AML. Leukemia 29: 2075-2085.
- Kawada H, Kaneko M, Sawanobori M, et al. (2013) High concentrations of L-ascorbic acid specifically inhibit the growth of human leukemic cells via downregulation of HIF-1α transcription. PLoS One 8: e62717.
- Wang Y, Liu Y, Malek SN, et al. (2011) Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 8: 399-411.
- Frolova O, Samudio I, Benito JM, et al. (2012) Regulation of HIF-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther 13: 858-870.
- Chen H, Shen Y, Gong F, et al. (2015) HIF-α Promotes Chronic Myelogenous Leukemia Cell Proliferation by Upregulating p21 Expression. Cell Biochem Biophys 72: 179-183.
- Zhang H, Li H, Xi HS, et al. (2012) HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood 119: 2595-2607.
- Rouault-Pierre K, Lopez-Onieva L, Foster K, et al. (2013) HIF-2α protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell Stem Cell 13: 549-563.
- Wellmann S, Guschmann M, Griethe W, et al. (2004) Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia 18: 926-933.
- Elias H, Verma A (2012) Activation of hypoxia signaling is marker of poor prognosis in myelodysplasia. Leuk Lymphoma 53: 2337-2338.
- Deynoux M, Sunter N, Hérault O, et al. (2016) Hypoxia and Hypoxia-inducible Factors in Leukemias. Front Oncol 6: 41.
- http://www.ascopost.com/issues/february-1-2013/cost-of-cancer-drugs-what-price-for-what-benefit
- http://chronicdisease.uchicago.edu/files/2014/09/Conti-pricing-trends-9-9-2014.pdf
- Wilson MK, Baguley BC, Wall C, et al. (2014) Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac J Clin Oncol 10: 22-37.
- Unlu A, Kirca O, Ozdogan M, et al. (2016) High-dose vitamin C and cancer. Journal of Oncological Science 1: 10-12.
- Nandal S, Ghalaut P, Shekhawat H, et al. (2014) High-dose intravenous vitamin C as an Anticancer Agent-A Literature Review. IJARESM 2: 10-17.
- Jacobs C, Hutton B, Ng T, et al. (2015) Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist 20: 210-223.
- Goodman A (2013) Vitamin C and Cancer. AIMS Medical Science 3: 41-51.
- Fritz H, Flower G, Weeks L, et al. (2014) Intravenous Vitamin C and Cancer: A Systematic Review. Integr Cancer Ther 13: 280-300.
- http://www.doctoryourself.com/vitciv.html.
- Duconge J, Miranda-Massari JR, Gonzalez MJ, et al. (2008) Pharmacokinetics of vitamin C: insights into the oral and intravenous administration of ascorbate. P R Health Sci J 27: 7-19.
- Hickey DS, Roberts HJ, Cathcart RF (2005) Dynamic Flow: A New Model for Ascorbate. Journal of Orthomolecular Medicine 20: 237-244.
- Carreau A, El Hafny-Rahbi B, Matejuk A, et al. (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15: 1239-1253.
- Malo C, Wilson JX (2000) Glucose modulates vitamin C transport in adult human small intestinal brush border membrane vesicles. J Nutr 130: 63-69.
- Washko P, Levine M (1992) Inhibition of ascorbic acid transport in human neutrophils by glucose. J Biol Chem 267: 23568-23574.
Corresponding Author
Domenico Mastrangelo, MD, Department of Medical, Surgical and Neurological Sciences, University of Siena, Polo Scientifico San Miniato, Via A, Moro 2, 53100 Siena, Italy.
Copyright
© 2017 Mastrangelo D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.