Laparoscopic Surgical Management of Symptomatic Anastomotic Ulcers after Gastric Bypass: A 10-Year Single-Center Experience
Abstract
Background
Marginal ulceration has a reported incidence up to 34% following Roux-en-y gastric bypass. Bleeding or perforation may be a medical emergency and can be managed via a wide range of techniques. Traditional laparoscopy or robotically assisted laparoscopy offer minimally invasive approaches. We reviewed the experience at a Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Center of Excellence.
Study Design
Our prospective database was retrospectively reviewed for all patients with anastomotic ulcers managed at our center over a 10-year period ending in 2017 to assess patient demographics and outcomes (surgical indication, urgency, approach, length of stay [LOS], 30-day readmissions and 30-day re-operations).
Results
One hundred and four operations performed on 84 patients were identified. Fifty-nine patients had 68 operations directly related to the ulcer: 47 were females (79.7%), mean age 51.7 years (range 32-73), and mean BMI 35.8 (range 20-60). Risk factors included: smoking (55.9%), alcohol (47.5%), and non-steroidal anti-inflammatory (15.3%) use. Failed medical therapy with proton pump inhibitor use was noted in 64.4% of patients. Over the 10-year period, 55 (93.2%) patients underwent 1 procedure and 4 (6.8%) underwent ≥ 2. Of the 68 procedures, common indications included: intractable pain (57.4%), bleed/anemia (13.2%), perforation (8.8%), and weight gain (7.4%). Thirteen operations (19.1%) were emergent. Surgical procedures included: anastomotic revision (91.2%) and omental patching (8.8%). 96% of cases were performed minimally-invasively (49% robotic, 47% laparoscopic) and 4% open. Mean LOS was 5.8 days (range 2-29). Thirty-day complications included 11 readmissions (16.2%), 4 reoperations (5.9%), and 0 mortalities.
Conclusions
Anastomotic ulcers can be successfully treated surgically via a minimally invasive approach with good outcomes in both the emergent and elective setting.
Keywords
Roux-En-Y gastric bypass, Anastomotic ulcer, Symptomatic ulcer, Bariatric surgery, Bariatric complication, Gastric bypass complication
Introduction
Obesity is a major healthcare concern around the world. According to the National Health and Nutrition Examination Survey, over one-third of American adults are obese, which accounts for 37.7% of adults in the United States (US) [1,2]. In 2000, the presence of morbid obesity was found to have a two-fold increase in odds of incurring healthcare expenditure compared to normal-weight adults and overall obesity-related healthcare costs in the US exceeded 11 billion dollars [3]. A 2016 meta-analysis and systematic review showed that these costs have risen to over 149 billion dollars annually [4].
Surgical intervention has been shown to be more effective than non-surgical interventions for sustained weight loss and the control of co-morbid conditions in the morbidly obese [5-7]. Bariatric surgery has also been well-established to improve survival for patients with obesity [8,9]. Laparoscopic Roux-en-y gastric bypass (RNY), which was introduced by Wittgrove, Clark, and Tremblay in 1994 [10], evolved to become the standard by which other bariatric procedures were measured. Since the Bariatric Revolution of 1998-2003 as described by Schirmer [11], the overall complication rates of laparoscopic RNY have decreased in the hands of the experienced surgeon. However, marginal ulcerations of the gastrojejunal anastomosis are still the most common complication following RNY and have a reported incidence of up to 34% [12-16]. No single etiology is known to cause marginal ulceration; however, several risk factors have been identified: the presence of Helicobacter pylori, infection, gastric pouch size and orientation, gastrogastric fistula, mucosal ischemia, staple-line disruption, the use of non-absorbable sutures for the inner anastomotic layer, lack of proton pump inhibitor use, smoking, and use of non-steroidal anti-inflammatory drugs (NSAID) [15,16] .The underlying mechanism remains elusive; thus, warranting further investigation. The most common presenting symptom is epigastric pain [15]. Other symptoms of marginal ulcers include nausea, vomiting, dysphagia, bleeding, and chronic anemia [16]. Symptoms indicating the diagnosis of marginal ulceration can be confounded by typical postoperative complaints, potentially attributed to overeating, or are simply not present. Up to 61% of patients with ulceration have been found to be asymptomatic [16] Perforation can occur in approximately 1.4% of marginal ulcers,[17] and should be a differential diagnosis when evaluating symptomatic patients with a history of gastric bypass. Indications for surgical intervention include: failure to respond to medical therapy (eg sucralfate, proton pump inhibitor), inadequate weight loss, weight regain, intractable bleeding, anemia from chronic gastrointestinal blood loss, the presence of a gastro-gastric fistula in addition to marginal ulceration, or intractable symptoms (eg abdominal pain, nausea, vomiting) [18,19]. The purpose of this study was to evaluate the experiences and outcomes of patients with anastomotic ulcers after prior RNY who underwent surgical intervention at our institution.
Methods
A prospectively-maintained, IRB-approved database of patients who underwent bariatric procedures was retrospectively reviewed for all patients with anastomotic ulcers managed at our Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Center of Excellence over a 10-year period ending in 2017. Data was collected regarding patient demographics, surgical details, and outcomes. Demographics included: patient age, gender, weight (pounds), body mass index (BMI), presence of comorbidities (hypertension [HTN], diabetes mellitus [DM], obstructive sleep apnea [OSA], gastroesophageal reflux disease [GERD], and osteoarthritis [OA]), smoking history (current, former, never), alcohol use (current, former, never), and current proton pump inhibitor (PPI) use (yes or no). Surgical details included operative indication, urgency, modality, number of procedures per patient, time to intervention from index RNY, and procedure performed. Outcomes included: hospital length of stay (LOS), 30-day readmissions, 30-day re-operations, 30-day mortalities. Surgical procedures not related to anastomotic ulcer management (e.g. lysis of adhesions, internal hernia, cholecystectomy) were excluded from the final analysis. Data were summarized using descriptive statistics, such as count, percent, mean, and range.
Surgical Technique
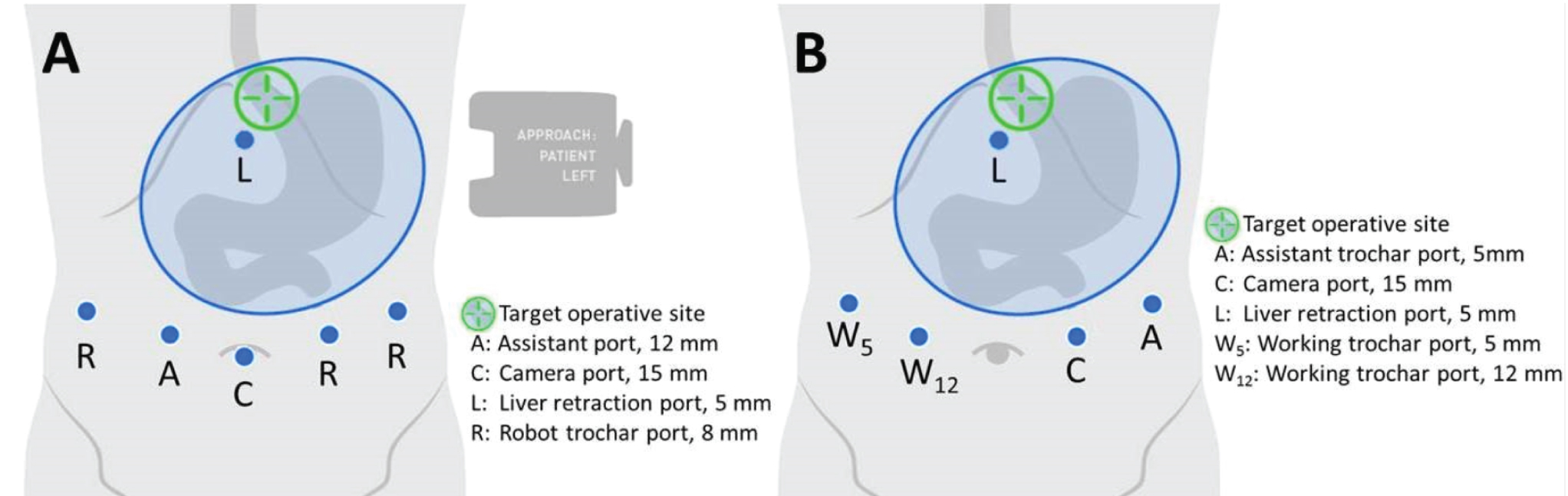
The surgical modalities utilized included traditional laparoscopy, robot-assisted laparoscopy (da Vinci® Si and Xi, Intuitive Surgical, Inc.), and open revision of the gastrojejunal anastomoses. The patient is usually placed in the reverse Trendelenburg position. For robot-assisted laparoscopy surgery cases, the trocar placement and operating setup used is shown in Figure 1A. Adhesiolysis was performed between the Roux limb, undersurface of the liver, gastric pouch, and gastric remnant. The jejunum was divided just distal to the anastomosis using a linear stapler. The mesentery was divided with ultrasonic shears and the pouch was divided 1-2 cm above the anastomosis with a peri-strip-reinforced (Peri-strips Dry® with Veritas®, Baxter Healthcare Corporation) stapling device. Staple cartridges for thick tissue were selected. The Roux limb was brought up and attached to the residual pouch by a single suture to prevent it from falling into the pelvis. The robot was docked and a running 2-0 synthetic, monofilament absorbable suture incorporating the staple line was placed attaching the roux limb to the pouch. A gastrotomy and enterotomy were made using the ultrasonic shears. The posterior mucosal layer of the anastomosis was made with a running 2-0 synthetic, absorbable, braided suture going from the greater to lesser curvature, then brought around starting from lesser to greater curvature utilizing the Connell suture technique. A second layer was started on the greater curvature going medially and an orogastric tube was advanced into the jejunum. The anterior mucosal layer was closed using 2-0 synthetic, absorbable, braided suture in a Connell fashion followed by closure of the anterior seromuscular layer with absorbable monofilament suture in a Lembert fashion. A leak test was performed via an orogastric tube using methylene blue. A fenestrated drain was placed in the left subhepatic space adjacent to the anastomosis in most cases.
For traditional laparoscopic surgery cases, ports were arranged as shown in Figure 1B. One of two methods were used to create the gastric bypass. In the first, an intra-abdominal circular stapler with a 25 mm anvil was used via oral entry into the newly formed gastric pouch and a fresh gastrojejunostomy was fashioned between the pouch and Roux limb. In the second approach, continuity was established using a linear stapler blue loads and pulled back to form a 2.5 cm anastomosis. The anterior mucosal layers were closed using absorbable suture, the seromuscular layer closed using silk sutures, and a fenestrated drain was placed alongside the anastomosis.
For open surgery cases, a mini laparotomy was performed in the epigastric region. The proximal Roux limb and gastric pouch were dissected and transected to include the ulcer. A gastrotomy and enterotomy were made and a common enterotomy fashioned using a thick-tissue stapler. Closure was achieved in a similar fashion to the laparoscopic technique.
Results
One hundred and four operations performed on 84 patients with anastomotic ulcers were identified. Thirty-six operations were performed on 25 patients for indications not related to the anastomotic ulcer and were excluded from the analysis. The 36 excluded procedures not related to the anastomotic ulcer included operations such as lysis of adhesions, repair of internal hernia and perforated duodenal ulcer, panniculectomy, appendectomy, and cholecystectomy. The remaining 59 patients had 68 operations directly related to the ulcer. Table 1 shows the demographics of the patients included in the study. Of note, 64% of patients who underwent operation for anastomotic ulcers failed medical management with PPI therapy. Table 2 shows the surgical details of the 68 procedures performed.
In this study emergent cases were defined as patients with diagnoses requiring operative intervention within 12 hours of presentation (eg visceral perforation, bleeding). A majority of the patients (93%) underwent a single procedure during the 10-year period, while 7% underwent two or more operations related directly to the anastomotic ulcer. The patient who underwent two procedures had a robotic revision of the gastrojejunal anastomosis due to a bleeding ulcer eight years after the index laparoscopic bypass, followed by a robotic revision of the jejunojejunal anastomosis two years later for symptomatic ulceration and weight re-gain. Two patients underwent three ulcer-related operations. One patient presented with an acutely perforated ulcer that was repaired with an omental patch two years after the index laparoscopic bypass. Three months later, the patient underwent open revision of the gastrojejunostomy for persistent abdominal pain followed by a repeat operation an additional eight months later. The second patient with three procedures initially had an open Roux-en-y gastric bypass then presented twice with a perforated anastomotic ulcer, once one year later then an additional six months later. She then underwent a complex laparoscopic revision of the gastrojejunostomy four years later for persistent abdominal pain. One patient underwent four ulcer-related procedures during the course of this retrospective study for persistent pain and PO intolerance. The first operation was a laparoscopic revision of the gastrojejunostomy eight years after the initial laparoscopic gastric bypass. One year later, a robotic revision of the gastrojejunostomy was performed followed by a robotic total gastrectomy with Roux-en-y esophagojejunostomy two years later. Continued pain and PO intolerance was presumed to be due to extensive intra-abdominal adhesions. A workup for Zollinger-Ellison syndrome was negative and the patient underwent a robotic revision of the esophagojejunostomy.
Outcomes are shown in Table 3. Reasons for 30-day readmission included PO intolerance (4; managed with esophagogastroduodenoscopy [EGD]), abdominal fluid collection [4], abdominal pain (perforated anastomotic ulcer), wound infection, and C.difficile. Reoperations were performed for two anastomotic leaks on postoperative day (POD) one, one ulcer perforation on POD 7 following a suture revision of the gastrojejunostomy, and one control of splenic capsule bleeding on POD 1, likely secondary to adhesiolysis performed during the revision of the gastrojejunostomy. There were no mortalities.
Discussion
Marginal ulceration continues to be a vexing problem for bariatric surgeons and will likely remain so until the true etiology is fully understood. The reported incidence of marginal ulceration is almost certainly underreported due to the large number of patients who are asymptomatic or manage their symptoms without medical consultation (ie over-the-counter medication for mild reflux or pain). The incidence of ulceration has been reported to peak during the first year after bypass, though ulcers have been diagnosed as late as 20-years postoperatively [17].
Surgeons are often presented with the severe cases of bleeding or perforated anastomotic ulcers. In this study, 13 cases were performed emergently, the majority of which (77%) were performed using a minimally invasive approach. Improved preventative measures can be implemented in an effort to decrease the incidence of ulceration, such as risk factor mitigation. The use of absorbable versus non-absorbable sutures to create the gastrojejunal anastomosis was shown to significantly reduce the incidence of ulceration from 2.6% to 1.3 [20]. Vigilance in marginal ulcer management is also crucial to reducing related morbidity and mortality. A more aggressive approach, such as earlier surgical intervention, may be used to manage high-risk patients. Carr et al presented an evidence-based algorithm to manage patients with marginal ulcers in which 6-12 months of empirical proton pump inhibitor therapy is recommended following RNY [17]. Notably in this study, 64% of the patients who were operated on had failed PPI therapy.
Risk factors commonly associated with the development of marginal ulceration include diabetes, history of peptic ulcer disease, smoking, NSAID use, and H. pylori infection. However, studies have not shown alcohol to be a statistically significant risk factor [14,21-24]. Uncommon causes of ulceration should also be ruled out during the preoperative workup. Zollinger-Ellison syndrome (ZE) is a condition caused by overproduction of gastrin due to a pancreatic or duodenal gastrinomas and has been linked to the formation of anastomotic ulcers following RNY [25,26]. In one such case, the authors emphasize the importance of ZE as a differential diagnosis due to the inability to perform endoscopic examination of the excluded gastric remnant [26]. A combination of elevated gastrin levels and a positive octreotide scan was used to make the diagnosis.
Surgical management of anastomotic ulcers can be completed using various techniques. Most often, resection of the ulcer and revision of the anastomosis is performed. In cases of perforation, omental patching can be used during the repair. In rare circumstances, resection of the ulcer with primary closure is done, typically in difficult cases with dense adhesions that are preventing safe dissection of the surrounding anatomy. Ulcer resection and primary closure has been described in the literature [19] and was performed for one patient at this institution with no adverse 30-day outcomes. Ulcer erosion in adjacent organs (eg liver, pancreas) can also occur. In those situations, we have found that the ulcer is usually visualized with a white base on EGD.
Limitations of this study include the small sample size, retrospective design, and single-site data. Interestingly, 80% of the patients in this study were females, which may suggest a correlation between gender and the propensity to develop marginal ulceration. In contrast, a recent review reported that males had a non-significant increased risk of ulcer development [27]. However, in our study the sample size is small and, in general, more females undergo RNY than men. Nonetheless, the contribution of gender to intractability needs to be further investigated to determine if a correlation exists and if so, the mechanism by which it occurs.
Conclusion
Surgical management of anastomotic ulceration can be performed safely using traditional laparoscopy or robot-assisted laparoscopy with a low incidence of complications in the elective and emergent settings. The exact mechanism of marginal ulcer development has yet to be fully elucidated; however, known patient risk factors can be mitigated perioperatively to decrease the incidence and associated morbidity.
References
- Hales CM, Carroll MD, Fryar CD, et al. (2017) Prevalence of obesity among adults and youth: United States, 2015-2016. Natl Cent Heal Stat Data Br 1-8.
- Fryar CD, Carroll MD, Ogden CL (2016) Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and Over: United States, 1960-1962 Through 2013-2014. Natl Cent Heal Stat.
- Arterburn DE, Maciejewski ML, Tsevat J (2005) Impact of morbid obesity on medical expenditures in adults. Int J Obes 29: 334-339.
- Kim DD, Basu A (2016) Estimating the medical care costs of obesity in the United States: Systematic review, meta-analysis, and empirical analysis. Value Heal 19: 602-613.
- Cheng J, Gao J, Shuai X, et al. (2016) The comprehensive summary of surgical versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomized controlled trials. Oncotarget 7: 39216-39230.
- Chang SH, Stoll CRT, Song J, et al. (2014) The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA Surg 149: 275-287.
- Gloy VL, Briel M, Bhatt DL, et al. (2013) Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 347: f5934.
- Arterburn DE, Olsen MK, Smith VA, et al. (2015) Association between bariatric surgery and long-term survival. JAMA 313: 62-70.
- Guidry CA, Davies SW, Sawyer RG, et al. (2015) Gastric bypass improves survival compared with propensity-matched controls: A cohort study with over 10-year follow-up. Am J Surg 209: 463-467.
- Wittgrove AC, Clark GW, Tremblay LJ (1994) Laparoscopic gastric bypass, Roux-en-Y: Preliminary report of five cases. Obes Surg 4: 353-357.
- Schirmer B (2006) Laparoscopic bariatric surgery. Surg Endosc 20: S450-S455.
- Azagury D, Abu Dayyeh B, Greenwalt I, et al. (2011) Marginal ulceration after Roux-en-Y gastric bypass surgery: Characteristics, risk factors, treatment, and outcomes. Endoscopy 43: 950-954.
- Adduci AJ, Phillips CH, Harvin H (2015) Prospective diagnosis of marginal ulceration following Roux-en-Y gastric bypass with computed tomography. Radiol case reports 10:1063.
- El-Hayek K, Timratana P, Shimizu H, et al. (2012) Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc 26: 2789-2796.
- Palermo M, Acquafresca PA, Rogula T, et al. (2015) Late surgical complications after gastric by-pass: A literature review. Arq Bras Cir Dig 28: 139-143.
- Coblijn UK, Lagarde SM, de Castro SMM, et al. (2015) Symptomatic marginal ulcer disease after roux-en-Y Gastric Bypass: Incidence, risk factors and management. Obes Surg 25: 805-811.
- Carr WRJ, Mahawar KK, Balupuri S, et al. (2014) An Evidence-based algorithm for the management of marginal ulcers following Roux-en-Y gastric bypass. Obes Surg 24: 1520-1527.
- Carrodeguas L, Szomstein S, Soto F, et al. (2005) Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: Analysis of 1292 consecutive patients and review of literature. Surg Obes Relat Dis 1: 467-474.
- Racu C, Mehran A (2010) Marginal Ulcers after Roux-en-Y Gastric Bypass: Pain for the Patient. Pain for the Surgeon?: Bariatric Times. Bariatr Times 7: 23-25.
- Sacks BC, Mattar SG, Qureshi FG, et al. (2006) Incidence of marginal ulcers and the use of absorbable anastomotic sutures in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2: 11-16.
- Spaniolas K, Yang J, Crowley S, et al. (2018) Association of long-term anastomotic ulceration after Roux-en-Y gastric bypass with tobacco smoking. JAMA Surg 153: 862-864.
- Sverdén E, Mattsson F, Sondén A, et al. (2016) Risk factors for marginal ulcer after gastric bypass surgery for obesity: A population-based cohort study. Ann Surg 263: 733-737.
- Aldoori WH, Giovannucci EL, Stampfer MJ, et al. (1997) A prospective study of alcohol, smoking, caffeine, and the risk of duodenal ulcer in men. Epidemiology 8: 420-424.
- Kato I, Nomura AM, Stemmermann GN, et al. (1992) A prospective study of gastric and duodenal ulcer and its relation to smoking, alcohol, and diet. Am J Epidemiol 135: 521-530.
- Court I, Zissman P, Rosenthal RJ (2010) Diagnosis and treatment of Zollinger Ellison syndrome in a morbidly obese patient after Roux-en-Y gastric bypass. Surg Obes Relat Dis 6: 714-717.
- Rosenthal R, Eckstein J, Patel S, et al. (2009) Zollinger-ellison syndrome presenting as recurrent marginal ulcers after gastric bypass. Cine-Med.
- Coblijn UK, Goucham AB, Lagarde SM, et al. (2014) Development of ulcer disease after Roux-en-Y gastric bypass, incidence, risk factors, and patient presentation: A Systematic review. Obes Surg 24: 299-309.
Corresponding Author
Collin EM Brathwaite, MD, FACS, FASMBS, Department of Surgery, NYU Langone Hospital—Long Island; Mineola, New York, USA.
Copyright
© 2020 Hall K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.