Surgical Crisis: Does the Robot Help or Hinder?
Abstract
Introduction
Robotic surgery is growing in popularity, but it is unclear whether the displacement of surgeon from the bedside affects outcomes when an unexpected and potentially catastrophic bleeding complication is encountered. This study aimed to compare the outcomes between the robotic and laparoscopic approaches when a surgical crisis was encountered during a major urologic procedure.
Material and methods
Using a nationwide database of patient-level billing data, we conducted a retrospective study of patients undergoing major urologic surgery (cystectomy, partial/radical nephrectomy, prostatectomy, and pyeloplasty) with concurrent surgical crisis between 2003 and 2013. Surgical crisis was defined as any blood transfusion on the day of index procedure along with the use of ≥ 3 suction canisters billed in the operating room. The primary outcome was 90-day major complication rates. Secondary outcomes were operating room time, length of stay, and 90-day readmission rates. Multivariable regression analyses were performed to evaluate the outcomes between robotic and laparoscopic approaches.
Results
A survey-weighted cohort of 582,844 patients was obtained, among which 0.23% encountered a surgical crisis (0.25% laparoscopic and 0.18% robotic). After adjusting for baseline characteristics, the robotic approach had higher odds of major complications (odds ratio: 2.6; p < 0.05) and longer operating room time (difference: 69 minutes; p < 0.05) than the laparoscopic approach; subgroup analysis revealed that this difference was most pronounced among in the healthiest patients (Charlson Comorbidity Index ≤ 1).
Conclusions
This study suggests potentially catastrophic surgical crises may be more difficult to manage with the robotic approach compared to the laparoscopic approach. The unique separation of patient and surgeon for the robotic procedure may thus benefit from additional training specifically to anticipate these rare events and improve outcomes for patients.
Keywords
Robotic, Laparoscopic, Crisis, Urology
Introduction
The United States Food and Drug Administration approved the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) for general laparoscopic surgery in 2000. While supporters of robotic technology argued that this system was an improvement over traditional laparoscopy, skeptics raised concerns. In particular, there was apprehension regarding the unique paradigm shift associated with the da Vinci robotic platform. Unlike traditional laparoscopic surgeries, the primary surgeon is seated at a console away from the patient and manipulates the robotic device docked to laparoscopic ports at the bedside. Critics felt that the surgeon lost independence and control, which could result in delays controlling a surgical catastrophe [1].
Despite these concerns, the da Vinci robotic platform has gained widespread acceptance. In the urologic community, robotic surgery has become a mainstay for many procedures [2,3]. The adoption of this new technology has occurred in part because of the large body of literature showing that robot-assisted surgery is relatively safe [3-6]. Nevertheless, the specific patient safety question of whether the displaced surgeon setup negatively impacts outcomes in the event of an intra-operative surgical complication remains unexplored. Prior studies evaluating robotic technology have been limited - primarily focusing on mechanical failure, providing few details regarding long term outcomes, and being based on self-reported events from facilities and Intuitive Surgical [7-9].
To clarify this issue, we studied surgical morbidity and mortality among major urologic procedures (cystectomy, partial/radical nephrectomy, prostatectomy, and pyeloplasty), which account for approximately 20% of robotic procedures in the United States [10]. This study aims to provide a better understanding of the implications of the da Vinci robotic platform on outcomes after an intraoperative surgical crisis as compared to the traditional laparoscopic approach. We also sought to propose a suggested algorithm which may be used to help address intraoperative surgical crisis during robotic cases.
Materials and Methods
Data source
Patient-level billing data were obtained from the Premier Hospital Database (Premier Inc, Charlotte, NC) from January 1, 2003 through December 31, 2013. Premier captures approximately 20% of inpatient discharges from non-federal institutions in the United States. All procedures and diagnoses were identified using the International Classification of Diseases, 9th revision, Clinical Modification (ICD9). This study was exempt from institutional review board approval given the de-identified nature of the data.
Study population
Patients were identified in the Premier database if they had a primary procedure code for urologic procedures performed via laparoscopic and robotic approaches: partial nephrectomy (ICD9 55.44), radical nephrectomy (ICD9 55.51), pyeloplasty (ICD9 55.87), cystectomy (ICD9 57.71), or prostatectomy (ICD9 60.5). The cohort was further limited to patients who underwent elective procedures based on administrative codes to exclude 1) Urgent or emergent surgeries; 2) Individuals who had surgery in the prior 3 months, and 3 those with concomitant surgical procedures as these patients likely have an inherently elevated risk for morbidity and mortality. Eligible cases were categorized as open, laparoscopic, and robotic approaches based on regular expression matching techniques following review of the chargemaster data [11]; open procedures were excluded.
We defined "surgical crisis" as significant intraoperative blood loss. Because there is no single ICD9 or administrative code to identify this specific clinical scenario, we reviewed the chargemaster data to flag patients who received a blood transfusion on the day of index procedure and required the use of ≥ 3 suction canisters in the operating room. The rationale for the above algorithm is based on the idea that laparoscopic and robotic procedure typically have relatively minimal blood loss and uncommonly involve copious irrigation thus the need for blood transfusion and an unusually high number of suction canisters would be limited to those patients with significant intraoperative blood loss.
Patient, hospital and surgical characteristics
Patient characteristics included age ( < 50, 50 to 59, 60 to 69, and ≥ 70 years), gender (male or female), race (White, Black, Hispanic, and Other), Charlson Comorbidity Index (CCI) (0, 1, and ≥ 2), and insurance type (Medicare, Medicaid, private insurance, and other). Hospital characteristics included teaching status (teaching or non-teaching), location (urban or not urban), hospital size (< 200, 200 to 399, and ≥ 400 beds) and United States Census geographic region (Midwest, Northeast, South, and West). Surgical characteristics included the type of index surgery (cystectomy, partial/radical nephrectomy, prostatectomy, or pyeloplasty).
Perioperative outcomes
The primary outcome was the rate of major post-operative complications up to 90 days after the index surgery. We identified the post-operative complications using ICD9 codes, which were further classified into no (grade 0), minor (grades I and II) and major (grades III to V) complications using the Clavien-Dindo system as described previously [12]. Outpatient complications were excluded due to the inability to reliably capture these in the data set. Secondary outcomes were operating room time, length of stay, and 90-day readmission rate. Billing data were used to determine the total time in the operating room. Length of stay was determined by calculating the number of days between admission and discharge dates.
Statistical analysis
Descriptive statistics were used to summarize patient, hospital, and surgical characteristics along with complication and readmission rates. Categorical variables were compared between laparoscopic and robotic approaches using the Chi-square test. We performed univariate quantile regression analysis for operating room time and negative binomial regression analysis for length of stay using the laparoscopic approach as the reference group. We also performed multivariate logistic regression for 90-day major complications and readmissions, generalized linear regression with a gamma distribution for operating room time, and negative binomial regression for length of stay, adjusting for patient, hospital, and surgical characteristics. All analyses incorporated survey weights and adjusted for hospital clustering. Data analyses were performed using Stata SE version12 (StataCorp LLC, College Station, TX). The level of significance was set at p < 0.05.
Robotic surgery crisis flowchart
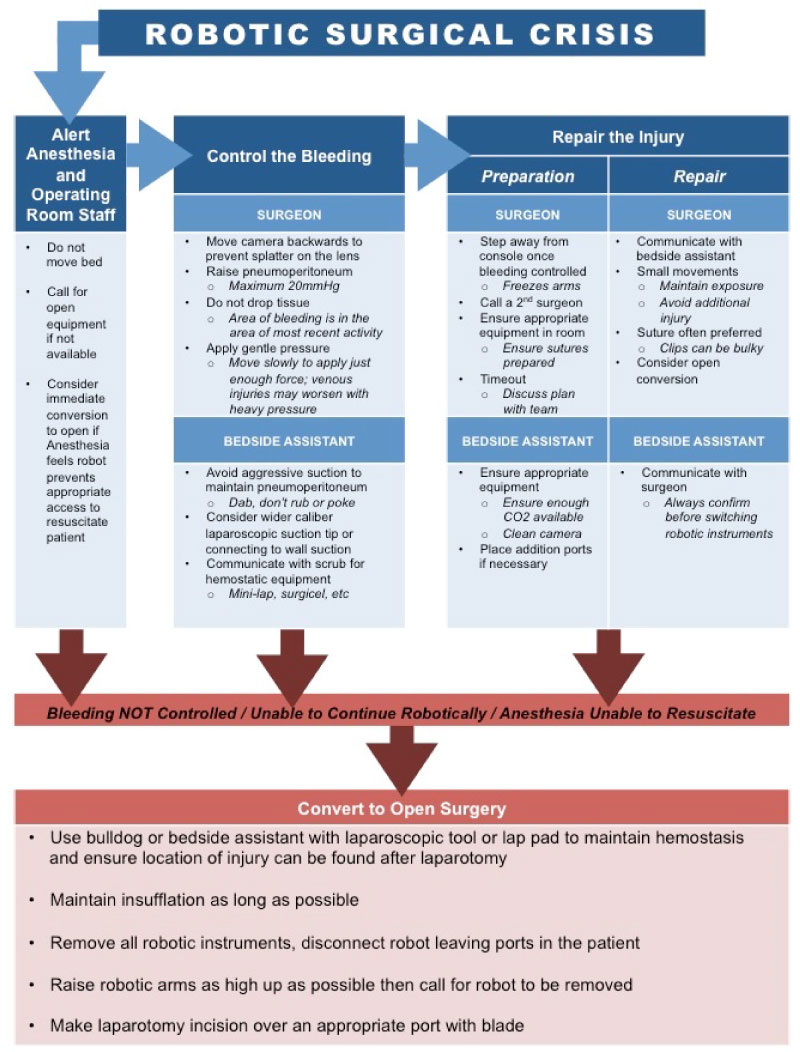
To help address how to best handle the issue of intraoperative surgical crisis during robotic procedures, the primary study authors created a flowchart to serve as a guide for initial management. This was then presented to a broader group of attending robotic surgeons at our collaborating institutions. The Delphi method was used to gain consensus between all surgeons, with a cutoff of 80% agreement for each bullet point [13]. The website SurveyGizmo (http://surveygizmo.com, SurveyGizmo, Boulder, CO) was then used to query experts throughout the field and solicit anonymous responses/suggestions to improve the flowchart. This survey process underwent two separate iterations before arriving at the final consensus flowchart.
Results
We evaluated a weighted cohort of 582,844 minimally invasive surgeries based on a total of 92,954 observations in the Premier dataset. Of these, 0.23% encountered a surgical crisis (0.25% laparoscopic, and 0.18% robotic) based on our algorithm yielding a final cohort of 1,317 patients (829 laparoscopic, and 488 robotic). Most patients with a crisis were at least 60-years-old, male, white, had a CCI < 2, and had Medicare insurance (Table 1). There was no significant difference in patient characteristics between the two minimally invasive surgical approaches. Most hospitals had > 200 beds and were located in urban areas. Compared to the laparoscopic approach, the robotic approach was more likely to be used in larger hospitals (p = 0.02) and in the Midwest region of the United States (p = 0.04). A trend toward increased use in teaching hospitals was seen, but did not reach significance (p = 0.054). Surgical crises mostly occurred during prostatectomy for the robotic approach compared to partial nephrectomy for the laparoscopic approach (p = 0.02).
Among patients in the study cohort, robotic surgeries, compared to laparoscopic surgeries, were found to have significantly higher unadjusted 90-day major complication rates (21.5% vs. 12.7%, p < 0.05), longer median operating room times (363 vs. 252 minutes, p < 0.05), and higher 90-day readmission rates (13.8% vs. 8.1%, p < 0.05). After adjusting for patient, hospital, and surgical characteristics, the robotic approach continued to have a higher odds of 90-day major complications (OR 2.6, p < 0.05) and longer median operating room time (+69 minutes; p < 0.05) as compared to the laparoscopic approach (Table 2 and Table 3). No statistically significant difference was found in either length of stay or 90-day readmission rate.
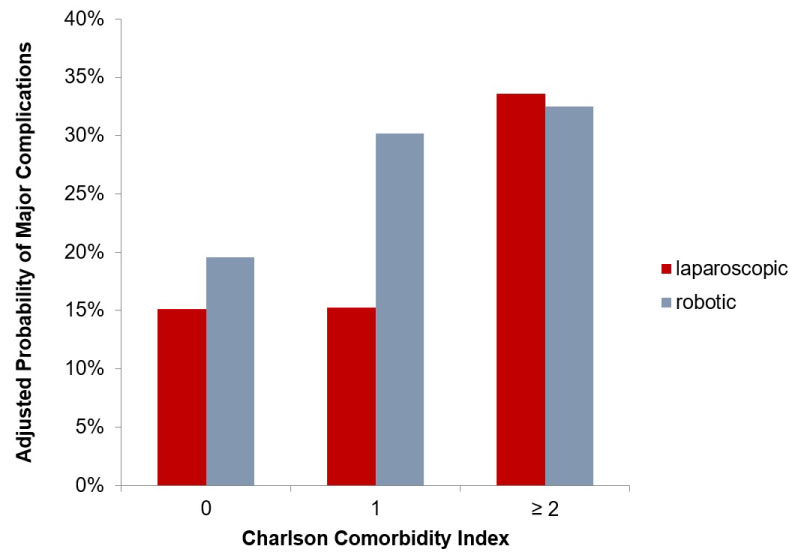
When stratifying based on CCI, the adjusted probability of 90-day major complication was statistically significantly higher for the robotic approach as compared to the laparoscopic approach in healthy patients (CCI ≤ 1) while there was no difference between surgical approaches was noted for those patients with a CCI ≥ 2 (Figure 1).
Subgroup analysis was performed to assess for differences in outcomes following surgical crisis among the individual procedure types. For those undergoing partial or radical nephrectomy, robotic surgery was associated with a significantly increased odds of adjusted 90-day major complication (OR 3.3, p = 0.01). Conversely, no difference was seen between the two surgical approaches for those undergoing prostatectomy (OR 1.0, p = 0.99). Too few observations were present to perform a subgroup analysis for cystectomy or pyeloplasty.
A procedural flowchart of how to best address intraoperative surgical crisis was devised by the primary study authors. This was then revised through an iterative process consulting with multiple experts at multiple institutions, resulting in the final flowchart presented in Figure 2.
Discussion
To our knowledge, this is the first population-based study of intraoperative surgical crisis associated with use of the da Vinci robotic platform which specifically looks at intra- and post-operative patient outcomes. Our analysis found that encountering a surgical crisis during major urologic surgery is very uncommon occurring in 0.23% of cases. However, this study revealed that utilizing a robotic approach was associated with an increased risk of 90-day major complications when compared to procedures performed with the conventional laparoscopic approach among patients who experienced a surgical crisis.
Because of the rarity of this type of event, we capitalized on the granular data in the Premier Hospital Database, a detailed nationwide discharge and billing database, to generate a cohort of sufficient size to perform a meaningful analysis. As rates of surgical crisis during minimally invasive procedures are relatively low, single- or multi-institutional series would likely lack the power to assess differences in outcomes. While the FDA MAUDE database attempts to collect adverse events nationwide, this data is electively submitted by medical facilities and is susceptible to reporting bias [9]. Additionally, use of the Premier database allows for tracking of unique patient identifiers which facilitates more accurate capture of 90-day complication rates.
Numerous critiques have been leveled at the master-slave configuration of the da Vinci system. First and foremost, the physical distance between the surgeon and the operative field has been viewed as a potential barrier to early recognition of, and response to, an injury or crisis [14,15]. This displacement of surgeon from bedside necessitates the surgeon become largely reliant on the ability and efficiency of their bedside assistant in carrying out many of the critical and time-sensitive technical aspects of mitigating a surgical crisis [1]. Even with a skilled and experienced assistant, the size, position, and relative immobility of the robotic platform itself may result in delays in clearing the operative field [16]. This has been previously shown in simulation work done on intra-operative cardiac emergency [17,18].
Due to the retrospective nature of the study design, we are unable to determine causation for the observed findings of the current study. However, we feel that it is reasonable to assume that the separation of surgeon from patient in robotic procedures likely contribute to the overall poorer outcomes following a surgical crisis during robotic surgery. It is plausible that a laparoscopic surgeon may minimize the sequela of an intraoperative crisis by being able to immediately convert to an open procedure to address ongoing bleeding. Conversely, with the robotic approach, smaller crises may evolve into larger ones in the time needed to undock the robot and have the primary surgeon scrub back into the case after leaving the console.
Interestingly, in this study we found a higher probability of major complications in the robotic approach among healthier patients but not less healthy patients (Figure 1). One possible explanation is that there are relatively fewer patients with significant comorbidities undergoing a robotic approach and thus it was not possible to discern a difference among those less healthy patients. Alternatively, we speculate that among healthier patients, there was a greater tolerance for blood loss potentially delaying conversion to an open procedure which, in turn, accentuated the problems of separating surgeon from patient thus increasing the risk of a poor outcome.
There exist a number of limitations to this study. The retrospective nature of this review and the use of an already populated commercial database limits what information we have available for analysis. Specifically, no data was available regarding pre-operative characterization of the patient disease status beyond generalized diagnosis codes. Without disease severity information (such as stage and grade for cancers), we lose some amount of resolution and introduce potential for unmeasured confounders. Another significant limitation in this study is in how we define "surgical crisis". The decision to use operative day transfusion in concert with ≥ 3 suction canisters used during the procedure limits us to hemorrhagic crises. This would, then, suggest that we may not be capturing all people due to our patient selection criteria.
Despite these limitations, and in light of the now widespread adoption of robotic surgery, our findings of worsened outcomes for those encountering surgical crisis during robotic procedures suggest this is an area ripe with potential for process improvement. Other authors have also seen this opportunity, with studies already published suggesting helpful intraoperative techniques to mitigate surgical crises [19,20]. Though these technical maneuvers are useful, we sought to address the issue in a more process driven manner with the creation of a robotic surgical crisis flowchart (Figure 2). The potential for success using a standardized process model is likely best exemplified by Gawande and colleagues' implementation of the surgical 'checklist' that we have all come to use as a standard of care. These safety checklists have been linked to improved patient outcomes in day-to-day operations, and have also been adapted to robotic surgery [21,22]. Similarly, checklists developed for intraoperative crises have been shown to improve adherence to critical steps even in the high stress environment of a simulated surgical crisis [23]. Garg, et al. have developed a similar checklist specifically pertaining to robotic crisis and implemented it in a simulated crisis environment [24]. Despite their reported success, these checklists tend to be brief and institution-specific making it difficult to adapt them to outside institutions. Therefore, we worked to develop a surgical crisis flowchart (Figure 2) to assist in the training of less experienced surgeons (i.e. house staff) in dealing with robotic surgical crises.
Conclusions
Though surgical crises during urologic procedures are rare events, we must continue to shed light on the issue and strive to broaden our understanding of its causes. This study provides thought-provoking conclusions that crises encountered during procedures performed via the robotic approach may carry with them worsened post-operative outcomes. As robotic surgery is here to stay, we should view this disparity as an area for improvement within our field. We hope that better training, including use of tools such as our surgical crisis flowchart, can help to minimize the risk of intraoperative robotic surgical crises and improve outcomes when such a crisis does occur.
Acknowledgements
Adam S. Kibel, Mark A. Preston, Caleb P. Nelson, Ruslan Korets, Christopher B. Allard, and Francisco J. Gelpi-Hammerschmidt.
References
- Loeb S, Catalona WJ (2007) Open radical retropubic prostatectomy. Urol Oncol 25: 494-498.
- Gandaglia G, Sammon JD, Chang SL, et al. (2014) Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. J Clin Oncol 32: 1419-1426
- Ghani KR, Sukumar S, Sammon JD, et al. (2014) Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: Results from the nationwide inpatient sample. J Urol 191: 907-912.
- Ficarra V, Minervini A, Antonelli A, et al. (2014) A multicentre matched-pair analysis comparing robot-assisted versus open partial nephrectomy. BJU Int 113: 936-941.
- Trinh QD, Sammon J, Sun M, et al. (2012) Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol 61: 679-685.
- Sivaraman A, Leveillee RJ, Patel MB, et al. (2012) Robot-assisted laparoscopic dismembered pyeloplasty for ureteropelvic junction obstruction: A multi-institutional experience. Urology 79: 351-355.
- Kim WT, Ham WS, Jeong W, et al. (2009) Failure and malfunction of da Vinci Surgical systems during various robotic surgeries: experience from six departments at a single institute. Urology 74: 1234-1237.
- Buchs NC, Pugin F, Volonté F, et al. (2007) Reliability of robotic system during general surgical procedures in a university hospital. Am J Surg 207: 84-88.
- Manoucheri E, Fuchs-Weizman N, Cohen SL, et al. (2014) Maude - analysis of robotic-assisted gynecologic surgery. J Minim Invasive Gynecol 21: 592-595.
- Intuitive Surgical (2013) Annual Report 2013.
- Wright JD, Ananth CV, Lewin SN, et al. (2013) Robotically assisted vs. laparoscopic hysterectomy among women with benign gynecologic disease. JAMA 309: 689-698.
- Leow JJ, Reese SW, Jiang W, et al. (2014) Propensity-matched comparison of morbidity and costs of open and robot-assisted radical cystectomies: A contemporary population-based analysis in the united states. Eur Urol 66: 569-576.
- Steurer J (2011) The Delphi method: an efficient procedure to generate knowledge. Skeletal Radiol 40: 959-961.
- Lavery HJ, Thaly R, Albala D, et al. (2008) Robotic equipment malfunction during robotic prostatectomy: a multi-institutional study. J Endourol 22: 2165-2168.
- Simorov A, Otte RS, Kopietz CM, et al. (2012) Review of surgical robotics user interface: what is the best way to control robotic surgery? Surg Endosc 26: 2117-2125.
- Zargar H, Krishnan J, Autorino R, et al. (2014) Robotic nephroureterectomy: A simplified approach requiring no patient repositioning or robot redocking. Eur Urol 66: 769-777.
- Huser AS, Müller D, Brunkhorst V, et al. (2014) Simulated life-threatening emergency during robot-assisted surgery. J Endourol 28: 717-721.
- Doumerc N, Yuen C, Savdie R, et al. (2010) Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int 106: 378-384.
- Sotelo R, Nunez Bragayrac LA, Machuca V, et al. (2015) Avoiding and managing vascular injury during robotic-assisted radical prostatectomy. Ther Adv Urol 7: 41-48.
- Siqueira TM, Kuo RL, Gardner TA, et al. (2002) Major complications in 213 laparoscopic nephrectomy cases: The Indianapolis experience. J Urol 168: 1361-1365.
- Howell AM, Panesar SS, Burns EM, et al. (2014) Reducing the burden of surgical harm: a systematic review of the interventions used to reduce adverse events in surgery. Ann Surg 259: 630-641.
- Ahmed K, Khan N, Khan MS, et al. (2013) Development and content validation of a surgical safety checklist for operating theatres that use robotic technology. BJU Int 111: 1161-1174.
- Arriaga AF, Bader AM, Wong JM, et al. (2013) Simulation-based trial of surgical-crisis checklists. N Engl J Med 368: 246-253.
- Garg T, Bazzi WM, Silberstein JL, et al. (2013) Improving safety in robotic surgery: intraoperative crisis checklist. J Surg Oncol 108: 139-140.
Corresponding Author
Matthew D Ingham, MD, Division of Urologic Surgery, Brigham and Women's Hospital 45 Francis Street, Boston, MA, 02115, USA.
Copyright
© 2019 Ingham MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.