Fruit Set, Yield, Quality, and Mineral Content Evaluation of 'Pricia' Apricot as Affected by Biostimulants Application
Abstract
A two-year trial was conducted during 2016 and 2017 seasons in an apricot (Prunus armeniaca L.) commercial orchard located at Cerignola (Foggia province, Puglia region, Southern Italy, 41°15'49''N; 15°53'59''E; 126 a.s.l.). The effects of three plant biostimulants (Hendophyt®, Ergostim® and Radicon®, based on biopolymers of polysaccharides, carboxylic acids and humic-fulvic acids, respectively) compared with control treatment, were evaluated on: Flowering, fruit set, yield, total polyphenols content and antioxidant activity, fruits physical-chemical traits and minerals content of the cultivar Pricia. Foliar spray of biostimulants were applied at three phenological stages (calyx perceptible, fruit-set and fruit development) during each growth season. Data showed some specific effects of biostimulants on the quanti-qualitative attributes of fruits. Flowering, fruit-set percentages, and yields were mainly affected by the season, probably because the different chilling hours accumulation during the winter period. Significant interaction between years and biostimulants application were observed. No effect of biostimulant treatments on total polyphenols content and antioxidant activity of fruit were noted in both the years. With regards of the mineral content, only potassium significantly changed.
Keywords
Organic fertilizers, Humic and fulvic acids, Polyglucosamine, Carboxylic acids, Flowering, Antioxidant activity
Introduction
At worldwide level, the largest production of apricot (Prunus armeniaca L.) is supplied from the European and Mediterranean countries, representing more than 75% of the world production [1,2] Italy, where this species has an important potential due to its genetic resources and ecological conditions, ranks first in Europe in fresh and dried apricot fruit production [2]. Italian apricot production is approximately 243,486 tons, on an area of 18,917 ha, mostly located in the regions Campania, Emilia Romagna, Basilicata, and Puglia. The production in this latter region of southern Italy accounts for 15,330 tons on 1125 ha [3]. Apricot being an attractive fruit is appreciated by consumers all over the world and has gained great economic importance over the years. It is consumed in fresh, dried, and frozen forms or used for preparation of jams, jellies, pulp, juices, nectars, and extruded products [1]. Apricot has a beneficial effect on human health and constitutes a rich source of compounds including polyphenols, vitamins, natural antioxidant, and mineral elements [4,5]. Mineral ions are very important for the nutritional value of the fruits, the major ones being K, Ca, Mg and P. Many of them are co-factors of different enzymes and amounts of these elements must be in human diet to pursue a good health [6]. Yield of the apricot tree depends on a number of factors, such as density of flower buds and number of flowers, chilling requirement, bud drop, fruit size, precipitation amount and orchard management, winter and late spring freeze damages, this last especially for early bloom cultivars. In apricot flowering takes place in winter or early spring, when adverse meteorological conditions can influence the pollination process and subsequently fruit set in many areas [7]. Higher temperatures during the vegetative period and the lack of sufficient chilling pose problems under most conditions in the temperate regions [8]. The failure in bud development (anomalies), inadequate bud production and high flower bud drop, consequently, have a negative influence on fruit production and yield as repeatedly reported in different cultivars and growing conditions [9]. Regular high fruit set and low fruit drop is desired for apricot growing.
As other perennial fruit tree species, the apricot in addition to climatic factors is also frequently exposed to other abiotic stresses, as nutritional, drought, or soil salinity which limit yield and fruit quality [10]. It is generally accepted that the appropriate orchard management is crucial for optimizing fruit crop production. Modern fruit tree physiology is currently focused, among others, on developing researches using new and mostly natural fertilizer called 'plant biostimulants' or 'Agricultural Biostimulants' (ABs), which are organic substances or microorganism applied to plants with the aim to enhance nutrition efficiency, abiotic stresses tolerance and/or crop quality traits, regardless of its nutrients content" [11]. The main categories of plant ABs include natural substances such as humic and fulvic acids, protein hydrolysates, compounds containing nitrogen, seaweed extracts, beneficial fungi, and bacteria [12-14]. They are applied to the soil and/or by foliar spraying. Taking into account the limitations of nutrients addition through soil, foliar sprays are an effective way to meet the plants nutrients requirements [15]. This fertilization method is more target-oriented and environmental friendly since the nutrients are applied in controlled quantities [16]. Studies on the effect of ABs on the growth and yield potential of plants have been conducted mainly on several vegetable crops [17-21]. However, availability of this information is relatively limited for fruit crops, with reports on soil or foliar application of humic acids on growth, yield and fruit quality of peach and apple [22], table grape [23,24] and apricot [25,26]. Moreover, they have shown that foliar application of seaweed extracts leads to enhanced root development in grape [27,28] and strawberry [29].
Enhanced fruit tree growth and yield by biostimulants have been accompanied in some cases by improved nutrient uptake. For example, leaves-applied humic substances in apricot trees stimulated mineral level (N, P, K, and Mg [30]. In addition, leonardite extract enhanced K, B, Mg, Ca, and Fe accumulation in olive leaves [31]. Considering the very few researches carried on the use of ABs on apricot, this study deals with the results of the effects of three commercial ABs (Hendophyt®, Ergostim® and Radicon®) application on flowering, fruit set, yield and quanti-qualitative characteristics of fruits of 'Pricia', an emerging apricot cultivar.
Material and Methods
Experimental conditions and biostimulant treatments
The experimental trial was conducted in two consecutive seasons, 2016 and 2017, in a commercial orchard located in the countryside of Cerignola (Foggia province, Puglia region, Southern Italy), 4-years-old apricot trees of 'Pricia' cultivar (publisher: Europepinieres, worldwide distributor: Vivai F.lli Zanzi). Pricia is a very early ripening cultivar that ripens about 35 days before cv San Castrese (reference cultivar for Italian apricot ripening calendar, last decade of June). The fruit has a large size, rounded-oblong shape, intensely orange skin with 50% red blush, firm flesh, and good aromatic flavor. Apricot trees were planted 6 × 6 meter apart with rows North-South oriented, drip irrigated and received the ordinary cultural practices (pruning, fruit thinning, fertilization, pest, and disease protection) of the area. In particular, for fertilization schedules, the doses per year applied in the experimental field were 95, 56 and 100 kg ha-1 for N, P2O5 and K2O, respectively. Fruit thinning in 2016 was not accomplished because of the low flowering intensity. The soil in orchard is deep, loamy, slightly alkaline, non-calcareous [32]. Trees used in the experiment were selected to be healthy and as uniform as possible.
For each season, the three commercial ABs, Hendophyt® PS (Iko-Hydro), Ergostim® XL (Isagro) and Radicon® (Fertek) were compared with a control. In (Table 1) the composition and the dose of the ABs used in the trials are reported. The ABs were applied in both years by foliar spraying, three times during the growing season (at red ball, fruit-set, and fruit development stages). The experiment was laid out in a randomized block design, with three replicates per treatment. One buffer row was located between replicates and blocks and two or more buffer rows around the perimeter of the experimental field. Each replicate had 15 plants and three centrally located plants per plot were used to collect vegetative and reproductive parameters. During the two experimental years, from June to May, daily main climatic parameters (maximum, minimum and mean temperatures, total rainfall and total "Class A" Pan evaporation) were recorded at the nearest meteorological station, few kilometers from the experimental area and supplied by Consorzio per la Bonifica della Capitanata of Foggia.
Flower buds and fruits set monitoring
Percentage of fruit set was estimated in each tree on four branches randomly selected in the four cardinal directions of the three central plants. Flower buds on each branch were counted distinctly for the three types of bearing shoots: long twigs, short twigs, and spurs. Approximately 50-80 flower buds were counted and recorded at the pre-flowering stage on each of these selected branches. Thus, 200-320 flower buds were counted on each tree. In each year the flower buds (on 14 February 2016 and 20 February 2017) and flowers (on 24 February 2016 and 2 March 2017) of each tagged branch were counted and flowering rate was calculated as flowers to flower buds numbers ratio. Fruit set, expressed as percentage of fruit per total open flowers [33,34] was evaluated twice, such as at the end of flowering (14 March 2016 and 20 March 2017, respectively) as initial fruit set and after physiological fruit drop (2 April 2016 and 10 April 2017) as final fruit set.
Fruits collection
In both years apricot fruits for each treatment were harvested, based on their skin ground color (i.e., fully colored), in one picking date at the commercial maturity stage (13 May and 15 May in 2016 and 2017, respectively). Yield per each tree was determined (kg/tree). The fruits were sorted to remove overripe and bruised fruits. Successively, samples of 15 fruits for each replicate were randomly collected, placed into shallow wood crates, and stored at 4°C and immediately carried to the lab for the subsequent analyses.
Morphological and chemical characteristics of the fruits
The following morpho-pomological and phisico-chemical traits were measured on the fruits: weight (g), length (mm), width (mm), thickness (mm), color index of the epicarp (CIE coordinates, L*, a* and b*), weight of the stone (g), sugar content (°Brix), pH, titratable acidity (% malic ac.), maturity index (ratio sugar content/titratable acidity), total phenols (mg GA 100 g-1 fresh weight (fw)) and antioxidant capacity (mg TE 100 g-1 fw). All the above parameters were evaluated using the methods and instruments as previously fully described for apricot [35].
Mineral elements in fruits
In order to determine dry matter, cations and anions, only for the 2017 harvest, samples of the pulp were dried to a constant weight in a forced-air oven at 65°C. The dry matter was expressed as %. The anions (nitrate, phosphate, and sulfate) and cations (sodium, potassium, magnesium, and calcium) content were determined by ion-exchange chromatography (mod. ICS-1100; Dionex Corporation, Sunnyvale, CA, USA). The anions were extracted from 0.5 g of dried and ground samples, with 50 mL of 3.5 mmol L-1 NaCO3 and 1.0 mmol L-1 Na2HCO3, and the extracts were analysed using a guard column and an analytical column (Thermo Fischer Scientific, MA, USA, mod. IonpacAG14 and AS14, respectively). The data were expressed as mg kg-1 fresh weight (fw). For the cations, 1.0 g dried and ground samples were used which were ash-reduced in a muffle furnace at 550°C and then digested in 20 mL 1.0 mol L-1 HCl in boiling water (99.5 ± 0.5°C) for 30 min. The resulting solution was filtered, diluted, and analysed using a guard column and analytical column (Thermo Fischer Scientific, MA, USA, mod. Ionpac CG12A and CS12A, respectively). The data are expressed as mg kg-1 fw [36]. Results were evaluated with one-way ANOVA using JMP® software (SAS Institute Inc., Cary, NC, USA) and average values were compared with Tukey test. Standard deviations were calculated using Excel software of the Office 2007® suite (Microsoft Corporation, Redmond, WA, USA). Percentage values were transformed to arcsin, prior to analysis of variance.
Results and Discussion
Climate conditions
The experimental site is characterized by mild winters and annual average rainfall of about 450 mm. The climatic parameters recorded during the 2015-2016 and 2016-2017 experimental seasons are reported in (Table 2). Major differences in terms of air temperature occurred between the two seasons, with the 2015-2016 winter relatively milder with respect to the 2016-2017 one. In particular, the average temperatures of December, January, and February of the first experimental season were higher of 1.2, 4.0 and 0.7°C than the corresponding months of the second season. The total seasonal rainfall and class "A" pan evaporation were quite similar between the two seasons while, as expected, more relevant differences among monthly values of the two seasons were recorded.
Fruit set and yield
Data of flowering, initial and final fruit set percentages, yield/tree resulted in significant differences between the two seasons (Table 3). In particular, flowering, initial and final fruit set percentages were significantly lower in 2016 season (47.5, 4.6 and 2.6%, respectively) than in 2017 (90.4, 41.5 and 14.4%, respectively). These different values may be explained by the high maximum and average temperatures occurred in December 2015, January, and February 2016, corresponding at the dormant pre-flowering period. In this regard, there could have been a negative effect on chilling units accumulated during winter (in the period starting from the fall of the leaves) that could explain the significantly lower flowering percentage in 2016 respect to 2017 season. The higher temperatures recorded in wintertime reduced the accumulation of chilling hours. Similar results have been obtained by the cultivar 'Orange rubis' in 2016 season at the same site [35]. Moreover, previous studies reported a significant negative effect in apricot of increased pre-flowering and flowering temperature on flower quality and fruit setting potential [37]. In 2016 season, when both lower flowering and fruit set percentage occurred, as previously mentioned, the fruit thinning was not applied but, nevertheless, very poor fruit yield was recorded (Table 3). Our data are in accordance with those of Alburquerque, et al. (2004) [38] who reported that when flower bud number was low, yield was proportionally low.
Considering the effect of ABs treatments on fruit-set some differences were observed in both years. In 2017 significant higher initial fruit set percentages (measured before thinning) were obtained using Ergostim® (45.0%), and Radicon® (47.0%) compared to Hendophyt® (39.0%), which was statistically similar to the control treatment (35.0%). But when considering the final fruit set percentage, non-significant differences among treatments were observed. On the contrary, in 2016 significant differences among treatments were reported at the beginning of fruit set, with higher values in Radicon® (6.0%) and Ergostim® (5.0%) with respect to control (3.0%). Final fruit set percentages resulted significantly higher for Hendophyt® (3.6%) and Ergostim® (3.0%) followed by Radicon® (2.3%) and finally by the control (1.3%).
Total fruit yield per tree was significantly higher in 2017 with an average of 46.5 kg per tree vs. 12.0 kg per tree in 2016. Differences among treatments were observed in 2016, when the fruit yield of Hendophyt® (13.4 kg per tree) and Ergostim® (13.1 kg per tree) were significantly higher than Radicon® (12.4 Kg per tree) and the control treatment showed the lowest value (10.3 kg per tree). In 2017, the Ergostim® treatment showed the highest yield (48.2 kg per tree) with respect to the other treatments.
Fruit quality attributes
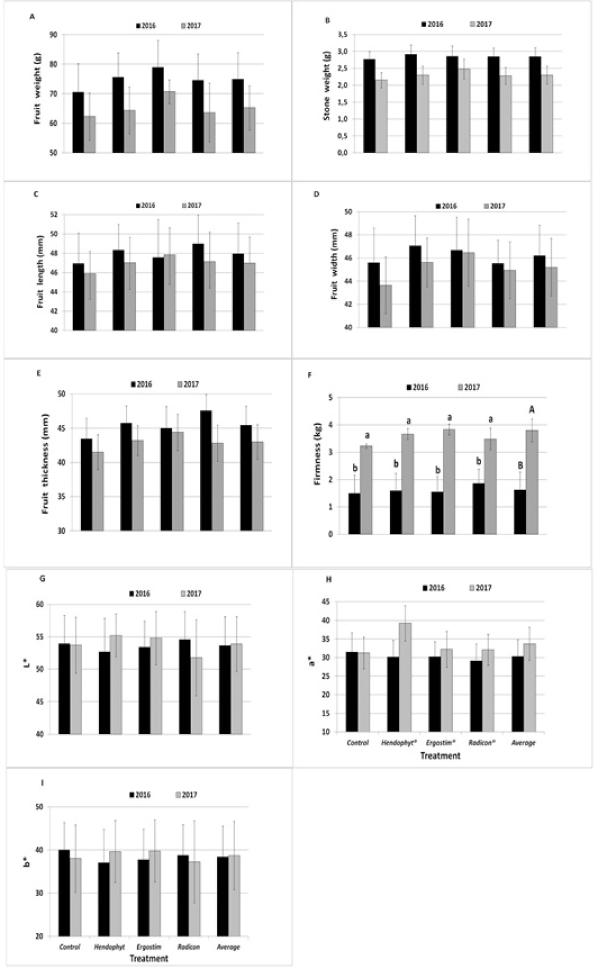
(Figure 1 and Figure 2) show the results of the pomological, chemical and nutraceutical parameters determined on the fruits by the effect of treatments in 2016 and 2017. Average annual values of each parameter were also showed. (Figure 1) shows the effect of treatments on pomological parameters (fruit and stone weight, length, width, thickness, firmness, and skin color) recorded in both seasons. The fruit weight (Figure 1A), stone weight (Figure 1B), fruit length (Figure 1C) and fruit width (Figure 1D) were not significantly different between years and among treatments, although the values tended to be higher in 2016 compared to 2017. The basically higher average values were obtained when the yield per tree was significantly lower (Table 3). In 2016, only fruit weight in Ergostim®, tended to be higher than other treatments. Also, for fruit thickness (Figure 1E), no differences among years and treatments were reported, although values were basically higher in the 2016 than 2017 and in all ABs treatments than the control. Only in 2016 thickness in Radicon® tended to be higher than other treatments. The fruit firmness (Figure 1F) was significantly higher in 2017 (average of 3.80 kg) than in 2016 (average of 1.62 kg). In both years no differences among treatments were observed.
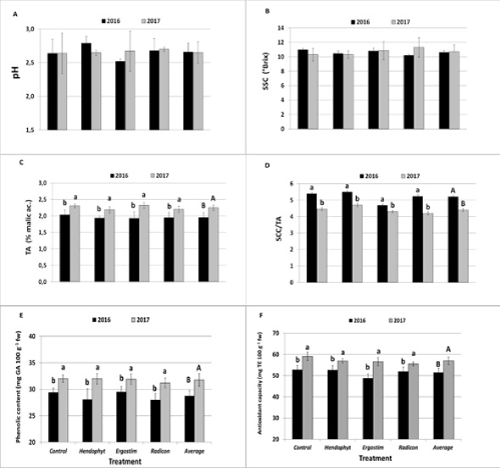
The average value of fruit firmness in 2017 is to be considered slightly higher with respect to the standard values suitable for ready-to-eat fruit reported by Scandella, et al. (1998) [39], who reported values between 1 and 3 kg cm-2 as more suitable for fresh consumption and processing. Regarding the fruit skin color, L* (lightness) (Figure 1G), a*(redness) (Figure 1H) and b* (yellowness) (Figure 1I) no statistical differences between years and among treatments were found. In any case the red over color, in all the treatments, was basically more pronounced in 2017 than in 2016, whereas in 2017 season it tended to be higher in Hendophyt® than other treatments. The chemical attributes of the fruits evaluated in both years are presented in Figure 2. Non-significant differences were observed for the pH (Figure 2A), with values ranging between 2.52 and 2.79. These values are slightly lowers than those of several genotypes reported in the literature [40,41]. Fruit SSC values (Figure 2B), being one of the most appreciable quality characteristics influencing notably the fruit taste and palatability, ranged between 10.20 and 11.30°Brix, with non-significant differences between years and among treatments. With regards of TA (Figure 2C), statistical difference between years were observed, with higher value in 2017 (average of 2.25%) than in 2016 (average of 1.96%). No differences among treatments were noted for each year. SSC and TA are hugely affected by fruit maturity stage to the point that, in practice, the ratio between these two parameters represents the main maturity index for most of edible fruits and is highly related with their sensorial quality. The SCC/TA ratio (Figure 2D) showed significant differences between 2016 (average of 5.20) and 2017 (average of 4.62). No differences among treatments were noted within each year.
With regard of phenolic content of fruits (Figure 2E), significant differences between seasons were obtained with a lower value in 2016 (average of 29.0 mg GA 100 g-1) than in 2017 (average of 32.3 mg GA 100 g-1). The higher value in 2017 could be due to the warm and dry climatic conditions occurring at the final stage of fruit growth in 2017, according to results found by other authors [42]. However, no differences were observed within each year among the treatments. As for the lower phenolic content observed in the first season compared with the second one, the total antioxidant capacity (Figure 2F) had a significantly lower value in 2016 (average 51.6 mg TE 100 g-1) compared to 2017 (average of 57.1 mg TE 100 g-1). The effect of the year on the antioxidant properties of fruit could be related to particular climatic conditions during fruit development stages (high temperatures and low rainfall, see the period march-may in (Table 2)).
Dry matter and minerals content
(Table 4) shows the mean values, standard deviations, and significance of the analyzed fresh fruit samples at harvest, for dry matter and concentration on fresh weight basis (mg kg-1 fw) of cations (Na, K, Mg and Ca) and anions (NO3, P and S) in 2017. Dry matter content averaged 13.2%, with values ranging from 12.7% to 13.7% and resulted not significantly different among treatments. Our data showed that apricot fruit of 'Pricia' cultivar contain significant amount of K, which is followed in the order by Ca, Mg, NO3, Na, P, S and N. K is an element usually present in large amount in vegetables and fruits, but apricot fruits were found to be particularly rich in K. It contributes to the regulation of water movement within the plant providing an electrical balance for anions in the vacuole of leaf cells and maintaining turgidity of cells. K appears to have a deep influence on fruit quality in particular on size, appearance, colour, soluble solids, acidity and vitamin content [43] In our study, K concentration was on average 4006.9 mg kg-1 with the highest value in Hendophyt® (4264.5 mg kg-1), significantly different with respect to values of other treatments which ranged from 3866.6 to 4059.8 mg kg-1. These K contents are similar to the data reported in the literature for different cultivars [44,45].
Ca is essential for structure and function of cell walls and membranes. It plays an important role in maintaining quality of fruits and in delaying senescence and controlling physiological disorder in fruits [46], influencing positively their shelf life. The mean Ca concentration of apricot samples was found 289.1 mg kg-1, with values ranging between 231.9 and 325.9 mg kg-1, with no differences among treatments. These Ca concentrations are close to the data of the investigations on different cultivars reported by Gergely, et al. (2014) [45], but are lower than those found by Haciseferogullarj, et al. (2007) [47].
Mg is the essential constituent of chlorophyll, thus contributes to carbohydrate production in leaves throw photosynthesis. The effect of Mg on 'good eat quality' is not well known except a negative impact of fruit firmness [48]. The Mg concentration in our study, similarly to Ca, was not significantly different among treatments, with values ranging between 193.8 and 235.8 and an average of 211.0 mg kg-1. Our values resulted lower than those (402.8 - 765.6 mg kg-1) reported by Haciseferogullari, et al. (2007) [47], but much higher than those (23.3 - 64.3 mg kg-1) reported by Wani, et al. (2015) [44]. Such huge differences could be related to many factors including different pedoclimatic environments, genotypes, and growing techniques.
Nitrate (NO3) is found naturally in foods and its content depends on a number of factors including species, cultivars, light, temperature, method of growth, fertilizers use and storage. NO3 content of apricots in our study tends to lower values in Radicon® (110.3 mg kg-1) and Hendophyt® (191.4 mg kg-1) with respect to control (211.3 mg kg-1) and Ergostim® (236.9 mg kg-1) treatments. These NO3 levels are quite consistent with those reported by Dogan, et al. (2008) [49]. who obtained values of this anion in the range 83.9 - 102.3 mg kg-1. Moreover, according to the NO3 contents classification, the apricot, like other fruits, are low nitrate containing foods (< 200 mg kg-1) [50], differently from certain vegetables, where NO3 is found naturally in high concentrations which are undesired because of their unfavorable effect on human health [51].
The Na concentration in apricots is generally low, which is recommended to reduce blood pressure and prevent heart disease [52]. The Na content ranged from 19.6 to 22.8 mg kg-1, and values were not significantly different among treatments. These values are similar to those obtained in other studies on different cultivars [44].
P concentration in apricots, when compared to other fruits, is generally higher, thus apricots are considered useful for their laxative, diuretic, and purifying properties. In the present study, P concentrations ranged between 174.5 and 189.7 mg kg-1 without differences among treatments. These P concentrations were in agreement with Ali, et al. (2015) [53] but were slightly lower of those reported by Mayer (1997) [54], and by Gergely (2014) [45] for different cultivars. S content of apricot samples ranged from 14.2 and 17.3 mg kg-1, averaging 16.0 mg kg-1, without differences among treatments.
Conclusions
The analysis carried out over two consecutive harvesting seasons on 'Pricia' apricot cultivar allowed establishing that apricot fruit set, yield and quality showed a high variability between the two years in relation to the climatic conditions and some differences among the biostimulant treatments were also observed. The relative mild environmental conditions of the winter prior the 2016 growing season negatively affected flowering, fruit set percentage and total yield per tree. Differences between the two years were also observed with regards of quality parameters.
With regards of the biostimulants, only Ergostim positively affected the yield in both seasons.
Only slight or not significant differences were noted for the other parameters determined.
Concerning biostimulant treatments, in 2016 positive effects were found for Hendophyt® and Ergostim® on crop yield, final fruit set, as well as, in this year, Radicon® contributed to increase tendentially fruit thickness. The total polyphenols content and antioxidant capacity, which in the fruits of the cv 'Pricia' were at high level, showed no difference between biostimulant treatments in both the years. Almost any difference was noted in mineral contents of fruits of compared treatments. Only Hendophyt® resulted to increase K content respect to other treatments.
In conclusion, the study suggested that biostimulants can play a role especially in protecting the apricot orchards in order to reduce adverse effects of fluctuations on climatic variable. Finally, the ability of plant biostimulants to attenuate abiotic and biotic stresses over the years should be also investigated on other apricot cultivars.
Author Contributions
Conceptualization, Annalisa Tarantino, Francesco Lops and Giuseppe Lopriore; Data curation, Annalisa Tarantino, Grazia Disciglio, Andrea Mazzeo and Giuseppe Lopriore; Formal analysis, Francesco Lops, Anna Gagliardi and Giuseppe Lopriore; Methodology, Annalisa Tarantino, Francesco Lops, Grazia Disciglio and Giuseppe Lopriore; Supervision, Annalisa Tarantino, Grazia Disciglio and Giuseppe Lopriore; Validation, Annalisa Tarantino, Grazia Disciglio, Giuseppe Ferrara and Giuseppe Lopriore; Writing - original draft, Annalisa Tarantino, Grazia Disciglio and Giuseppe Lopriore; Writing - review & editing, Annalisa Tarantino, Grazia Disciglio and Giuseppe Lopriore. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We sincerely thank Mr. Rocco Cascavilla for their kind hospitality at his farm and his effective cooperation in carrying out project activities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leccese A, Bartolini S, Viti R (2007) Total antioxidant capacity and phenolics content in apricot fruits. International Journal of Fruit Science 7: 3-16.
- Leccese A, Bureau S, Reich M, et al. (2010) Pomological and nutraceutical properties in apricot fruit: Cultivation systems and cold storage fruit management. Plant Foods Hum Nutr 65: 112-120.
- ISTAT (2021) Electronic information system on agriculture and livestock. Italian National Statistical Institute, Rome.
- La Vecchia C, Altieri, A, Tavani A (2001) Vegetables, fruit, antioxidants and cancer: A review of Italian studies. Eur J Nutr 40: 261-267.
- Bartolini S, Leccese AM, Viti R (2015) Quality and antioxidant properties of apricot fruit at ready-to-eat: Influence of the weather conditions under Mediterranean coastal area. J Food Process Technology 7: 1-10.
- Valvi SR, Rathod VS (2011) Mineral composition of some wild edible fruits from Kolhapur District. International Journal of Applied Biology and Pharmaceutical Technology 2: 392-396.
- Ledbbetter CA (2008) Apricot. In: Hancock JF (edn), Temperate fruit crop breeding germplasm to genomics. Springer, New York, 39-82.
- Frank G, Dennis JR (2000) Flowering, fruit set and development under warm conditions. In: Amnon Erez (edn), Kluwer Academic Publishers, Temperate fruit crops in warm climates, 101-122.
- Gallotta A, Palasciano M, Mazzeo A, et al. (2014) Pollen production and flower anomalies in apricot (Prunus armeniaca L.) cultivars. Sci Hortic 172: 199-205.
- Drobek M, Frac M, Cybulska J (2019) Plant Biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress-A Review. Agronomy 9: 335.
- Du Jardin P (2015) Plant biostimulants: De?nition, concept, main categories and regulation. Sci Hortic 196: 3-14.
- Battacharyya D, Babgohari MZ, Rathor P, et al. (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196: 39-48.
- Canellas LP, Olivares FL, Aguiar NO, et al. (2015) Humic and fulvic acids as biostimulants in horticulture. Sci Hortic 196: 15-27.
- Colla G, Hoagland, L Ruzzi M, et al. (2017) Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front Plant Sci 8.
- Wójcik P (2004) Uptake of mineral nutrients from foliar fertilization. J Fruit Ornam Plant Res 12: 201-218.
- Fernández V, Eichert T (2009) Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and respective of foliar fertilization. Crit Rev Plant Sci 28: 36-68.
- Colla G, Rouphael Y, Canaguier R, et al. (2014) Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front Plant Sci 5: 1-6.
- Disciglio G, Frabboni L, Tarantino A, et al. (2014) Applying natural fertilizer to herbaceous crops. J Life Sci 8: 504-510.
- Tarantino E, Disciglio G, Frabboni L, et al. (2015) Effects of biostimulant application on quali-quantitative characteristics of cauliflower, pepper and fennel crops under organic and conventional fertilization. World Acad Sci Eng Technol Int J Agric Biosyst Sci Eng 9: 734-738.
- Rouphael Y, Colla G, Giordano M, et al. (2017) Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci Hortic 226: 353-360.
- Ertani A, Sambo P, Nicoletto C, et al. (2015) The use of organic biostimulants in hot pepper plants to help low input sustainable agriculture. Chem Biol Technol Agric 2: 1-10.
- Fathy MA, Eissa-Fawzia MM, Yahia MM (2002) Improving growth, yield and fruit quality of 'Desert Red' peach and 'Anna' apple by using some biostimulants. Minia J Agric Res Dev 22: 519-534.
- Ferrara G, Brunetti G (2008) Influence of foliar applications of humic acids on yield and fruit quality of table grape cv. Italia. J Int Sci Vigne Vin 42: 79-87.
- Ferrara G, Brunetti G (2010) Effects of the times of application of a soil humic acid on berry quality of table grape (Vitis vinifera L.) cv Italia. Spanish Journal of Agricultural Research 8: 817-822.
- Eissa-Fawzia M, Fathi MA, El Shall SA (2007) Response of peach and apricot seedlings to humic acid treatment under salinity condition. J Agric Sci Mansoura Univ 32: 3605-3620.
- Fathy MA, Gabr MA, El Shall SA (2010) Effect of humic acid treatments on "Canino" apricot growth, yield and fruit quality. N Y Sci J 3: 109-115.
- Mancuso S, Azzarello E, Mugnai S, et al. (2006) Marine bioactive substances (IPA extract) improve foliar ion uptake and water stress tolerance in potted Vitis vinifera plants. Adv Hortic Sci 20: 156-161.
- Mugnai S, Azzarello E, Pandolfi C, et al. (2008) Enhancement of ammonium and potassium root influxes by the application of marine bioactive substances positively affects Vitis vinifera plant growth. J Appl Phycol 20: 177-182.
- Alam MZ, Braun G, Norrie J, et al. (2013) Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can J Plant Sci 93: 23-36.
- Fatma KMS, Morsey MM, Thanaa SMM (2015) Influence of spraying yeast extract and humic acid on fruit maturity stage and storability of "Canino" apricot fruits. Int J Chem Tech Res 8: 530-543.
- Fernández-Escobar R, Benlloch M, Barranco D, et al. (1996) Response of olive trees to foliar application of humic substances extracted from leonardite. Sci Hortic 66: 191-200.
- Tarantino E, Frabboni , Giuliani M, et al. (2008) Opuscolo divulgativo Lotta alla desertificazione. 1-81.
- Williams RR (1970) Factors affecting pollination in fruit trees. In: Luckwill LC, Cutting CV (edn), Physiology of Tree Crops. Academic Press, London, 193-207.
- Alburquerque N, Burgos L, Egea J (2003) Apricot flower development and abscission related to chilling, irrigation and type of shoots. Sci Hortic 98: 265-276.
- Tarantino A, Lops F, Disciglio G, et al. (2018) Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of 'Orange rubis®' apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci Hortic 239: 26-34.
- Renna M, Gonnella M, Giannino D, et al. (2013) Quality evaluation of cook-chilled chicory stems (Cichorium intybus L., Catalogna group) by conventional and sous vide cooking methods. J sci Food Agric 94: 656-65.
- Polat AA, Caliskan O (2014) Fruit set and yield apricot cultivars under subtropical climate conditions of Hatay Turkey. J Agric Sci Tech 16: 863-872.
- Alburquerque N, Burgos L, Egea J (2004) Influence of flower bud density, flower bud drop and fruit set on apricot productivity. Sci Hortic 102: 397-406.
- Scandella D, Sibille I, Venien S, et al. (1998) Apricot : Evaluation des Atouts Organoleptiques. Infos-Ctifl 141: 22-25.
- Leccese A, Bartolini S, Viti R (2012) From genotype to apricot fruit quality: The antioxidant properties contribution. Plant Foods Hum Nutr 67: 317-325.
- Mratinic E, Popovski B, Milosevic T, et al. (2011) Evaluation of apricot fruit quality and correlations between physical and chemical attributes. Czech J Food Sci 29: 161-170.
- Bartolini S, Leccese AM, Viti R (2015) Quality and antioxidant properties of apricot fruit at ready-to-eat: Influence of the weather conditions under Mediterranean coastal area. J Food Process Technol 7: 538.
- Ramesh Kumar A, Kumar N, et al. (2006) Role of potassium in fruit crops-A review. Agr Rev 27: 284-291.
- Wani SM, Masoodi FA, Wani TA, et al. (2015) Physical characteristics, mineral analysis and antioxidant properties of same apricot varieties grown in North India. Cogent Food & Agric 1: 1-10.
- Gergely A, Papp N, Stefanovits-Bànyai E, et al. (2014) Assessment and examination of mineral elements in apricot (Prunus armeniaca L.) cultivars: A special attention to selenium and other essential elements. Eur Chem Bull 3: 760-762.
- Bakshi P, Masoodi FA, Chauhan GS, et al. (2005) Role of calcium in post-harvest life of temperate fruits: A review. J Food Sci Tech Mys 42: 1-8.
- Haciseferogullarj H, Gezer I, Özcan MM, et al. (2007) Post harvest chemical and physical- mechanical properties of some apricot varieties cultivated in Turkey. J Food Eng 79: 364 -373.
- Gerendás J, Führs H (2013) The significance of magnesium for crop quality. Plant Soil 368: 101-128.
- Dogant A, Kazankara A, Balta MF, et al. (2008) Nitrate and nitrite levels of some fruit species grown in Van Turkey. Asian J Chem 20: 1191-1198.
- Zengin MM, Gülderen MS (1997) Nitrate and nitrite contents of some fruits sold in bazaars in konya city Turkey. J Agric For 21: 463-468.
- Santamaria P (2006) Nitrate in vegetables: Toxicity, content, intake and EC regulation. J Sci Food Agric 86: 10-17.
- WHO (1988) Guidelines for drinking-water quality. Vol 2, Health criteria and other supporting information: addendum, 2nd (edn), Geneva.
- Ali S, Masud T, Abbasi KS, et al. (2015) Apricot: Nutritional potentials and health benefits-A review Annals Food Sci Technol 16: 175-189.
- Mayer AM (1997) Historical changes in the mineral content of fruit and vegetables. Brit Food J 99: 207-211.
Corresponding Author
Annalisa Tarantino, Department of Agriculture, Food, Natural resources and Engineering (DAFNE), University of Foggia, via Napoli 25, I-70100, Foggia, Italy.
Copyright
© 2022 Tarantino A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.