Ex-Situ Induction of Sprouting on Euadenia trifoliolata Cuttings Harvested from Four Pedoclimatic Areas of Côte d'Ivoire
Abstract
Euadenia trifoliolata (Capparaceae) is a tropical forest plant. It is used in the pharmacopoeia for its medicinal properties and for that it is overexploited. Information on propagation techniques of E. trifoliolata domestication and its wild management are still missing. The study conducted on nurseries aimed to obtain some young plants from cutting stems. For this purpose, cuttings harvested in different areas (Adzopé, Banco, Ehotilé Islands and Marahoué) were used and some parameters like viability, time of emission of shoot and the first leaf, number of leaves, length and width of leaves, length of stems and diameter of stems were evaluated according to the origin and treatment of the cuttings and the growing medium (unheated soil, heated soil (45 °C), soil treated with extracts of Ricinus communis (Euphorbiaceae) without heating and heated soil (45 °C) and treated with extracts of R. comminus). Results showed that the viability varied according locations and treatments. Shoot emission time varied from 13.32 ± 1.12 days to 12.94 ± 1.38 days. The emission time of the first leaf ranged from 22.65 ± 3.82 to 24 ± 6.32 days. There was also variation in the number of leaves (from 5.32 ± 0.19 to 5.13 ± 0.27), the leaf length (from 50.74 ± 1.50 to 53.20 ± 2.47 cm) and leaf width (from 25.96 ± 1.37 to 39.14 ± 2.26 cm). The stem length oscillated between 25.68 ± 1.28 and 29.01 ± 1.50 cm and the stem diameter fluctuated from 12.52 ± 0.51 to 12.06 ± 0.57 cm. E. trifoliolata can be regenerated from cuttings and maintained its therapeutic potential. Cuttings treated with newsprint gave the best results whatever the culture medium.
Keywords
Induction, Ex-situ, Sprouting, Cutting, Euadenia trifoliolata, Areas Pedoclimatic
Introduction
Medicinal plants are valuable resources for the vast majority of rural populations in Africa. More than 80% of this population use these resources for human and animal health care [1,2]. In addition, non-timber products have attracted considerable interest in Africa in recent years for their contribution to the household economy and the conservation of plant biodiversity [3]. Thus, the strong demand for medicinal plants becomes a threat to the plant species. According to Mehdioui and Kahouadji [4], intensive plant exposure can become harmful if it exceeds the tolerable threshold of renewal of regeneration of the resources used. Regeneration is the process by which the plant gives a new individual by artificial induction. There are several methods of regeneration including cuttings that are the removal of a stem or root fragment from which rhizogenesis is induced [5-5]. Several works have been done on cuttings of plants such as Garcinia kola [7], Vitex doniana [8] and Lawsonia inermis [9]. The success of cuttings depends on endogenous factors (age, size and appearance) and exogenous factors (soil (substrates), cultural practices, light and temperature [10]. The combined action of these factors allows to patrol some stresses in order to carry out the vegetative multiplication. In Côte d'Ivoire, investigations on medicinal plants are mainly focused on floristic diversity [11], ethnobotany and phytochemical potential [12-15], biological and ethnopharmacological activity [16,17] and biofungicide effects [18]. However, studies on the regeneration of medicinal plants are still limited. This is the case of Euadenia trifoliolata (Capparaceae), a medicinal plant used in the pharmacopoeia to treat otitis, anemia, respiratory diseases, male and female fertility disorders and especially as an aphrodisiac [19-21]. In fact, E. trifoliolata is overexploited for its medical properties and is becoming more and more rare. Despite this threat, literature on regeneration of E. trifoliolata is still missing. This study aimed at investigating the efficacy of "cutting method" in the regeneration of E. trifoliolata. We hypothesized that E. trifoliolata can be regenerated through cutting to ensure it's survive and good management.
Material and Methods
Study areas
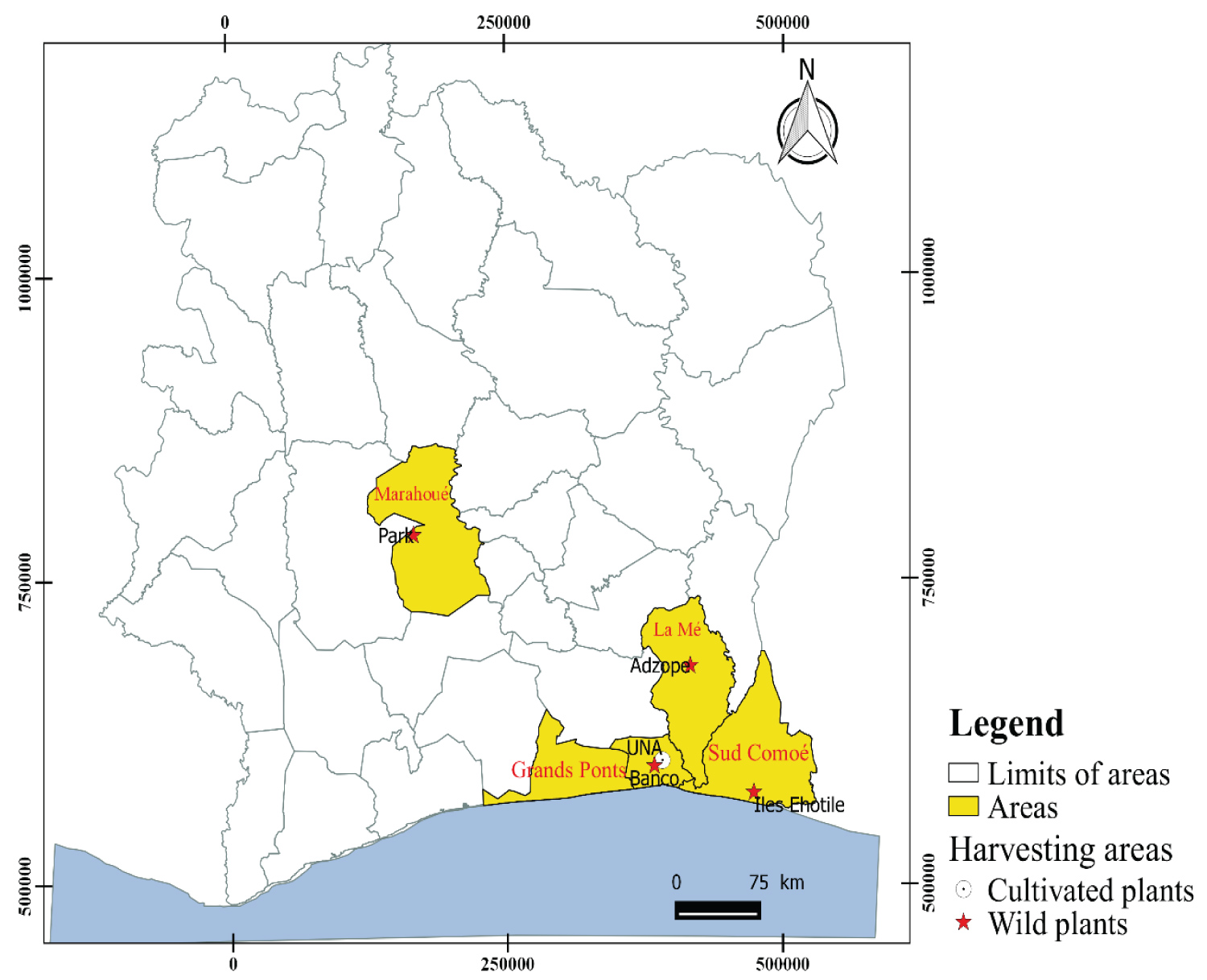
Cutting samples of Euadenia trifoliolata were harvested in Adzopé, Banco, Ehotilé Islands and Marahoué. Adzopé is situated between latitudes 6°06 N - 6°25 N and longitudes 3°51 W - 3°36 W. Banco is located between latitudes 5°23 N - 5°40 N and longitudes 4°03 W - 4°07 W. Éhotilé Islands are situated between latitudes 5°10 N - 5°37 N and longitudes 3°13 W - 3°44 W. Marahoué is between latitudes 7°05 N - 7°49 N and longitudes 6°01 W - 6°32 W and the UNA is located between latitudes 5°23 N - 5°43 N and longitudes 4°17 W - 5°43 W. The climate of the harvested and regeneration zones is equatorial. Rainfall oscillated between 1800 and 2000 mm. The relative humidity was 85%. Soils of harvest areas were ferruginous and hydromorphic with acidic pH in general [22]. The samples harvested were authenticated at the "Centre National Floristique de l'Université Felix Houphouet Boigny de Cocody". Figure 1 shows the different harvesting and growing areas of cuttings of E. trifoliolata.
Plant material and its regeneration tests
The plant material consisted of cuttings from four areas (Adzopé, Banco, Ehotilé Island and Marahoué), which constituted four populations of Euadenia trifoliolata (Schum & Thonn) Oliv (Capparaceae). Four hundred young and old cuttings with external and internal diameters respectively between intervals [1, 22] cm and [1, 20] cm were used. Their length was 10.5 cm. These cuttings consisted of large and small caliber. The cuttings were taken from end branches of wild plants from June 2015 to September 2015. For the experiment, cuttings were soaked in water from the harvested site in coolers. These cuttings were divided into three groups. The first group had its upper surface in contact with ambient air when the upper surface of the second group and the third group was protected with newspaper and transparent plastic bag on 2 cm length respectively. Cuttings treated were grouped into 4 batches and were sown obliquely in black nursery bags containing untreated soil, heated soil (45 °C), soil treated with extracts of Ricinus comminus (Euphorbiaceae). The combination of soil treatments and cuttings also generated the following groups of individuals:
■ Cuttings that had upper surface free and planted in the soil without treatment (BLS),
■ Cuttings that had upper surface free and planted in the heated soil (BLC),
■ Cuttings that had upper surface free and planted in the soil treated with extract of ricin (BLR),
■ Cuttings that had upper surface free and planted in the heated soil and treated with extract of ricin (BLCR),
■ Cuttings that had upper surface protected with newspaper and planted in the soil without treatment (BJS),
■ Cuttings that had upper surface protected with newspaper and planted in the heated soil (BJC),
■ Cuttings that had upper surface protected with newspaper and planted in the soil treated with extract of ricin (BJR),
■ Cuttings that had upper surface protected with newspaper and planted in the heated soil and treated with extract of ricin (BJCR). The treatment of cuttings with transparent plastic bag gave also four groups. They were BTS, BTC, BTR and BTCR.
The bags were placed in the shade. Bio-pesticides applied at the time of cuttings were extracts of R. comminus and Azadirachta indica (Anacadiaceae) [23,24]. Each day, castor and neem extracts were applied on plants at a dose of 1 kg/L of fresh crushed leaf to protect them against pests. The nursery was watered daily to keep the humidity at 85% and weed control was regular. For humidity measurement, the "Extech model RH210" manual device was used. Droppings of guinea fowl were used as compost (fertilizer of the culture medium). The experiment lasted 90 days and Parameters measured were the viability of the cuttings (VC), the time of shoot emission (TSE), time of emission of first leaf (TEFL), number of leaves (NL), the length of leaf (LeL) and width of leaf (WiL), the length of stems (LeS) and the neck diameter of stems (ND). The regenerated plants were subjected to qualitative phytochemical analysis and compared to wild plants. In fact, samples of leaves and roots of Euadenia trifoliolata harvested in the different environments were dried at room temperature and then crushed with a grinder "Culatti typ MFC" brand. The different powders obtained were macerated in distilled water using 400 g of powder for l L during 24 hours and then filtered. The residues were macerated again for 24 hours twice always in solvent. After filtration, the filtrates were dried in an oven at 40 °C to obtain acqueous extracts that were subjected to phytochemical tests according to methods described by Boua, et al. [25].
Statistical analysis
Data obtained were analyzed using Statistical software 10.0228.2. GLM statistical tests (generalized linear model) ANOVA was used to determine the differences between modalities and Turkey test was applied for averages comparison. Significant differences between means were determined by P-values < 0.05 (i.e. α = 0.05).
Results
Viability rate of cuttings
Cuttings viability rate at 45 and 90 days after burial is summarized in Table 1. There was no significant difference between viabilities at 45 days and 90 days for the same origin. The cuttings without plastic remain viable whatever their origin during the period of experimentation according to Turkey test. The duration of the experiment had no effect on the cuttings. They kept their appearance on the 90 days experimentations with a rate remaining higher than 50%.
Average viability according to diameters
There was a variation in viability according to cutting diameters (Table 2). For cuttings with diameter from 1 to 22 cm, the viability ranged from 8.9 to 11.81. The highest viability was obtained with cuttings from Marahoué and the lowest from Ehotilé Islands. However, there was no significant difference between the viability of cuttings from Adzopé and that of cuttings from Banco. Similar observations were made while using cuttings of diameter varying from 1 to 20 cm.
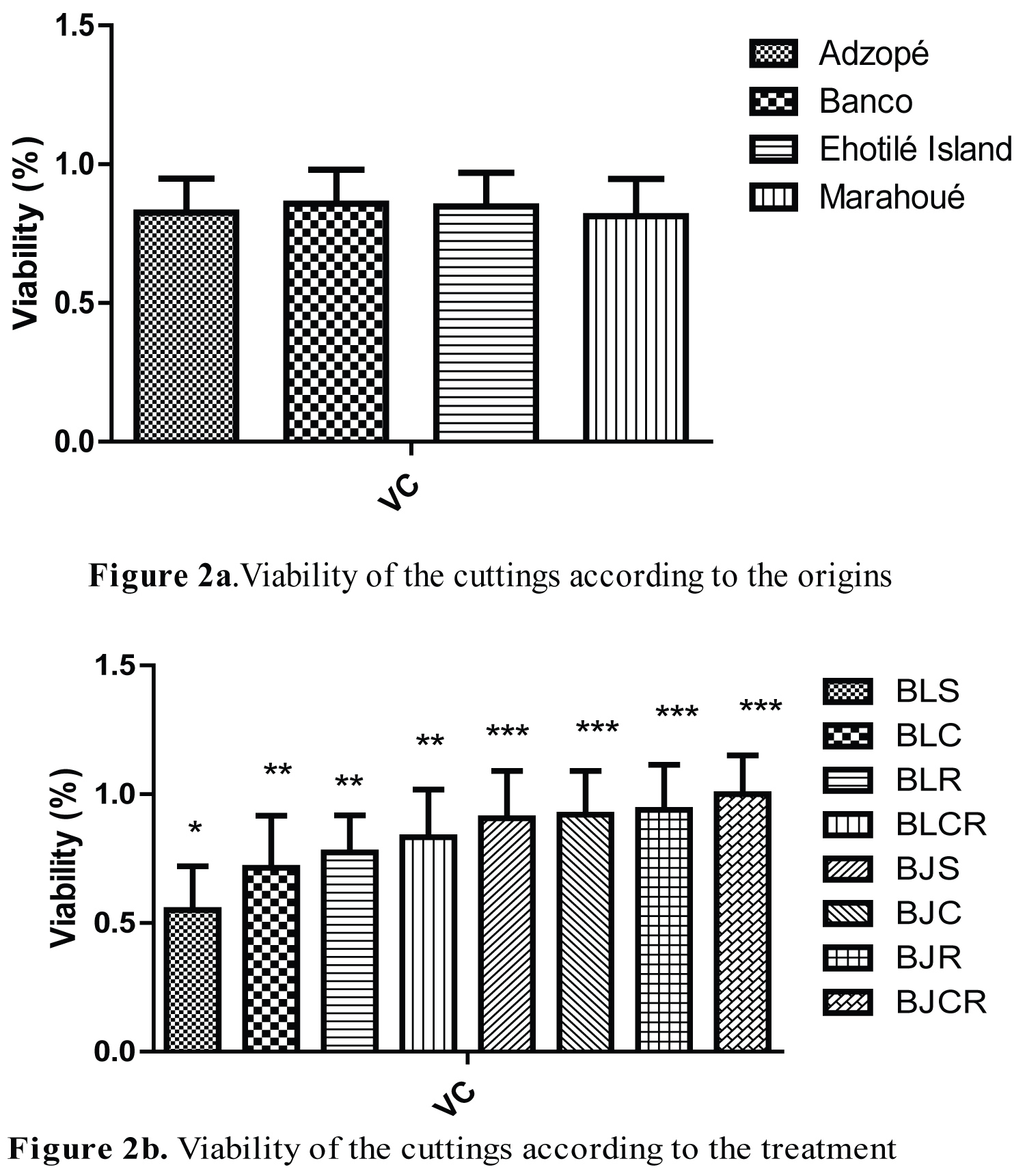
Viability of cuttings during their growing
The influence of cuttings origin on viability is represented on Figure 2 and shows that the origin of cuttings provenance does not influence their viability. Cuttings remained viable whatever their origin (Figure 2a). This viability differed according to the origin and varied from 0.81% to 0.85%. The highest viability was obtained with cuttings from Banco and the lowest with cuttings from Marahoué. Viability was 0.82 ± 0.12 for Adzopé, 0.85 ± 0.12 for Banco, 0.84 ± 0.12 for Ehotilé Island and 0.81 ± 0.13 for Marahoué. The treatment influenced the viability of the cuttings (Figure 2b), with a rate ranging from 0.55% to 100%. The averages for the treatments were respectively 0.55 ± 0.17 for BLS, 0.71 ± 0.20 for BLC, 0.77 ± 0.14 for BLR, 0.83 ± 0.18 for BLCR, 0.90 ± 0.18 for BJS, 0.92 ± 0.17 for BJC, 0.94 ± 0.17 for BJR, and 1 ± 0.15 for BJCR. There was no viability for the culture media as BTS, BTC, BTR and BTCR. Globally, it appears that the viability of the cuttings depends on the treatment and not on the origin.
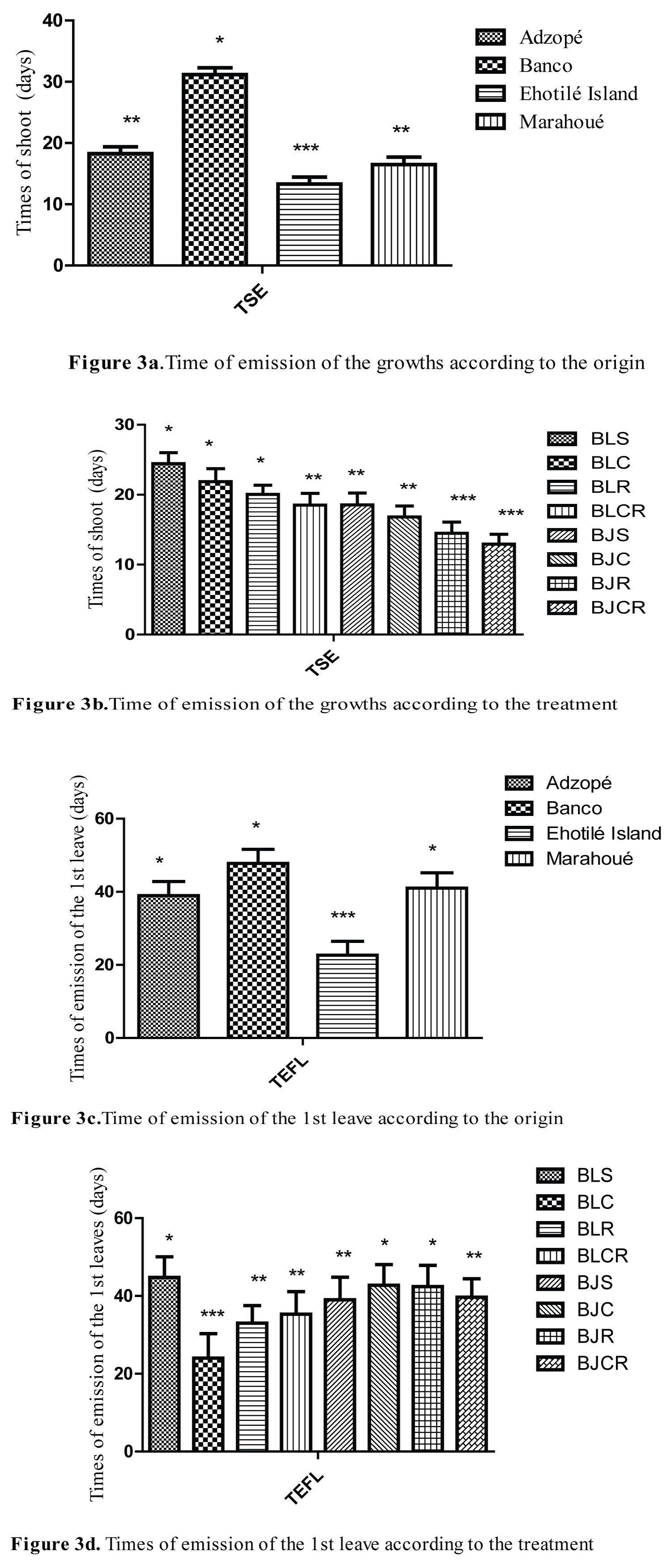
Timing of shoot and first leave emission
Figure 3 shows the emission timing of shoots and first leave in function of cuttings origin and treatments. Plants from Banco had the longest shoot time (31.17 ± 1.12 days) compared to other localities. Plants from Ehotilé Islands had the shortest shoot time (13.32 ± 1.12 days) (Figure 3a). The timing for shoots emission in function of treatments varied from one culture medium to another. The longest time was recorded for BLS treatment (24.42 ± 1.57 days) while the BJCR treatment got the shortest shoot time emission (12.94 ± 1.38 days) (Figure 3b). The emission of the first shoot and leaf on the cuttings from Ehotilé Island was faster than in those of other origins. Cuttings from Banco took the longest time for the emission of the shoot according to Turkey's test. The emission times of the first leaf of the cuttings differed significantly (Figure 3c and Figure 3d). Emission time of the first leaf of the cuttings from Ehotilé Island (22.65 ± 3.82 days) was shorter than that of the cuttings from Banco (47.77 ± 3.82 days) (Figure 3c). Concerning the cuttings from Adzopé and Marahoué, the emission time of the first leaf was the same statistically. The emission time of the first leaf of the BLC treatment (24.00 ± 6.32 days) was shorter compared to those of other treatments. BLS treatment recorded the longest time (44.75 ± 5.34 days) (Figure 3d). Also, the emission of the first shoot was done in a different way and gave three groups of plants. Group 1 or early group was constituted of BJCR and BJR, and group 2 or medium group gathered BJC, BJS and BLCR, when group 3 or group that lasts consisted of BLR, BLC and BLS.
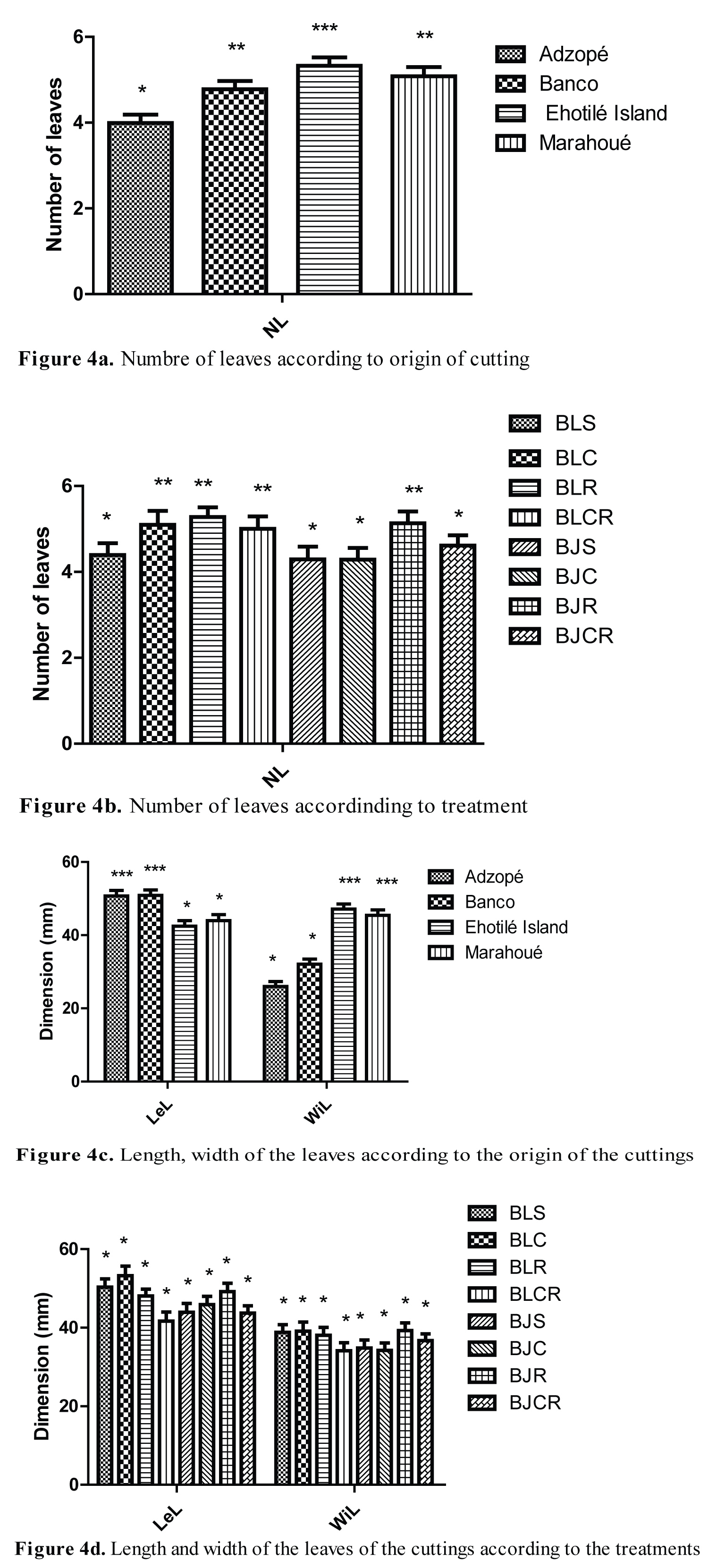
Number, length and width of leaves
The variation in the number of leaves, the length of leaves, the width in function of the origin of cuttings and treatments are shown on Figure 4. The plants of Adzopé had fewer leaves (3.99 ± 0.19) than those of Banco, Ehotilé Island and Marahoué following Turkey test. However, the plants of Ehotilé Islands had the highest number of leaves (5.32 ± 0.19) (Figure 4a). The average number of leaves per cutting ranged from 4 to 5. Plants from BLS, BJS and BJC treatments produced fewer leaves than the other treatments (BLC, BLR, BLCR, BJR and BJCR) (Figure 4b). The leaves of Adzopé (50.74 ± 1.50 mm) and Banco (50.87 ± 1.50) were longer than they were wide while those of the Ehotilé Islands (47.14 ± 1.37 mm) and Marahoué (45.80 ± 1.50 mm) were wider than they were long (Figure 4c). The leaves from the plants of the BLCR and BJCR treatments were the widest while the plants of the other treatments produced the longest leaves (Figure 4d).
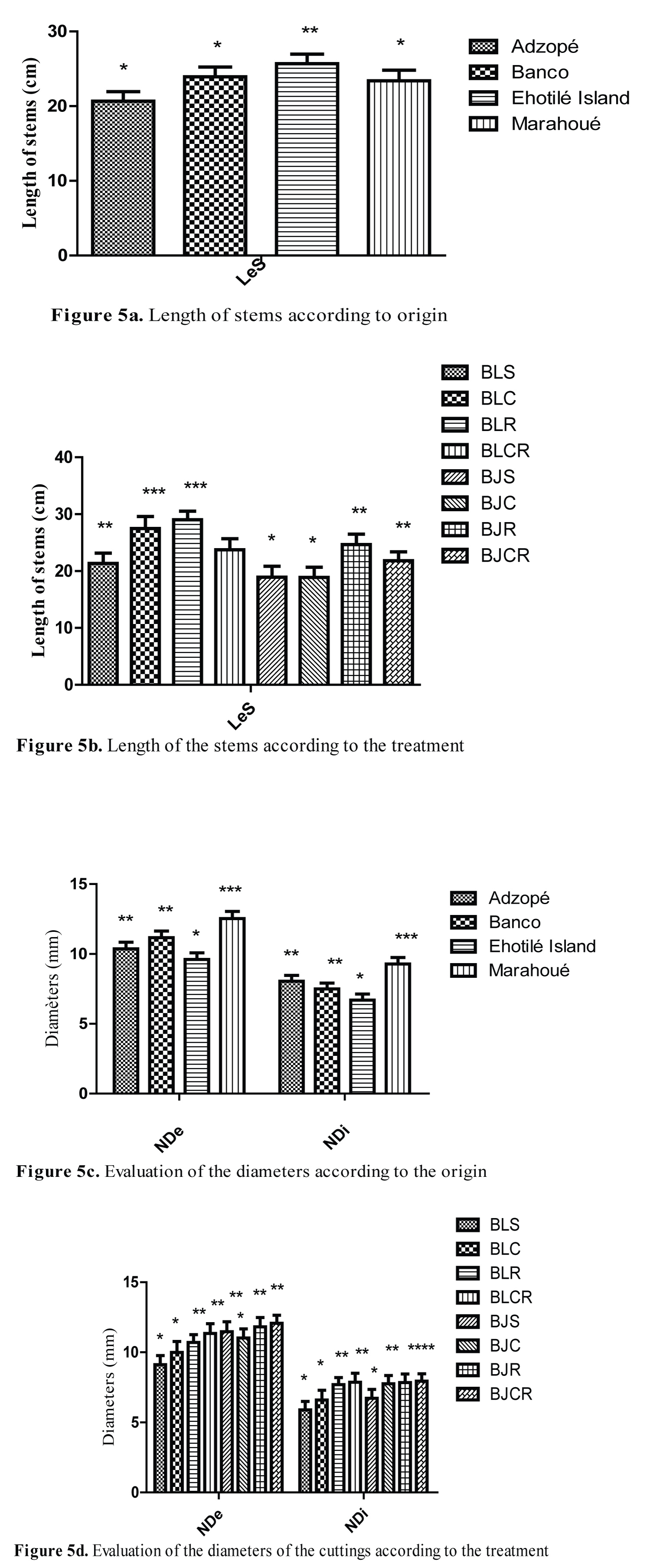
Stems length and diameters evaluation of regenerated plants
Figure 5 presents the influence of origin and treatments on stems length and diameters of regenerated plants. The stems of the Adzopé cuttings were shorter than those of Banco, Ehotilé Islands and Marahoué (Figure 5a). However, the stems of the plants of the Ehotilé Islands were the longest (25.68 ± 1.28 cm). The stems of the plants from the BLC, BLR treatments were larger than those of the BJS and BJC treatments. BLS, BLCR, BJR and BJCR treatments resulted in medium size of stems (Figure 5b). The external neck diameter of the plants varied from 9 to 12 cm and the internal diameter from 5.89 to 9.93 cm (Figure 5c and Figure 5d). Plants from Marahoué cuttings had the largest diameter, followed respectively by those from Banco, Adzopé and Ehotilé Islands cuttings. Thus, the BJR and BJCR treatments had the largest external diameter followed by BLCR, BJS, BLR and BJC respectively. BLS treatment produced the smallest external diameter. Concerning the internal diameters, the BLR, BLCR, BJC, BJR and BJCR treatments gave the largest diameters. The BLC and BJS treatments produced medium diameters, and BLS treatment gave the smallest diameters (9.11 ± 0.65, 5.89 ± 0.59 mm) (Figure 5d).
Phytochemicals
Table 3 presents the qualitative composition of Euadenia trifoliolata. These results showed that regenerated and wild plants contain polyphenols, tannins, flavonoids, coumarins, saponins, quinones, alkaloids, cardiac glycosides, anthracene compounds, reducing compounds, sterols and polyterpenes. From this study, it appeared that the regenerated and wild plants had the same phytochemicals composition.
Discussion
Good management of genetic resources of medicinal plants is an alternative to their disappearance, erosion and threat. Thus, vegetative propagation has been undertaken to facilitate the reproduction of plants. The multiplication or regeneration of Euadenia trifoliolata has been studied through viability, shoot emission time, first leaf emission time, leaf number, leaf length and width, stem length and neck diameter of stem. A quality control of regenerated and wild plants was carried out through phytochemical tests.
The viability of the cuttings during handling showed that this parameter was similar despite the difference in cuttings origin. This analysis showed that the cuttings remained viable at 45 and at 90 days after burial, although the viability at 45 were greater than that at 90 days. These results could be explained by the adaptation of the plant to its new environment which may have allowed it to resist during the regeneration period. The viability expressed with respect to cuttings' diameters showed a significant difference between the parameters. This difference could be attributed to geographical and genetic factors. Indeed, the Ehotilé Islands contain island populations. This geographical position may favor the establishment of plants of small diameters. The study of the viability according to the origin of the cuttings indicated that there was no significant difference but it varied significantly according to treatments. This difference could be due to the treatment of cuttings, biotic and abiotic factors. Regarding the treatments, the use of transparent bag would have prevented the cuttings from breathing. Remaining free or wrapped in newspaper might allow plants to breathe and therefore increase their viability.
The shoot emission timing data on cuttings indicated that there was a significant difference in origin and treatments. From this analysis, it appeared that the shoot growth of the cuttings depended on their origin and treatment. The time of shoots emission of plants from Ehotilé Islands were shorter than others. This result suggested that plants in this locality have adapted to the new environment. The time of shoots emission from Banco cuttings were the longest. These results could be explained by the effect of the environment that, through biotic and abiotic factors, favors the emergence of shoots. Similar observations were made by Ky-Dembele, et al. [26] during the vegetative propagation of Detarium microcarpum. According to these authors, the humid environment of the greenhouse favors the emission of shoots compared to outdoor. In this study, the emission time of shoots from cuttings according to the treatments varied significantly. The BLS treatment took the longest time when that of the BJCR treatment were the shortest. These results could be assigned to the fact that the cuttings from the first medium did not receive any treatment. In the BJCR treatment, protection of upper surface by the newspaper would have created a microclimate that would have kept humidity constant and favored the rapid emission of shoots. Thus, culture conditions may affect shoot release on the organ used, the phenological stage, the level of growth hormone contained in the substrate, and the growing season [27-30]. Regarding the emission time of the first cuttings leaf, there was a significant difference in function of origins on one hand and in function of treatments on the other hand. This difference could be explained by the nature of the cuttings. The cuttings from island populations would have acquired the capacities necessary to support the stress related to the cultural practices. These cultural practices would have stimulated the rapid appearance of leaves in the individuals of the Ehotilé Islands. However, BLC treatments were not promptly for the expression of this parameter. The late reaction of the BLC treatment could be explained by heat that inhibited certain components involved in the rapid appearance of the leaves.
The number of leaves, the length and width of leaves varied in function of the origin and the soil treatment. These results could result from the effect of the environment and genetic factors. Indeed, the synergistic action of these two factors would have stimulated the massive production of leaves. Also, in plants from Ehotilé islands, these environmental and genetic factors would have an optimal effect which would explain a high number of leaves. Our results do not corroborate with those of Kshatri, et al. [31] obtained with the species Ficus benjamina. In fact, Kshatri, et al. [31] observed an average number of leaves varying between 1 and 3 with F. benjamina. The observed difference in leaves number could be assigned to the composition of growing medium. The results concerning the length and width of the leaves indicated no significant difference between treatments. However, leaf shape varied significantly in function of origin from one treatment to another. This result could be explained by the edaphic and genetic factors. These factors would have favored long-leaf growth in the Adzopé, Banco, BLS, BLC, BLR, BJS, BJC and BJR plants as they were allowed for growth in width for BLCR and BJCR treatments. In addition, the cultivation methods by using compost would have brought nutriments (nitrates) and increased the production of proteins implied in the synthesis of genes that determine differentiation of leaves. Those practices favored a new architecture to the plants in different growth media. The architecture of a plant is the whole of the shapes structural observable at one time. It results from the simultaneous activity of the apical and underground air meristems of the plants. That would have explained the different length of leaves within the regenerated plants. Stems length and diameters changed significantly from one origin to another. These observations could be explained by the application of compost, the ability of the roots to draw nutrients from the soil and the bioavailability of these nutrients. Indeed, the application of compost may have fertilized the substrate, which allowed the roots to absorb the nutrients necessary for the vertical growth of plants. Since the plants were from different origin and cultivated in different environments, this could explain the difference in size. The use of compost as fertilizer would have increased the level of nitrogen which is the limiting factor of growth and production of plants [32]. The large size of the plants of the Ehotilé Islands and treatments BLC and BLR would be due to threshold content of nitrogen that brought compost to these environments from which the plants derived. This content of nitrogen would have favored rapid growth, hence the production of large plants [33].
Concerning the diameter, it differed significantly in function of origin of treatment. This difference could be due to genetic, physiological factors (photosynthesis and root sucking) and nitrogen in the culture medium [32,34]. The growth in thickness could result from the activity of the genes involved in photosynthesis which allowed gas exchange between the environments necessary for the synthesis of nutrients. In addition, by the phenomena of root sucking, plants would draw nutrients that would have favored the growth in diameters. The nitrogen supply by composting may have contributed to the increase of the diameter of plants.
According to the qualitative analysis, phytochemical elements were detected in all the parts of the regenerated plants as wild plants. This result explains the capacity of cuttings regeneration to conserve its properties. In fact, the regenerated plants are clones of wild plants, and consequently have expressed the same phytochemical constituents. Our results are different from those reported by Rathnayake, et al. [35] when working on Momordica charantia. According to these authors, the wild species contains more phytocompounds than the cultivated one. This difference could result in the regeneration medium that allow the maintenance of these compounds in the regenerated plants.
Conclusion
From this study, it appears that the regeneration of Euadenia trifoliolata can be performed using stem cuttings. But the viability and diameter parameters of the cuttings remain important factors for achieving this regeneration. Thus, the cuttings of big caliber produced shoots in order to have plants. Cuttings from Ehotilé Islands had a better regenerative ability. BLS, BLC, BLR, BLCR, BJS, BJC, BJR and BJCR treatments were favorable for vegetative propagation by cuttings. No important changes have been observed in phytochemical compounds of regenerated plants compared to wild plants.
Acknowledgement
The authors express their gratitude to the research center for natural substances for carrying out phytochemical tests, the Ivorian Office of Parks and Reserves (OIPR) for their technical support and access authorization during fieldwork.
References
- Jiofack T, Fokunang C, Guedje N, et al. (2009) Ethnobotany and phytomedicine of the upper Nyong valley forest in Cameroon. African Journal of Pharmacy and Pharmacology 3: 144-150.
- Dro B, Soro D, Koné MW, et al. (2013) Evaluation de l’abondance de plantes médicinales utilisées en médecine traditionnelle dans le Nord de la Côte d’Ivoire. Journal of Animal & Plant Sciences 17: 2631-2646.
- Betti JL (2002) Medicinal plants sold in yaounde markets, Cameroon. African Study Monographs 23: 47-64.
- Mehdioui R, Kahouadji A (2007) Etude ethnobotanique auprès de la population riveraine de la forêt d’Amsittène: Cas de la commune d’imi n’tlit (Province d’Essaouira). Bulletin de l’Institut Scientifique, Rabat, section Sciences de la Vie 29: 11-20.
- Nguema NP, Bouanga EB, Massounga YC, et al. (2013) Comparative study of three methods of propagation of Jatropha curcas L. in the climatic conditions of southeastern Gabon. Journal of Applied Biosciences 65: 4989-4998.
- Agbogan A, Bammite D, Tozo K, et al. (2014) Contribution à la multiplication,par graines et par boutuage de segments de tigeset de racines, de trois fruitiers spontanésdela régiondes savane sau Togo: Haematostaphis barteriI Hook. F., Lannea microcarpa Engl. & K. Krauss et Sclerocarpa birrea. European Scientific Journal 10: 1-17.
- Kouakou K, Dao J, Kouassi K, et al. (2016) Propagation of Garcinia kola (Heckel) by stem and root cuttings. Silva Fennica 50: 1-17.
- Mapongmetsem PM, Fawa G, Noubissie Tchiagam J-B, et al. (2016) Vegetative propagation of vitex doniana sweet from root segments cuttings. Bois et forêts des tropiques 327: 29-37.
- Badji M, Sanogo D, Akpo LE (2008) Possibilité de bouturage in situ de Lawsonia inermis L. (henné). Bois et Forêts des Tropiques 297: 35-41.
- Leakey RRB (2014) Plant Cloning: Macropropagation. In: Encyclopedia of agriculture and food systems. Elsevier 4: 349-359.
- Koulibaly AV (2008) Caractéristiques de la végétation et dynamique de la régénération, sous l’influence de l’utilisation des terres, dans des mosaïques forêts-savanes, des régions de la réserve de Lamto et du parc national de la Comoé, en Côte d’Ivoire. Thèse docteur de L’ Université de Cocody Abidjan (Cote d'Ivoire).
- Koné MW, Kamanzi AK, Traoré D, et al. (2005) Anthelmintic activity of medicinal plants used in Northern Côte d’Ivoire against intestinal helminthiasis. Pharmaceutical Biology 43: 72-78.
- Békro YA, Mamyrbékova_Békro JA, Boua BB, et al. (2007) Etude ethnobotanique et screening phytochimique de Caesalpinia benthamiana (Baill.) Herend. et Zarucchi. Science Nature 4: 217-225.
- Touré IAK (1989) Evaluation de l’efficacité thérapeutique d’une recette traditionelle améliorée « le Gastrosedal » dans le traitement des gastrites. Thèse, Bamako (Mali).
- Gnagne AS, Camara D, Fofie NBY, et al. (2017) Étude ethnobotanique des plantes médicinales utilisées dans le traitement du diabète dans le Département de Zouénoula (Côte d’Ivoire). Journal of Applied Biosciences 113: 11257-11266.
- Koffi E, Sea T, Dodehe Y, et al. (2010) Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. Journal of Animal and Plant Sciences (JAPS) 5: 550-558.
- Soro TY, Mian Jc, Coulibaly S, et al. (2016) Anti-inflammatory activity of the aqueous extract of Daniellia oliveri (Fabaceae). International Archives of Integrated Medicine 3: 1-9.
- Kassi F, Badou O, Tonzibo Z, et al. (2014) Action du fongicide naturel NECO contre la cercosporiose noire (Mycosphaerella fijiensis Morelet) chez le bananier plantain (AAB) en Côte d’Ivoire. Journal of Applied Biosciences 75: 6183-6191.
- Dickson R, Fleischer T, Ekuadzi E, et al. (2012) Anti-inflammatory, antioxidant, and selective antibacterial effects of euadenia eminens root bark. Afr J Tradit Complement Altern Med 9: 271-276.
- Sofidiya MO, Oloruntola OM, Sofola I, et al. (2016) Antinociceptive activity of Euadenia trifoliolata (Schum. & Thonn.) Oliv. leaves and roots in mice. Journal of Traditional and Complementary Medicine 6: 289-293.
- Fah MA, Coulibaly SS, Kouakou KL, et al. (2019) Influence of the type of organ, the origin and regeneration method of Euadenia trifoliolata (Capparaceae) Schum & Thonn. Oliv. on the sexual behavior of rats. Journal of Medicine and Biology 2: 5-16.
- Lassoudière A (1973) La culture bananière sur sols hydromorphes dans la zone du Nieky (Agneby) en Côte d’Ivoire. Fruits 28: 85-102.
- Nayak N, Sharma JL (2015) Evaluation of neem parts on egg hatching of Meloidogyne incognita. Global journal of bio-science and Biotechnologogy 4: 242-246.
- Rocha VS, Costa AGF, TrovãO DMBM, et al. (2016) Management of volunteer castor bean in the glyphosate-resitant soybean crop. Planta Daninha 34: 545-553.
- Boua BB, Mamyrbekova-Bekro JA, Kouame BA, et al. (2013) Criblage phytochimique et potentiel érectile de Turraea heterophylla de Côte d’Ivoire. Journal of Applied Biosciences 68: 5394-5403.
- Ky-Dembele C, Tigabu M, Bayala J, et al. (2010) Clonal propagation of detarium microcarpum from root cuttings. Silva Fennica 44: 775-786.
- Ede FJ, Auger M, Green TGA (1997) Optimizing root cutting success in Paulownia spp. Journal of Horticultural Science 72: 179-185.
- Stenvall N, Haapala T, Pulkkinen P (2004) Effect of genotype, age and treatment of stock plants on propagation of hybrid aspen (Populus tremula x Populus tremuloides) by root cuttings. Scandinavian Journal of Forest Research 19: 303-311.
- Tsipouridis CG, Schwabe WW (2006) Studies on the regeneration of peach cultivars and rootstocks from root cuttings in comparison with aerial cuttings. Australian Journal of Experimental Agriculture 46: 1091-1095.
- Snedden J, Landhäusser S, Lieffers V, et al. (2010) Propagating trembling aspen from root cuttings: Impact of storage length and phenological period of root donor plants. New Forests 39: 169-182.
- Kshatri BB, Kemp PD, Devkota JHN (2005) Vegetative propagation of Ficus benjamina using non-sterile sand and hardwood cuttings. Agronomy New Zealand 35: 19-22.
- Tendonkeng F, Boukila B, Pamo TE, et al. (2011) Effet direct et résiduel de différents niveaux de fertilisation azotée sur la croissance et le rendement de Brachiaria ruziziensis à différents stades phénologiques. Tropicultura 29: 197-204.
- Stout MJ, Brovont RA, Duffey SS (1998) Effect of nitrogen availability on expression of constitutive and inducible chemical defense in tomato Lycopersicon exculentum. Journal of Chemical Ecology 24: 945-963.
- Obulbiga MF, Kaboré-Zoungrana CY (2007) Influence de la fumure azotée et du rythme d’exploitation sur la production de matière sèche et la valeur alimentaire de Andropogon gayanus Kunth au Burkina Faso. Tropicultura 25: 161-167.
- Rathnayake AMGN, Abeysinghe DC, Dharmadasa RM, et al. (2018) Phytochemical, physiochemical and mineral contents of domesticated and non domesticated populations of Momordica charantia L. seeds harvested at two maturity stages. World Journal of Agricultural Research 6: 77-81.
Corresponding Author
Sifolo Seydou Coulibaly, Department of Biological Sciences, University "Peleforo Gon Coulibaly", BP 1328 Korhogo, Côte d'Ivoire
Copyright
© 2021 Fah MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.