Embryo Rescue of Aged Cucurbita pepo Seeds Using Squash Rescue Medium
Abstract
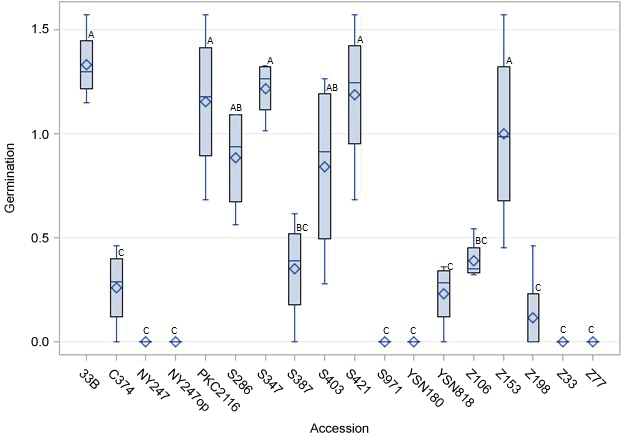
Plant seed is both an important food source and an indispensable propagation material for many cultivated crop species. The ideal conditions required for long-term storage of seeds are expensive, exacting, and not always tenable, particularly for small breeding programs. In most plant species, viability is significantly reduced in old, sub-optimally stored seeds due to depleted energy reserves and biochemical degradation. However, revival of such old germplasm is often desirable for research and germplasm improvement purposes. In the current study, germination response of eighteen 20-year-old squash (Cucurbita pepo L.) breeding lines was investigated in potting mix, water fortified with hydrogen peroxide, and in vitro in squash rescue (SR) medium. For the in vitro method, SR medium was delivered either as a gel (with or without a towel) or as liquid. No germination was observed in the hydrogen peroxide treatment, while only one seedling emerged in the potting mix. However, for the in vitro method, significant differences (P < 0.05) in germination rates (0-92.2%) were observed among the accessions with 33B, S347, S421, PKC2116 and Z153 showing the highest overall germination. No significant differences in germination rates were observed among in vitro treatments (gel or liquid). The current study demonstrates usefulness of SR medium in breeding projects using old, rare and valuable squash germplasm.
Keywords
Cucurbits, E21, Germination, Old seeds, SRM, Tetrazolium
Introduction
Adequate seed viability is of paramount importance in any seed-propagated species, including members of the Cucurbitaceae such as squash (Cucurbita sp.), watermelon (Citrullus lanatus), cucumber (Cucumis sativus) and melon (Cucumis melo) [1]. In addition to their use in propagation, seeds of Cucurbitaceae crops are rich in oil, protein and unsaturated fatty acids, and are an important source of nutrition and income globally [2-6]. Seed viability in Cucurbitaceae is a function of many factors including seed maturity at fruit harvest, extraction and drying procedures, moisture content at time of storage, pathogen contamination and the storage environment [1,7]. Since interactions among these factors can make seed viability difficult to predict, it is critical to follow optimal seed production, processing and storage protocols. For most cucurbits, mature seeds (~5% moisture content) [8] can remain viable in storage for a long time if kept under conditions that minimize age-related deterioration, such as low temperature and low relative humidity (RH) [9,10]. For example, cucumber seeds maintained viability for thirteen years when stored at 3 ℃ and 38% RH [11], while squash (Cucurbita sp.) seeds maintained viability after over 40 years of storage, initially at 5 ℃, then at -18 ℃ [12]. In contrast, viability of such seeds under natural conditions is limited to about 4 years, assuming that the seeds are kept relatively cool and dry [13].
Optimal storage conditions are not always possible, however. Infrastructure constraints, natural disasters and other limitations may prevent or interfere with seed storage facilities and protocols. In the case of rare or experimental germplasm that may have been compromised by sub-optimal storage (high temperatures or humidity), the development of methodologies to revive even a bit of these materials could prove to be extremely valuable. Viability is markedly reduced in old and/or sub-optimally stored seeds due to depleted energy, low enzyme levels and hypoxic conditions during germination [14]. In such cases, intervention measures aimed at providing missing elements are necessary to achieve germination. For instance, Liu, et al. [14] successfully germinated 4-year-old squash (C. pepo) seeds using hydrogen peroxide as a source of bioavailable oxygen. Similarly, Duval and NeSmith [15] used hydrogen peroxide to improve germination in 'Genesis' triploid watermelon cultivar.
In addition to these approaches, in vitro tissue culture techniques may be used for embryo rescue of immature and old cucurbit seeds [11,16]. For this technique, embryos are extracted from the seed and placed in a nutritionally rich medium under sterile conditions to stimulate germination. Success of embryo rescue partly depends on the type, concentration and combination of plant growth regulators (PGRs) used. Ali, et al. [11] successfully regenerated cucumber seeds using 1-naphthaleneacetic acid in combination with 6-benzylaminopurine. Similarly, epibrassinolide at low concentrations was shown to improve germination of C. pepo seeds [17]. Utilizing gibberellic acid (GA3) has been shown to enhance seed germination, although the concentration used is critical to success [18,19]. For example, GA3 was found to enhance germination of watermelon and muskmelon at certain concentrations (0.1 or 0.32 mM) [18,19] but have inhibitory effects at others (2.9 mM) [20]. In addition, other forms of gibberellin, such as GA4/7 may be more effective than GA3 in promoting cucurbit seed germination [20]. The osmotic condition of seeds during germination is also important. Wu, et al. [21] reported that polyethylene glycol enhanced germination of aged cucumber seeds.
The aim of the current study was to evaluate germination response of eighteen sub-optimally stored squash breeding lines in potting mix, water fortified with hydrogen peroxide, and in vitro using squash rescue (SR) medium.
Materials and Methods
Plant material and sterilization
Seeds used in this experiment were derived from the cucurbit-breeding program at the University of Florida (UF), USA. These seeds were extracted from fruits of summer squash (C. pepo) harvested in spring 1998 at a UF research station. The storage conditions of this material over the last two decades is largely unknown, but the seeds were intermittently stored in coolers (~8 ℃) at three different research stations, most recently (since 2015) at the UF Tropical Research and Education Center, Homestead, FL. From this population, a subset of 18 breeding lines, hereafter referred to as accessions, were used in the current experiment (Table 1). For sterilization, seeds were removed from refrigerated storage and surface-sterilized in a 10% bleach (8.25% sodium hypochlorite active ingredient) solution containing Tween® 20 (10 drops/liter) for 30 minutes, following a modified cucumber seed sterilization protocol [11]. The solution was decanted, and the seeds were transferred into a sterile beaker. They were then rinsed three times with sterile deionized water.
Tetrazolium tests
Prior to germination experiments, viability tests using 2,3,5-triphenyl tetrazolium chloride (TTC) were performed on an aliquot of most of the accessions. The TTC test provides a reproducible estimate of relative seed vigor [22]. For this test, seeds were washed with detergent and soaked overnight in deionized water. The embryos were extracted and gently rubbed to remove the persisperm membrane. They were then soaked overnight at 30 ℃ in 0.5% TTC [21]. The next day, the samples were assessed for degree of staining on a scale of 0-3, where a score of 0, 1, 2 and 3 represented seed lots with 0-25%, 26-50%, 51-75% and > 75% of the seeds stained pink, respectively (Figure 1). For one of the accessions (NY247), the seeds available were too few to spare for a TTC test.
Germination in soil
For each accession, fifty seeds were sowed in cells (5.98 × 3.68 × 4.69 cm) filled with Fafard 3B (Sun Gro Horticulture, Agawam, MA, USA) potting mix amended with Osmocote Classic (Scotts-Sierra Horticultural Products, Marysville, OH, USA) (1.38 g/kg N, 1.38 g/kg P, 1.38 g/kg K) in the greenhouse maintained at 25-30 ℃. For half of the seeds, small portions of the distal ends (furthest from the embryo) were sliced off with a scalpel to facilitate water imbibition. Germination percentage was determined every 7 days for a period of eight weeks.
Germination in hydrogen peroxide
Hydrogen peroxide was used as a source of bioavailable oxygen during germination according to protocols developed by Liu, et al. [14]. Briefly, fifty seeds of each line were soaked for 24 h in 50 mL of 0.5 mm Ca(NO3)2 (Mallinckrodt Inc., Paris, KY, USA) fortified with 0.15% hydrogen peroxide (Vi-Jon, St. Louis, MO, USA) in a 95 × 15-mm petri dishes at room temperature (25 ± 1 ℃). Distal portions of half of the seeds were removed as described previously to promote imbibition. The seeds were rinsed off four times with distilled water and placed in fresh petri dishes with napkins saturated with 0.5 mm Ca(NO3)2. The petri dishes were sealed with parafilm and placed at room temperature in the dark. Germination data was recorded as previously described.
In vitro germination
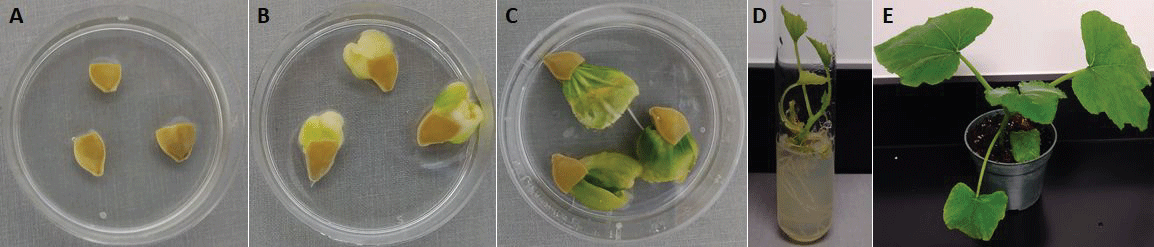
Under sterile conditions, seeds were treated in one of two ways: Either the distal portion of the seed was sliced off with a scalpel, or the zygotic embryo was extracted entirely from the seed coat. The sliced seeds were plated onto squash rescue (SR) medium in one of three forms. Treatment 1 consisted of SR medium in gel form poured onto a petri dish (60 × 15 mm, Fisher Scientific), as is routine in our laboratory [treatment squash rescue gel (SR-G)]. For treatment 2, approx. 2 ml of SR medium was pipetted, before it had gelled, onto a sterile, approx. 2 × 2 cm square of Scott® Shop Towel (Kimberly-Clark, Neenah, WI, USA) that had been placed into a petri dish [treatment squash rescue gel towel (SR-GT)]. The final treatment consisted of a piece of sterile towel onto which approx. 2 ml of liquid SR medium was added with a pipette [treatment squash rescue liquid towel SR-LT]. Extracted embryos were plated as in treatment 1 (SR-G). The number of empty seeds in the subsample was determined at this time.
SR medium is a variation of the E21 medium developed by Sauton and Dumas de Vaulx, [23], supplemented with some of the components added by Nuñez-Palenius, et al. [24], specifically indole-3-butyric acid and 6-benzyladenine. It is composed of 0.5x E-20A major salts, 1x E-20A minor salts, 0.5x E-20A organics and 1.2% sucrose (Table 2). After pH adjustment to 5.7, 2 g/L gellan gum (Caisson Laboratories, Smithfield, UT, USA) was added to the media for SR-G and SR-GT treatments, and then autoclaved. Ampicillin (50 mg/L), cefotaxime (300 mg/L) and Plant Preservative Mixture™ (PPM) (5 ml/L) were added via filter-sterilization to all media, as preliminary germination experiments in SR medium using some of these aged seed had yielded some positive results, but massive bacterial contamination levels had been observed (data not shown). Fungal contamination was also observed, but not at a high a frequency; PPM was added to minimize fungal contamination and to augment bacterial suppression efforts. For in vitro treatments, the number of seeds used per accession varied depending on seed availability (Table 1).
Statistical analysis
Prior to statistical analysis, data were arcsine square root transformed since it was expressed as a proportion of the initial seed quantity. Pearson correlations were performed in JMP Version 9 (SAS Institute Inc., Cary, NC). Analysis of variance and mean separation of germination rates was performed using PROC GLM procedure in SAS v.9.4. Chi-square was implemented in SAS using PROC FREQ to determine the no-germination/germination rate for the tested accessions.
Results
Tetrazolium tests
Seed viability was dependent on accession type as revealed by TTC test (Table 1). Majority (44.4%) of the seed lots had a rating scale of 1 (26-50% of seeds stained), while only 16.7% had more than 75% of the seeds stained. In one of the accessions (NY247op), fungal contamination was observed in the subsample undergoing TTC testing. This accession also showed high levels (100%) of contamination in vitro (Table 1). Variation in the number of empty seeds, either embryo absent or shriveled, ranged from 0% to 16.2% (Table 1).
Germination
Of the seeds sowed in potting mix, only one emerged from the S286 accession. On the other hand, no germination was observed in the hydrogen peroxide treatment for all accessions. Therefore, data were not further analyzed for these two treatments.
For in vitro experiments, first visible germination occurred between two and eight weeks after the seeds were introduced into the treatments (data not shown). Visible germination was defined as swelling of cotyledons and development of green tissue. Significant differences (P < 0.05) in germination rates (0-92.2%) across the three in vitro treatments (SR-G, SR-GT and SR-LT) were observed among the accessions (Figure 2). The Chi-square predicted germination rate for the tested accessions was 29.4%/70.6% (not-germinated/germinated). Fourteen of the accessions performed worse than this value (Table 1), while four had a better response [33B (92.2%), S347 (86.9%), S421 (79.9%), and PKC2116 (77.5%)].
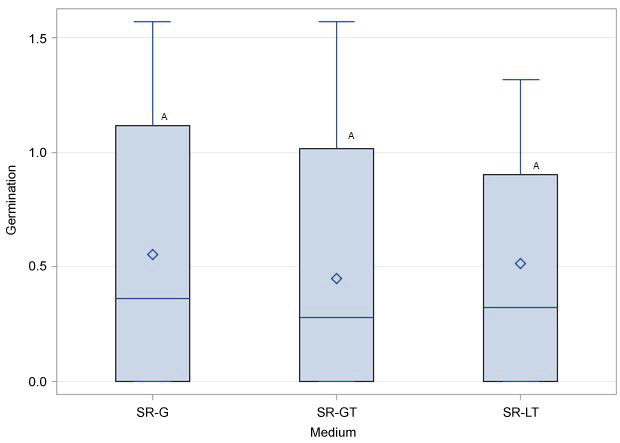
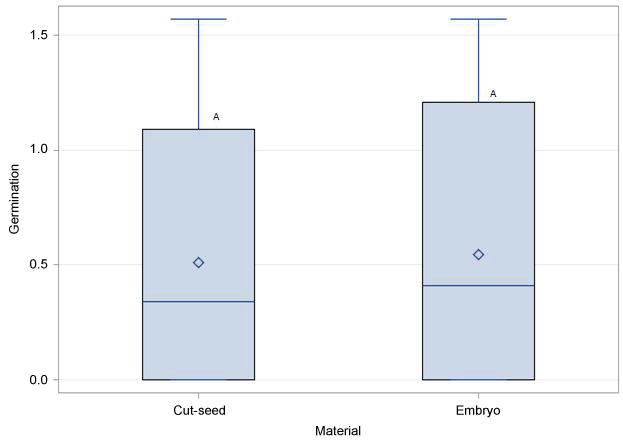
No significant differences in germination rates was found among SR-G, SR-GT and SR-LT treatments (Figure 3). Similarly, in vitro germination response was not influenced by whether the seed coat was partially or completely removed prior to plating (Figure 4). As expected, percent seed germination was negatively correlated (P < 0.05) with contamination (mostly fungal) levels (R2 = -0.64). Conversely, a significant positive correlation (R2 = 0.63) was found between TTC test and germination rates. Following acclimatization, the plantlets were transferred into pots where they developed into healthy plants (Figure 5).
Discussion
The tetrazolium test is a quick and easy estimation of seed viability [22,25]. It is based on knowledge that living seed tissue contains dehydrogenase enzymes, which reduce tetrazolium chloride to formazan, a reddish/pink, water-insoluble compound. Therefore, the level of staining is indicative of the presence and amount of living cells releasing hydrogen during respiration [25]. In the current study, most of the accessions had a mixture of viable (stained pink) and non-viable seeds (unstained), with the latter dominant. In a few cases, the seeds showed spotty, red and unstained patches, suggesting incomplete perisperm removal, or presence of dead and living tissue in the embryos [11,26]. A significant positive correlation between TTC test and germination rate support reliability of the test as a tool for rapid estimation of seed viability [22,25]. However, TTC test does not provide information on seed dormancy or chemical damage [27]. For example, one of the accessions (S286) exhibited poor TTC test yet had a > 60% germination, suggesting that the seed tissue was viable but dormant.
Direct sowing of seeds in potting soil or treatment of seeds with hydrogen peroxide for increased bioavailable oxygen resulted in poor germination or no germination, respectively. A previous experiment using hydrogen peroxide yielded high (> 90%) germination for 4-year-old squash seeds [14]. However, the seeds used in the current study were at least 5 times older than those used in Liu, et al. [14] study, thus hindering direct comparison. The inability to obtain desirable germination in potting soil or hydrogen peroxide suggested a need for exogenous nutrient or PGRs for seed nourishment. Indeed, the overall seed germination in the current study was improved by providing exogenous energy source (sucrose), salts, iron and PGRs. However, germination rates were largely genotype dependent and ranged from 0 to 92.2%. Genotype factors that may influence germination include growing environment during fruit/seed development, age of the fruit at harvest, seed moisture at storage and internal seed contamination with pathogen [1]. However, since this information is lacking for breeding lines used in the current study, it is difficult to draw any firm conclusions regarding influence of these factors on germination.
Although the accessions in the current study were under storage for only 20 years, they exhibited lower overall germination (31.5%) than that reported for accessions of Cucurbita pepo (76%), C. moschata (75%) and C. maxima (63%) stored for ~43 years in the USDA National Plant Germplasm System (NPGS) [12]. A plausible explanation for this disparity is the difference in storage conditions between USDA NPGS (-18 ℃) and UF facilities (~8 ℃). In addition, desirable storage conditions at UF facilities may have been interrupted by extended periods of power loss, particularly during natural disasters such as hurricanes. Moreover, the breeding material under this study has been transferred between several research stations over the years, and temporary storage conditions during movement are largely unknown. Exposure of cucurbit seeds to high (> 30 ℃) temperatures is known to accelerate ageing and reduce germination viability [1,21]. Such high temperatures are common in Florida, particularly in summer where average temperatures can reach ~33 ℃ [28].
Conclusions
All in vitro treatments (SR-G, SR-GT and SR-LT) resulted in seed germination (average 28.5-36.3%), but none significantly fared better over the other. However, use of sliced seeds in SR-G treatment was more robust and quicker than other treatments since it did not require extra steps for preparing paper towels or extracting embryos. Therefore, this method will be adopted for routine germination of old seeds in our breeding program. Future work will explore the influence of type, concentration and combination of PGR and sugars on germination of old cucurbit seeds.
References
- Nerson H (2007) Seed production and germin ability of cucurbit crops. Seed Sci Biotechnol 1: 1-10.
- Baxter GG, Murphy K, Paech A (2012) The Potential to produce pumpkin seed for processing in northeast Victoria. Rural Industries Development Corporation 11: 5-36.
- Fruhwirth GO, Hermetter A (2007) Seeds and oil of the Styrian oil pumpkin: components and biological activities. Eur J Lipid Sci Technol 109: 1128-1140.
- Meru G, McGregor C (2013) Genetic mapping of seed traits correlated with seed oil percentage in watermelon. HortScience 48: 955-959.
- Meru G, McGregor C (2014) Quantitative trait loci and candidate genes associated with fatty acid content of watermelon seed. J Amer Society HortSci 139: 433-441.
- Nakić SN, Rade D, Skevin D, et al. (2006) Chemical characteristics of oils from naked and husk seeds of Cucurbita pepo L. Eur J Lipid Sci Technol 108: 936-943.
- Demir I, Ellis RH (1993) Changes in potential seed longevity and seedling growth during seed development and maturation in marrow. Seed Sci Res 3: 247-257.
- Anderson JD, Baker JE (1983) Deterioration of seeds during aging. Phytopathology 73: 321-325.
- Matthews S (1985) Physiology of seed aging. Outlook on Agriculture 14: 84-89.
- Styer RC, Cantliffe DJ, Hall CB (1983) The relationship of ATP concentration to germination and seedling vigor of vegetable seeds stored under various conditions. J Amer Society HortSci 105: 298-303.
- Ali N, Skirvin R, Splittstoesser WE, George WL (1991) Germination and regeneration of plants from old cucumber seed. HortScience 26: 917-918.
- Walters C, Wheeler LM, Groterhuis JM (2005) Longevity of seeds stored in a genebank: Species characteristics. Seed Sci Res 15: 1-20.
- Romer J (1999) Life expectancy of vegetable seeds. Horticulture and Home Pest News, Iowa State University, Ames, IA.
- Liu G, Porterfield M, Li Y, et al. (2012) Increased oxygen bioavailability improved vigor and germination of aged vegetable seeds. HortScience 47: 1714-1721.
- Duval JR, NeSmith DS (1998) Germination response of 'Genesis' triploid watermelon (Citrullus lantanus (Thunb. Matsum&Nakai) to hydrogen peroxide and seed coat alteration. HortScience 33: 546.
- Visser DL, Franken J (1979) Use of In vitro culture for inducing germination of Cucumis seed. Cucurbit Genetics Cooperative Rep 2: 46-47.
- Sayed HF, Ibrahim HK, El-Shadey AS, et al. (2009) Effects of pre-soaking Cucurbita pepo L. (C. pepo) seeds in two different concentrations of epibrassinolide (Eb) on seed germination and seedling growth. Australian J Basic Applied Sci 3: 4465-4477.
- Evensen KB, Loy JB (1978) Effects of gibberellic acid and gold light on germination, enzyme activities, and amino acid pool size in a dwarf strain of watermelon. Plant Physiol 62: 6-9.
- Nerson H, Cantliffe DJ, Paris HS, et al. (1982) Low temperature germination of birds nest-type muskmelons. HortScience 17: 639-640.
- Puchalski JT, Robinson RW (1980) Gibberellic acid treatment to improve germination of cucurbit seed. Cucurbit Genetics Cooperative Rep 3: 43.
- Wu C, Qiao A, Sun M (2009) RAPD studies on genomic DNA damages during artificial aging and its osmoprimed repair with PEG in cucumber seeds. J Changjiang Vegetables 14: 15-18.
- Delouche JC, Caldwell WP (1960) Seed vigor and vigor tests. Proceedings of the Association of Official Seed Analysts. Association of Official Seed Analysts.
- Sauton A, Dumas de Vaulx R (1987) Induction of gynogenetic haploid plants in muskmelon (Cucumismelo L.) by use of irradiated pollen. Agronomie 7: 141-148.
- Nuñez-Palenius HG, Klee HJ, Cantliffe DJ (2006) Embryo-rescue culture of the 'Galia' muskmelon (Cucumismelo L. var. reticulatus Ser.) male parental line. Plant Cell Tiss Organ Cult 85: 345-352.
- Tetrazolium Subcommittee of the Association of Official Seed Analysts (2001) Tetrazolium Testing Handbook. In: Peters J, Handbook on Seed Testing. Association of Official Seed Analysts, Lincoln, NE, 1-21.
- Welbaum GE (1999) Cucurbit seed development and production. HortTechnology 9: 341-348.
- Busso C, Torres Y, Ithurrart L, et al. (2015) The TTC-technique might not appropriately test the physiological stage of plant tissues. Russ J Plant Physiol 62: 551-556.
- US climate data.
Corresponding Author
Geoffrey Meru, Department of Horticultural Sciences, Tropical Research and Education Center, University of Florida, IFAS 18905 SW 280th St., Homestead, FL 33031, USA.
Copyright
© 2018 Moon P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.