Impact of Two Hydrothermal Carbonization Filtrates on Soil Greenhouse Production
Abstract
Hydrothermal carbonization (HTC) is a thermochemical treatment process that allows for the conversion of wet biomass slurries to new liquid and solid products. A majority of the research to date has focused on the solid HTC product (hydrochar). Less attention has been paid to the utilization of the HTC filtrate, which comprises the larger mass fraction of the process. Finding value added products for this liquid phase is pivotal for HTC to be an economically viable treatment option for waste biomass. Laboratory soil incubations were performed to determine alterations in microbial functionality by quantifying greenhouse gas (CO2, CH4, and N2O) production and nitrogen mineralization rates following filtrate application. Results of these studies confirmed inhibitory effects of high application rates of condensed distiller soluble HTC filtrate (> 0.2 mL g-1) on agricultural soil microbes for a 2-week period after application, but this inhibitory effect was not observed for a swine manure filtrate. On the other hand, lower rates of application stimulated mineralization of the filtrate and increased soil N-availability during the 2-week period. More importantly, the simple storage of filtrate in an open container for 90 days altered the observed impact on soil microbes, reducing the initial inhibitory effects. These observations lead to the conclusion that there are volatile organic chemicals present in the original filtrate that are responsible for the observed negative effects which are lost though storage (volatilization or microbial mineralization). Even though the effects observed here are dependent on feedstock type, applied concentration, and post-treatment of the applied filtrate, this data does support the continued examination of the utilization of HTC filtrates as a renewable source of agricultural nutrients.
Introduction
The conversion of biomass by thermal-chemical processing has a very long history, dating back to the earliest period of our civilization. The Egyptians used the liquid product from the thermal pyrolysis of biomass as an embalming fluid, since it was known to slow microbial degradation reactions [1]. This pyrolysis liquid, sometimes called wood vinegar, and was our source of chemical resources (e.g., acetic acid) prior to the discovery of lower cost fossil fuel resources [2]. In recent times, there has been an extensive characterization and evaluation of these pyrolytic liquids as potential fuel precursors, pesticides, antibiotics, fungicides, and even potentially plant growth stimulants [2-7].
However, pyrolysis liquids do differ in chemical composition compared to hydrothermal carbonization (HTC) filtrates, even for the same feedstock [8,9]. While much attention has been focused on generation of solid products derived from HTC (hydrochars), less effort has been directed to the development of value-added liquid products from the HTC filtrate [10-14]. A majority of examined uses has been related to its use as a liquid fuel for energy [14,15]. Since the aqueous phase of the HTC process is the primary mass fraction of the final HTC products (70-90%), coupled with the fact that the majority of nutrient resources (i.e., nitrogen, potassium, and phosphorus) of the feedstock material is in the filtrate [11,16]; it is important that other uses of this liquid HTC by-product be developed to take advantage of the sustainable agricultural benefits that HTC treatment of waste biomass offers [17].
Several studies to date have focused on the microbial impacts of hydrochar (the solid HTC phase) as a soil amendment. Laboratory incubations have been conducted with the hydrochar to determine toxicity on soil microorganisms [18,19], as well as its impacts on seed germination/plant growth [20-23]. However, only two studies have specifically examined HTC filtrates, the first of which utilized HTC filtrates by first mixing them with the respective hydrochar and other organic raw materials, and then subjecting the mix to composting prior to soil application [20]. The other study determined the effect of only the HTC filtrate application and aging on plant germination and growth [24], but there were no assessments of the microbial system responses. The results of the Bargmann, et al. [18], study confirms the potential toxic and inhibitory nature of some HTC filtrates on seed germination and seedling growth. Other than those identified works, very limited research has been conducted on the aqueous HTC filtrate with regards to its use as a soil amendment, despite the linkage of inhibitory effects of sorbed organic compounds on hydrochar [18,22,25,26]; HTC filtrate is a complex assortment of a variety of organic acids, aldehydes, ketones, alcohols, and dissolved inorganic salts [27].
Due to the sealed nature of HTC reactors, there is a reduced loss of plant nutrients due to volatilization as compared to thermal pyrolysis conversions [28]. For this reason, there is justification for examining this liquid product as an agricultural fertilizer. However, understanding the response within the agricultural soil in relation to HTC filtrate application is an essential step in determining the feasibility and sustainability of this waste treatment option. It is imperative that filtrates are well characterized and the effects of the chemical matrix within the HTC filtrates on soil microbes and plant growth are well understood. This research is solely the first step in the process of examining the GHG production potential impacts.
Past research conducted with pyrolysis wood vinegar and hydrochar with regard to effects on soil microbial populations have shown that primary variables that affect soil bacteria are amendment feedstock type and applied concentration. Furthermore, it has been shown that a post-treatment of hydrochar (such as aging) also diminishes toxic effects [20,25,29]. Therefore, we hypothesize that there is a strong dependency on HTC feedstock and applied filtrate amounts that will impact microbial soil functionality, and aging of the filtrate will reduce initial inhibitory impacts. To understand how filtrate type and concentration affect the metabolic rate of agricultural soil microbes, varying concentrations of two HTC filtrates types were investigated. Filtrates were generated through HTC reaction of swine manure and condensed distiller solubles (CDS) from the dry-grind ethanol industry and then applied to an agricultural soil. We examined how feedstock and application rate affects the metabolic rate of agricultural soil microbes assessed through the monitoring of the produced gases (CO2, N2O, and CH4) and net N-mineralization rates. Furthermore, tracking greenhouse gas (GHG) production rates allows for insights into the soil system's response to HTC filtrates.
Materials and Methods
HTC filtrate preparation
Swine manure and corn distiller's solubles (CDS) were used as starting materials for a 2-hour HTC processing at 225 ℃. All HTC reactions were conducted in a laboratory-scale stirred stainless steel reactor fitted with a heating mantel system (1000 mL; Parr Instruments, Inc.; Moline, IL). The raw feedstock (≈500 mL) was poured into the reactor, stirred at 88 RPM, and heated to the specified temperature for the defined time, as outlined in Table 1. No supplemental pressure was applied (autogenous). After the reaction time was reached, the heating mantel was disengaged from the HTC reactor, and the reaction unit was cooled using a fan to 40 ℃. At this time, the reactor was disassembled and the contents were filtered [VWR Filter Paper, 415. Cat 28320-020] [30]. The end result was the production of a solid hydrochar and the aqueous filtrate products. This conversion process was conducted on both feedstock types.
Filtrate characterization
Filtrates were first filtered through a 0.45 µm filter (Pall Acrodisc; PN 4184) and then analyzed for ammonium, nitrate and phosphate via spectroscopy (Lachat Instruments, Loveland, CO). Filtrate pH was measured in the undiluted state. An aliquot of the filtrate was taken for chemical analysis of total carbon (TC) by a UV-persulfate TOC analyzer (Tekmar Dohrmann - Phoenix 8000, Mason, Ohio). Filtrate samples were also analyzed for ammonium [NH4] and the sum of nitrite and nitrate [NO2 and NO3] using a flow-through injection analyzer (Lachat, Instruments, Loveland, CO). Sample extracts were subsequently analyzed solely for nitrite [NO2] and the amount of nitrate was calculated by difference. Due to the elevated levels of NH4, dilution of the original sample was required for analysis.
HTC filtrate aging
Half of the generated filtrate (450 mL) was used to evaluate the impact of aging. We conducted an aging experiment to simulate both open and closed tank storage conditions. The filtrate was split between an "open" and a "closed" treatment (225 mL each). A 500 mL wide mouth glass bottle was used to simulate a storage tank (Thermo Scientific, part #2100-0016). For each treatment, 250 mL of filtrate was initially placed in each bottle. For the open treatment, the lid was left open to allow evaporation and volatilization to occur. The closed treatment was identical except the container was sealed. To minimize the confounding effects of concentration differences between open and closed treatments due to liquid evaporation, the open treatments were topped up every week with ultrapure HPLC water (Aqua Solutions, Deer Park, TX.; W1089-4L), until the total mass (volume) of the container was the same as it was at the start of the aging process. The mass of each open container undergoing an aging period was recorded before and after H2O addition. The filtrates were aged in this manner for 3 months.
Soil incubation preparation
Two separate incubations were conducted to test microbial activity of agricultural soil microbes in response to application of two types of HTC filtrates at various concentrations. Due to the fact that a portion of the filtrate was aged, the fresh and aged experiments were conducted at different times. The first experiment analyzed metabolic activity of soil microbes in response to an application of fresh CDS and swine HTC filtrates, and the second determined the metabolic activity in response to the same filtrates previously aged for three months in closed and open containers. Individual soil controls were conducted with each set.
Both of these experiments were similarly conducted to determine microbial activity, 5 g of sieved (< 2 mm) agricultural soil (Rosemont, MN; bulk surface sample 0-5 cm; Waukegan silt loam) was placed into 125 mL glass vials (Wheaton). A series of dilutions of each filtrate was then applied in triplicate to the serum vials in 1 mL total volume, using 1:50, 1:20, 1:10, 1:5, 1:2, and 0-fold dilutions. These application rates correspond to approximate field application rates of 1, 2.3, 4.6, 9, 22.8, and 45.6 g m-2, respectively, for a soil bulk density of 1.5 g cm-3 and assuming incorporation across a 15 cm depth (Table S1). A triplicate set of deionized water was used as a control of the microbial activity (no filtrate addition). The vials were then sealed and allowed to incubate at room temperature (22 ℃ ± 2 ℃) on a laboratory bench enclosed in an opaque plastic container.
Greenhouse gas production (CH4, N2O, and CO2) within the headspace of the vials was assumed to be a surrogate for the overall microbial activity. Differences between the control and filtrate additions were an indication of the potential of the filtrates to stimulate or inhibit microbial functionality, as has been performed in other studies [31-33].
Gas headspace sampling and analysis
The vial headspace was sampled periodically via a syringe. Initially, the syringe was purged seven times with ambient laboratory air between each sampling. To prevent a vacuum within the incubation vials, 5 mL of laboratory air was initially injected into each vial, then mixed (syringe was pumped up and down 5 times). After which, 5 mL of headspace gas was sampled and injected into a sealed 10 mL vial (Agilent), which was previously flushed with helium for 20 seconds at a rate of 2-3 L min-1.
The collected samples were then loaded into the headspace autosampler (HP 7694E; Palo Alto, CA) and analyzed for O2, N2, CO2, CH4, and N2O via gas chromatography (GC). From each sample vial, three independent gas samples were transferred into three separate columns in two GC units (HP5890; Agilent) for the analysis of O2, N2, CO2 (CTR-1, Grace, TCD), CH4 (Porapak T; FID) and N2O [Porapak Q; ECD with a nafion dryer (Perma pure)] by separate sample loops. Full details of the GC system are found in Spokas and Bogner [34]. The system was checked daily for accuracy with NIST traceable gas standards (Minneapolis Oxygen, Minneapolis, MN).
These incubations were sampled 5 times through the 14 day incubation (1, 3, 5, 7, and 14 days). If any of the incubation vials dropped to anoxic levels (< 15% O2), all vials of the set were decapped for 15 minutes, and allowed to equilibrate to atmospheric levels in order to replenish headspace oxygen to atmospheric levels (~21%). To calculate cumulative gas production or consumption graphs, interval production was either subtracted or added to the cumulative production, depending on whether the respective gas was being produced or consumed. The incubations were run for 14 days, following a 5 d equilibrium period to minimize the impact of the initial spike in gas production on rewetting impacting the GHG results [35,36].
Soil extractions for nutrient analysis
Following the incubation period, all vials were extracted to determine the amount of inorganic N (ammonium and nitrate) present. To perform the nutrient analysis, 30 mL of 2 M KCl was added to each of the 125 mL vials that contained the 5 g of soil, and agitated on a reciprocal shaker for 30 minutes. Following the extraction, the supernatant was filtered through medium porosity filter paper (Whatmann, No.1), and subsequently analyzed on the flow-injection auto analyzer (Lachat Instruments, Loveland, CO) for ammonium and nitrate. The rate of mineralization was calculated by the following formula:
,
Where Ntotal, final is the sum of the inorganic N at the final extraction and Ntotal, initial is the sum of inorganic-N at the start of the incubation, t is time (in days), and gsoil is the mass of dry soil. This N-mineralization rate allows for an assessment of the observed differences between the treatments as well as the overall assessment of soil N-mineralization dynamics and corresponding N-nutrient availability.
Statistics
Means and standard deviations were calculated for all samples. All microbial and nutrient assessments were conducted on triplicate replicates. Independent variables evaluated were filtrate type, applied amount, and aging effects, and dependent variables which underwent statistical analyses were concentrations of produced and consumed gases (CO2, N2O, and CH4) as well as N-mineralization rates. Statistical analysis was conducted with R. A 3-way analysis of variance (ANOVA) was performed. This analysis first identified significant interactions among filtrate type, applied amount, and aging treatments impacts on GHG production rates. If higher interactions were observed to be significant, then 2-way ANOVAs were performed on the subdivided data by the significant interaction variable (i.e. filtrate type or treatment). If a significant difference was observed in the ANOVA, then Tukey-Kramer Multiple Comparisons Tests were performed to assess significant differences in the individual factors. Values are presented on the data subgroups when the interactions were significant and on the pooled data, when interactions were not significant. In order to assess the impact of aging, a pair-wise t-test was performed on the aged filtrates compared to the fresh to assess significant differences resulting from aging. A P-value of < 0.05 was used to assess statistical significance.
Results
Filtrate chemistry
Table 1 illustrates the comparison between the initial concentrations of the filtrates used in this experiment. The most notable differences were the higher dissolved P and TOC concentrations in the CDS filtrate as compared to swine manure filtrate (Table 1). The only statistically significant difference as a consequence of aging was the aged swine filtrates contained a higher amount of NH4 (P < 0.05; Table 1). There were no other statistical differences observed in the chemistry parameters between the two different aging treatments (open or closed) for either filtrate. This most likely was due to the acidic nature of the filtrate limiting ammonia volatilization losses coupled with the hydrothermal processing reducing microbial activity [37]. In addition, there were no detectable levels of nitrate within either of the filtrates.
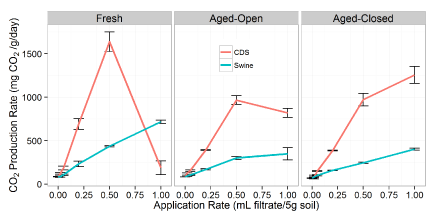
CO2 production
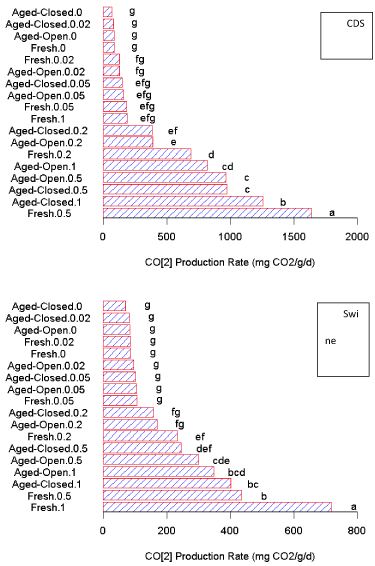
An overall three-way ANOVA (dilution; filtrate type; aging treatment) suggests significant interactions between all the factors. The CDS filtrate additions resulted in statistically higher CO2 production rates when compared overall to the swine filtrate across all levels, which agrees with the TOC results of the filtrates (Table 1). We observed that the impacts of the two filtrates are similar in the fact that CO2 production increases with higher amounts of filtrate (Figure 1). This increase in CO2 activity is significant for additions more concentrated than the 1:20 dilution (Figure S1). However, the CDS filtrate has a localized maximum in production. Due to the presence of these interactions, we will ignore these effects across the entire datasets and instead separate by filtrate to examine the impact of treatment (aging) and application rate.
For the fresh swine manure filtrate, the increase in CO2 production was well correlated well with application amount (R2 = 0.995). In fact, the linear regression shows an average response of 646 µL CO2 per mL swine filtrate. On the other hand, for the CDS filtrate there is a significant interaction of filtrate rate on the observed CO2 production as well as a rate x treatment effect (the localized maximum in the production curve). For the CDS filtrate, application amount was a significant factor influencing observed CO2 production (P < 0.005). As observed in Figure 1, higher amounts of fresh CDS filtrate caused a sharp decline in the CO2 production at additions above the 0.5 mL (1:2 dilution), suggesting an inhibitory effect on microbial respiration once filtrate concentration exceeds 0.1 mL g-1. The exact cause of this decrease was not evaluated in this study.
Overall, there was no difference between the aging treatments (P > 0.05), but both the open and closed treatments were significantly different than the fresh filtrate (P < 0.01; Figure S1). With undiluted filtrates (1 mL addition level) the microbial population did respond differently as a result of the aging (P < 0.01; Figure S1). The aging process reduced the amount of the CO2 produced during this incubation period compared to the fresh filtrate. This suggests that the easily degradable substrates were lost during aging and these could be the easily degraded components that contributed to the higher CO2 production observed from the fresh filtrate treatments. It should be noted, that any pretreatment of organic wastes prior to soil application, whether hydrothermal carbonization, pyrolysis, or composting, alters the chemistry and composition of the wastes [38], and thereby changes the impact of the amendment on the soil microbial community.
For the fresh filtrates, the calculated zero and first-order CO2 mineralization rates are presented in Table 2. The magnitude of these first-order rate constants are in agreement with other carbon mineralization studies (non-filtrate), such as the 3 to 8 × 10-2 d-1 of Ajwa and Tabatabai [39] and slightly higher than the rates of 0.01 to 0.7 × 10-2 d-1 of Gale and Gilmour [40]. The observed first-order degradation rate (k) was nearly constant for the swine filtrate, with no clear correlation with application rate (all within the same order of magnitude; Table 2). On the other hand, the CDS rate shows the doubling of the degradation rate at 1:20 dilutions (0.05 mL), but then a notable drop in the CO2 production rate at higher additions, with the degradation rate of the undiluted filtrate being 4 × 10-8 d-1 which clearly demonstrates an inhibitory effect [4 orders of magnitude reduction compared to the control rate (3.5 × 10-4 d-1; Table 2)]. However, there was a poor first-order model fit to this undiluted data (R2 = 0.7; Table 2).
The degradation rate for the swine filtrate was more consistent, suggesting a lower filtrate impact on the kinetics of carbon turn-over in these incubations. Given the fact that both filtrates were acidic and contained nearly similar amounts of N, the most likely explanation is the presence of an inhibitory compound in the dissolved organic phase of the CDS. As shown in Table 1, there was an order of magnitude difference between the total organic carbon (TOC) concentrations of the two filtrates, which is comprised of different water-soluble organic compounds.
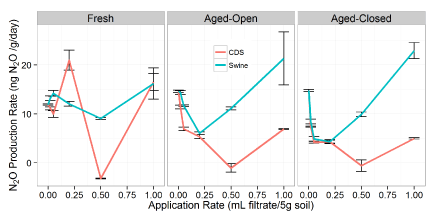
N2O production
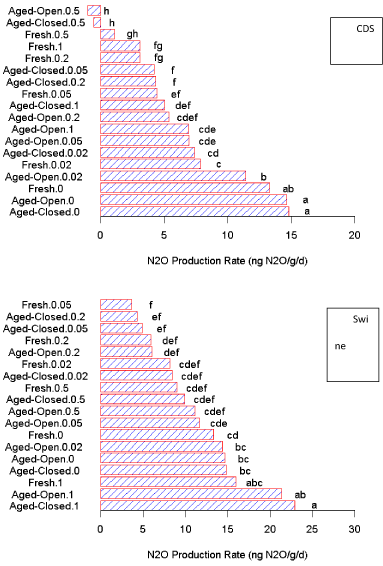
Similar to CO2 production, the overall three-way ANOVA suggests significant interactions between all the factors (Figure S2). There were significant interactions of filtrate type (P < 0.01), treatment, and application rate (P < 0.01), as well as the two-way interaction between filtrate type x rate (P < 0.01) observed in the N2O production rates. Due to the presence of these interactions, we will ignore these overall effects and instead divide our dataset by filtrate to examine the impact of treatment (aging) and application rate for each individual filtrate.
N2O production rates as a function of application amounts (Figure 2), we observed that the impacts of the two filtrates are similar in the fact that N2O production decreases with higher additions of filtrate, but then increases after the 1:2 dilution level. This is in agreement with the conceptual model that the filtrate provided additional organic matter that is being mineralized by the soil microbes, leading to a reduction in denitrification rates.
Other studies have observed this decreased N2O production as a function of easily degradable organic matter additions [41]. However, after a certain level, the N-added in the filtrate begins to influence the overall C:N ratio of the soil system and the N2O production increases (Figure 2).
For the CDS filtrate, N2O production of the amended samples was consistently lower than the N2O production of the soil control (Figure 2). This trend was consistent across the entire dataset. For the swine manure filtrate, N2O production of the amended samples was also lower than the N2O production of the control at dilutions greater than 1:2 (Figure 2). This trend was consistent despite the increasing CO2 production that was observed as a function of application amounts (Figure 1). This suggests that these lower levels of N2O production could be due to N-immobilization, which would be consistent with increasing CO2 respiration and increased C:N ratio of the soil mixture at low filtrate additions. Eventually, the added inorganic N with the swine reached a point (> 0.5 mL filtrate 5 g-1) where the initial N-immobilization created by the additional C sources was overcome due to the higher N supply in the filtrate. However, the main observation is a different trend for the N2O and CO2 production as a function of filtrate amount.
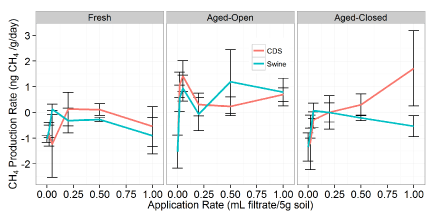
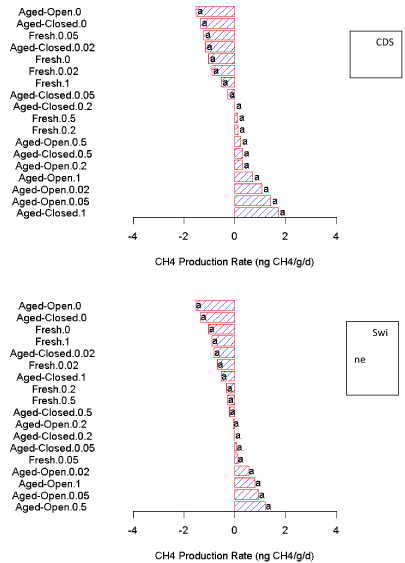
CH4 oxidation production
From the overall ANOVA, there were significant interactions of application rate (P < 0.01) and treatment (P < 0.01) on the observed net balance of CH4 (Figure 3). There were no significant differences between filtrates (Figure S3). The addition of higher amounts of filtrate reduced the observed net oxidation rate, due to either to CH4 production resulting from the new organic compounds or a decrease in the rate of CH4 oxidation activity. The exact cause could not be elucidated from this data. Statistically, there were no significant differences observed in the CH4 data (Figure S3).
N-mineralization
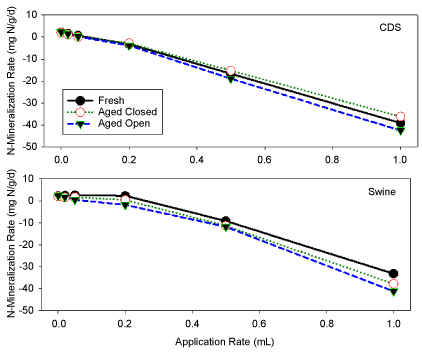
In general, nitrate levels (NO3) were the highest at the lowest treatment concentrations for filtrate applications, and decreased with increasing filtrate application amounts (Table 3). The highest dilution factors (1:20 and 1:50) possessed the highest nitrate concentrations, and correspondingly the highest rate of N-mineralization. Whereas, the more concentrated filtrate additions had low nitrate and often exhibited N-immobilization (Table 3). Undiluted fresh swine filtrate (~3900 ± 390 mg L-1) prior to soil incubation had a slightly higher initial NH4 concentration than undiluted CDS filtrate (~3500 ± 350 mg L-1), although the differences were within the same order of magnitude (Table 1).
Statistically, there was no difference observed in the final NH4 concentrations between the open and closed CDS treatments (Table 3). The amount of nitrate in the final incubation was negatively correlated to the dilution amount, indicating that higher amounts of filtrate reduced the N-mineralization and the formation of nitrate (Figure 4).
The type of aging of the filtrates did not produce a significant impact on inorganic-N concentrations or N-mineralization rates. The only statistically different final NH4 concentration between the open and closed swine treatments was observed in 1:5 treatments (Table 3), where the open treatments had a 40% lower concentration (P < 0.05) than the closed treatments. The results of increased N-mineralization rates suggest that the soil microbial population prefers the aged filtrate in the open container. All soil incubations treated with swine HTC filtrates contained greater final NH4 levels than those treated with CDS filtrates. Levels of NO3 extracted from all vials treated with swine HTC filtrate were also considerably higher than the corresponding CDS filtrate applications (Table 3).
Discussion
Overall, there was an observable difference in microbial activity in soil incubations of both filtrate types, under all three treatment conditions: Fresh filtrates, and those aged in open and closed containers. CDS HTC filtrate at 1:2 dilution proved to create an ideal environment for filamentous microbial growth, whereas no obvious growth was seen in any incubations containing swine HTC filtrate. This should be further studied, as some types of microbial growth, particularly fungi, can be detrimental to crop production [42] and can also be the cause of fungi induced denitrification and N2O emissions [43].
Comparison of cumulative CO2 production of CDS vs. swine treatments suggests that there was higher microbial respiration in the 1:2 CDS treatments than those at any concentration of swine filtrate. These results are similar to the observations made by Kammann, et al. [44] and Fornes, et al. [45] of the stimulatory effect of solid hydrochar additions. Further comparison of cumulative CO2 production indicates that aging filtrate in open containers has a significant reduction on the inhibitory impact of HTC filtrates. This is supported by the fact that more CO2 was produced in aged filtrates in open containers, and therefore suggests reduced inhibitory effects of the filtrate on microbial respiration activity. The cumulative levels of CO2 in the CDS open vials were 67% greater than those from the closed aging tests. Similarly, the rate of CO2 production was 27% greater in the CDS undiluted filtrate open aging than that of the corresponding closed treatment.
Soil extractions for N-mineralization shed more light on the differences between filtrate types and the impacts of aging. In general, overall final NH4 concentrations for soil incubations were greater in swine filtrate treatments than CDS treatments (Table 3). N-mineralization was greater for all soil incubation treatments containing swine filtrate, than the corresponding concentrations of CDS filtrate treatments, regardless of post-aging condition (Table 3). Comparing the starting levels of NH4 in both filtrate types, with CDS containing ~3,900 mg/L and swine filtrate containing ~3,500 mg/L, to the concentrations of NH4 following incubations, shows that there was greater utilization of NH4 in CDS filtrate incubations than those of swine filtrate. This information, coupled with the higher rate of CO2 production observed in CDS filtrates points to a possible limiting factor within the swine filtrate to microbial growth. It is likely that the greater microbial activity in CDS filtrate incubations (omitting 1:2 level which were dominated by fungi) caused a greater shift towards N-immobilization as available carbon was being actively depleted. However, due to the differences observed as a function of filtrate type and storage condition, additional work is needed to understand how long the observed repeated applications affect soil quality. Such studies should also include an evaluation of the potential salt accumulation that could occur with some HTC filtrate types.
Conclusions
Soil incubations with CDS and swine filtrates have demonstrated that soil microbes can utilize both filtrate types. Overall, the greatest amount of microbial activity between the two filtrates occurred in the 1:2 CDS treatments. However, CDS filtrate does have a threshold at which microbial activity is greatly inhibited; whereas, the same threshold was not reached with swine filtrate. This observed difference implies the presence of a potential microbial inhibitor that increases at higher concentrations of CDS filtrate. Aging of the filtrates in an open container proved to enhance nutrient mineralization of ammonium into nitrate after soil application. N2O production stimulated by microbial degradation of the filtrates should be considered when performing life cycle analyses in regard to utilization of HTC filtrates as soil amendments. N2O is produced at levels greater than the soil control at some HTC filtrate application rates. There was reduced N-mineralization rates observed in this study as a function of applied filtrate.
Despite high metabolic activity in response to increased application amounts, lower filtrate applications appear to provide the most optimal conditions for N-mineralization and reduced impact on the GHG production. These initial observations show that soil amendments with HTC filtrates do show initial promise, but there is no universal effect across different types of microbial functionality.
References
- Baumann B (1960) The botanical aspects of ancient egyptian embalming and burial. Economic Botany 14: 84-104.
- Hawley LF (1926) Fifty years of wood distillations. Ind Eng Chem 18: 929-930.
- Mu J, Uehara T, Furuno T (2004) Effect of bamboo vinegar on regulation of germination and radicle growth of seed plants ii: Composition of moso bamboo vinegar at different collection temperature and its effects. Journal of Wood Science 50: 470-476.
- Xu Sy, Chen Jj, Cao Dr (2006) Analysis of components in wood vinegar. Guangzhou Chemistry 31: 28.
- Harada K, Iguchi A, Yamada M, et al. (2013) Determination of maximum inhibitory dilutions of bamboo pyroligneous acid against pathogenic bacteria from companion animals: An in vitro study. J Vet Adv 3: 300-305.
- Mac Culloch J (1814) I On certain products obtained in the distillation of wood, with some account of bituminous substances, and remarks on coal. Transactions of the Geological Society of London S1-S2: 1-28.
- Yatagai M, Nishimoto M, Hori K, et al. (2002) Termiticidal activity of wood vinegar, its components and their homologues. Journal of Wood Science 48: 338-342.
- Karagöz S, Bhaskar T, Muto A, et al. (2005) Comparative studies of oil compositions produced from sawdust, rice husk, lignin and cellulose by hydrothermal treatment. Fuel 84: 875-884.
- Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 20: 848-889.
- Saetea P, Tippayawong N (2013) Recovery of value-added products from hydrothermal carbonization of sewage sludge. ISRN Chemical Engineering 2013: 268947.
- Heilmann SM, Jader LR, Sadowsky MJ, et al. (2011) Hydrothermal carbonization of distiller's grains. Biomass and Bioenergy 35: 2526-2533.
- Humphrey AE (1979) Hydrolysis of cellulose: Mechanisms of enzymatic and acid catalysis. American Chemical Society 25-53.
- Russell JA, Molton PM, Landsman SD (1980) Chemical comparisons of liquid fuel produced by thermochemical liquefaction of various biomass materials. 3rd Miami international conference on alternative energy sources, Miami Beach, FL, USA.
- Goudriaan F, Peferoen DGR (1990) Liquid fuels from biomass via a hydrothermal process. Chemical Engineering Science 45: 2729-2734.
- Yin C (2012) Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresource Technology 120: 273-284.
- Heilmann SM, Jader LR, Harned LA, et al. (2011) Hydrothermal carbonization of microalgae ii. Fatty acid, char, and algal nutrient products. Applied Energy 88: 3286-3290.
- Libra JA, Ro KS, Kammann C, et al. (2011) Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2: 71-106.
- Bargmann I, Rillig MC, Kruse A, et al. (2014) Effects of hydrochar application on the dynamics of soluble nitrogen in soils and on plant availability. Journal of Plant Nutrition and Soil Science 177: 48-58.
- Rillig MC, Wagner M, Salem M, et al. (2010) Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Applied Soil Ecology 45: 238-242.
- Busch D, Stark A, Kammann CI, et al. (2013) Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicology and Environmental Safety 97: 59-66.
- Gajić A, Ramke HG, Hendricks A, et al. (2013) Microcosm study on the decomposability of hydrochars in a cambisol. Biomass and Bioenergy 47: 250-259.
- Jandl G, Eckhardt KU, Bargmann I, et al. (2013) Hydrothermal carbonization of biomass residues: Mass spectrometric characterization for ecological effects in the soil-plant system. J Environ Qual 42: 199-207.
- Becker R, Dorgerloh U, Helmis M, et al. (2013) Hydrothermally carbonized plant materials: Patterns of volatile organic compounds detected by gas chromatography. Bioresour Technol 130: 621-628.
- Bargmann I, Rillig MC, Buss W, et al. (2013) Hydrochar and biochar effects on germination of spring barley. Journal of Agronomy and Crop Science 199: 360-373.
- Fuertes AB, Arbestain MC, Sevilla M, et al. (2010) Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Australian Journal of Soil Research 48: 618-626.
- Cao X, Ro KS, Chappell M, et al. (2011) Chemical structures of swine-manure chars produced under different carbonization conditions investigated by advanced solid-state 13c nuclear magnetic resonance (nmr) spectroscopy. Energy Fuels 25: 388-397.
- Stemann J, Putschew A, Ziegler F (2013) Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresource Technology 143: 139-146.
- Reza MT, Lynam JG, Uddin MH, et al. (2013) Hydrothermal carbonization: Fate of inorganics. Biomass and Bioenergy 49: 86-94.
- Pham M, Schideman L, Sharma BK, et al. (2013) Effects of hydrothermal liquefaction on the fate of bioactive contaminants in manure and algal feedstocks. Bioresour Technol 149: 126-135.
- Wood BM, Jader LR, Schendel FJ, et al. (2013) Industrial symbiosis: Corn ethanol fermentation, hydrothermal carbonization, and anaerobic digestion. Biotechnol Bioeng 110: 2624-2632.
- Czimczik CI, Trumbore SE, Carbone MS, et al. (2006) Changing sources of soil respiration with time since fire in a boreal forest. Global Change Biology 12: 957-971.
- Vasconcellos RLF, Bonfim JA, Andreote FD, et al. (2013) Microbiological indicators of soil quality in a riparian forest recovery gradient. Ecological Engineering 53: 313-320.
- Elmajdoub B, Barnett S, Marschner P (2014) Response of microbial activity and biomass in rhizosphere and bulk soils to increasing salinity. Plant and Soil 381: 297-306.
- Spokas KA, Bogner JE (2011) Limits and dynamics of methane oxidation in landfill cover soils. Waste Manag 31: 823-832.
- Fierer N, Schimel JP (2003) A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Science Society of America Journal 67: 798-805.
- Cabrera ML (1993) Modeling the flush of nitrogen mineralization caused by drying and rewetting soils. Soil Science Society of America Journal 57: 63-66.
- Namioka T, Morohashi Y, Yoshikawa K (2011) Mechanisms of malodor reduction in dewatered sewage sludge by means of the hydrothermal torrefaction. Journal of Environment and Engineering 6: 119-130.
- Kirchmann H, Witter E (1992) Composition of fresh, aerobic and anaerobic farm animal dungs. Bioresource Technology 40: 137-142.
- Ajwa HA, Tabatabai MA (1994) Decomposition of different organic materials in soils. Biol Fert Soils 18: 175-182.
- Gale PM, Gilmour JT (1986) Carbon and nitrogen mineralization kinetics for poultry litter. Journal of Environmental Quality 15: 423-426.
- Yao Z, Zhou Z, Zheng X, et al. (2010) Effects of organic matter incorporation on nitrous oxide emissions from rice-wheat rotation ecosystems in china. Plant and Soil 327: 315-330.
- Koehler B, Holbert JR (1930) Corn diseases in illinois; their extent, nature, and control. Bulletin. Illinois Agricultural Experiment Station 354: 164.
- Baggs EM (2011) Soil microbial sources of nitrous oxide: Recent advances in knowledge, emerging challenges and future direction. Current Opinion in Environmental Sustainability 3: 321-327.
- Kammann C, Ratering S, Eckhard C, et al. (2012) Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J Environ Qual 41: 1052-1066.
- Fornes F, Belda RM, Lidón A (2015) Analysis of two biochars and one hydrochar from different feedstock: Focus set on environmental, nutritional and horticultural considerations. Journal of Cleaner Production 86: 40-48.
Corresponding Author
Kurt A Spokas, Soil and Water Management Research Unit, United States Department of Agriculture - Agricultural Research Service; Department of Soil, Water, and Climate, University of Minnesota, 1991 Upper Buford Circle, 439 Borlaug Hall, St. Paul, MN 55108, USA, Tel: (612)-626-2834, Fax: (651)-649-5175.
Copyright
© 2018 Georgiy VV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.