The Effect of Doppler Ultrasonography on Pregnancy Outcomes in Fetal Cardiac Activity Evaluations
Abstract
Doppler ultrasound is widely used in obstetrics. However, the biological effects of high energy from Doppler ultrasonography have not been clearly revealed. Animal studies have shown negative effects on the fetus, but studies on humans are limited. The aim of this study is to evaluate the effect of Doppler ultrasonography used in early pregnancy weeks (6-12 week) to detect fetal cardiac activity on pregnancy outcomes (number of births and abortions, type of delivery, cesarean indications, birth week, preterm labor rates, birth weights, low-birth-weight infant). For fetal cardiac activity, 359 patients examined with Doppler ultrasound constituted the study group and 252 patients examined without Doppler ultrasound constituted the control group. The pregnancy outcomes were compared. According to the results of the study, no adverse effects of Doppler ultrasound were found. These results were thought to be related to the use of Doppler according to safety recommendations.
Keywords
Doppler ultrasonography, Fetal cardiac activity Obstetric-neonatal results
Introduction
In the first trimester, worrying about the well-being of the fetus is common in expectant mothers. Having a poor obstetric history and vaginal bleeding cause anxiety for the patient.Therefore, it is important to evaluate fetal cardiac activity in patients who present to clinics. For this purpose, Doppler ultrasonography has been used for years. There is consensus that B-mode and M-mode ultrasonography are safe in every period of pregnancy due to their limited acoustic output [1, 2]. Doppler ultrasonography has a higher energy output, thus more biological effects are expected. In particular, the effect on the examination of a small area may be more pronounced [3, 4]. Therefore, there is an opinion that Doppler examination should be performed only if clinically necessary in the first trimester of pregnancy. The main reason for advocating the prudent use of Doppler ultrasound in early pregnancy is not knowledge that it harms the fetus, rather because we do not know if it is safe, and that the first trimester is a particularly vulnerable fetal life period.
The number of human studies confirming the reliability of Doppler ultrasound is limited. Studies have mostly been done on animals. In our study, the obstetric results of patients who were followed up by clinicians who used Doppler ultrasound to confirm fetal cardiac beat in the first trimester were compared with those of clinicians who did not use Doppler ultrasound. And thus, it was evaluated whether the Doppler ultrasound had a negative effect on fetal cardiac results in the first trimester, or if it affected obstetric results.
Materials and Methods
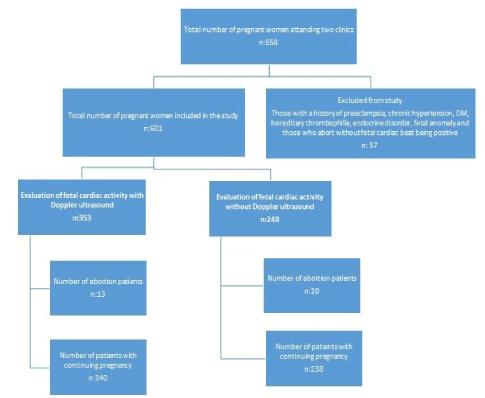
Ethics committee approval of the study was obtained from Amasya University on November 7, 2019. The ethics committee number for the study was 48. The study was conducted with pregnant women who came to the Amasya Sabuncuoğlu Şerefeddin Training and Research Hospital Gynecology and Obstetrics clinic for the first pregnancy visit between 1 January 2018 and 31 December 2018. According to the clinical preference, 353 pregnant women who were examined by a physician who used Doppler ultrasound to confirm fetal heartbeat at 6-12 weeks of pregnancy formed the study group. As a clinical preference, 248 pregnant women, whose fetal viability was evaluated by seeing fetal heart movements without using fetal Doppler ultrasound for the confirmation of fetal heartbeat, constituted the control group. The records of the patients belonging to the groups were analyzed retrospectively. Pregnant women with a history of preeclampsia, chronic hypertension, diabetes mellitus, hereditary thrombophilia, endocrine disorders (such as thyroid function disorder, Addison's disease) and fetal anomaly were not included in the study. Pregnancy results such as whether pregnancies resulted in birth or miscarriage, the type of delivery, cesarean rates, birth weeks, birth weights, preterm labor and intrauterine growth retardation were compared. Pregnancies that resulted in abortion without any fetal cardiac activity detected were not included in the study. A Mindray DC-7 Diagnostic Ultrasound System device was used for both groups in the study. The Doppler of this device is colored flow and pulsed Doppler. The duration of Doppler ultrasound used to assess fetal cardiac beat was between 15 and 60 seconds. If pregnant women included in the study presented with pelvic pain or vaginal bleeding and did not exceed their 12th week, the number of ultrasound and Doppler ultrasound scans performed on these patients were also noted. The duration of the ultrasound and Doppler ultrasound application of those who came for the second or third time was also the same. In order to better show the effects of Doppler ultrasound, the results of birth or miscarriage, the type of delivery, cesarean rates, birth weeks, birth weights, preterm labor and intrauterine growth retardation of these patients who were examined for the second and third times were evaluated separately.
Power Analysis
The sample size for the research was determined with the G * Power 3.1 program. In the study of Stroux, et al. [5], considering the Doppler-based fetal heart rate research for IUGR detection, 0.28 effect size was calculated according to the double-tailed hypothesis method. Confidence interval was determined as 95% and margin of error as 5%. As a result of the calculation, it was determined that there should be 230 women for the control group, 230 women for the experimental group and 460 women in total.
Statistical Analysis
Data were analyzed with IBM SPSS V23. Compliance with normal distribution was examined by Kolmogorov-Smirnov test. Chi-square test and Fisher's Exact test were used to compare categorical variables according to groups. Mann-Whitney U test was used to compare non-normally distributed data according to paired groups, and independent two sample t test was used for comparison of normally distributed data. Analysis results are mean ± s for quantitative data. For categorical data, deviation, median (minimum - maximum) and frequency (percentage) are given. The significance level was taken as p < 0.05.
Results
The demographic characteristics of the groups (age, height, weight, BMI, parity, education level, previous surgery, chronic disease) were homogeneous, as shown in Table 1.
A total of 353 pregnant women whose fetal cardiac beat was evaluated by Doppler ultrasound in the first trimester constituted the study group. Of these, 246 (67.9%) were viewed once by Doppler ultrasound, 97 (27.5%) twice and 10 (2.8%) three times. A total of 248 pregnant women whose fetal cardiac activity was evaluated by ultrasound examination constituted the control group. 169 (68.2%) of these pregnant women were examined by ultrasound once, 72 (29.0%) twice and 7 (2.8%) three times. There was no significant difference between the groups in terms of the number of ultrasound / Doppler ultrasound examinations (p = 0.916) (Table 2). Spontaneous abortion developed in 13 (3.7%) pregnant women in the study group in which Doppler ultrasound was used, and 10 (4.0%) in the control group in which ultrasound was used. There was no difference between the groups in terms of development of abortion (p = 0.826) (Table 3).
Pregnancy results of the groups are shown in Table 3. Since spontaneous abortions would not have gestational consequences, evaluation was made after spontaneous abortions were removed from the groups. In the study group in which Doppler ultrasound was used, the pregnancy outcomes of 340 pregnant women were compared with the results of 238 pregnant women who had ultrasound. Mean birth weight was 3252.03 ± 431.94g in the study group and 3275.29 ± 443.15g in the control group. There was no significant difference between the groups in terms of mean birth weight (p = 0.618). Mean birth week was 39.00 ± 1.17 weeks in the study group and 38.93 ± 1.28 weeks in the control group. There was no difference between the groups in terms of mean birth week (p = 0.883). 142 (41.8%) pregnant women in the study group and 94 (39.5%) pregnant women in the control group delivered by cesarean section. There was no difference between the groups in terms of cesarean delivery (p = 0.585). Six (1.8%) patients in the study group and 10 (4.2%) patients in the control group had preterm labor. There was no difference between the groups in terms of preterm delivery (p = 0.077). Intrauterine growth retardation was higher in the study group, but there was no significance between the groups (n: 7 (2.1%) and n: 5 (2.1%), respectively) (p = 1.00) (Table 3).
In order to better show the effects of Doppler ultrasound, the results of the pregnant women who had 2 or 3 ultrasound / Doppler ultrasound examinations were evaluated separately (Table 4). 107 pregnant women in the study group were examined by Doppler ultrasound and 79 pregnant women in the control group were examined by ultrasound two or three times. While spontaneous abortion developed in 4 (3.7%) pregnant women in the study group, it occurred in 3 (3.8%) pregnant women in the control group. There was no significant difference between the groups in terms of spontaneous abortion (p = 1.00). Pregnancy outcomes of patients who had 2 or 3 ultrasound / Doppler ultrasound examinations were evaluated by removing spontaneous abortions. Pregnancy outcomes of 103 pregnant women in the study group were compared with those of 76 pregnant women in the control group. While the mean birth weight was 3285.73 ± 368.67g in the study group, it was 3190.39 ± 389.79g in the control group. There was no significant difference between the groups in terms of mean birth weight (p = 0.097). Mean birth week was 39.06 ± 1.10 weeks in the study group and 38.96 ± 1.19 weeks in the control group. There was no significant difference between the groups in terms of mean birth week (p = 0.970). 42 (40.8%) pregnant women in the study group and 29 (38.2%) pregnant women in the control group delivered by cesarean section. There was no difference between the groups in terms of cesarean delivery (p = 0.723). One pregnant woman (1.0%) in the study group and 2 (2.6%) pregnant women in the control group delivered preterm. There was no difference between the groups in terms of preterm labor (p = 0.575). There were 3 (2.9%) IUGGs in the study group and 2 (2.6%) in the control group. There was no significant difference between the groups in terms of IUGR (p = 1.00).
Discussion
To evaluate the effect of Doppler ultrasound on the fetal heart and fetus in general in the first trimester, we compared the results of pregnant women followed by clinicians who evaluated fetal cardiac beat with Doppler ultrasound with the results of pregnant women followed by clinicians who did not use Doppler ultrasound. We compared abortion rates to evaluate early effects, birth weight, birth week, delivery type, preterm labor rates and intrauterine growth retardation to evaluate late effects. When we evaluated the results of the study, no significant difference was found between the two groups (Table 1). When we compared the numbers of Doppler ultrasound and ultrasound, though the exposure time to Doppler ultrasound may potentially increase the damage that may occur to the fetus, no significant difference was found between the groups (Table 3). Since the exposure time of Doppler ultrasound is important, we evaluated the pregnancy results of those who were examined with 2 or 3 ultrasound / Doppler ultrasound separately. As seen in Table 4, there was no significant difference between the groups in terms of spontaneous abortion, birth weight, birth week, delivery type, preterm labor and intrauterine growth retardation (p < 0.05).
We have been using ultrasound for diagnostic and screening purposes for 60 years. Although there is no evidence of immediate or long-term harm to the fetus, its reliability is still in doubt [6]. Although B mode is said to be safe, the reliability of diagnostic ultrasound and Doppler ultrasound is doubtful [3,7,8]. Especially with advances in the technology (high-output ultrasound energy levels), the development of modern medical devices with high resolution has led to an increase in high output energy levels used in ultrasound. This level is much higher with color Doppler and pulsed Doppler [9,10].
The 'as low as reasonably achievable' (ALARA) principle is recommended for the use of ultrasound within safe limits [11]. In a fetus with normal screening results, it may be easy to comply with the ALARA principle. However, major cardiac defects can be detected between 11 and 13 + 6 weeks [12]. In addition, it is difficult to comply with the ALARA principle with the need to use cardiac and intracardiac Doppler ultrasound for cardiac pathologies that frequently coexist in aneuploidies (especially in trisomy 21) and in the evaluation of aneuploidy in pregnancies that are not scanned during these weeks [13,14].
Human studies evaluating the effects of Doppler ultrasound on the fetus are very limited. Most of the information obtained on this subject has come from animal experiments, in vitro experiments and epidemiological studies. Adverse effects of Doppler ultrasound in the animal fetus have been demonstrated. For example, it has been determined that the application of pulsed Doppler for 3 seconds to 10 minutes to the ductus venosus of rat fetuses increases apoptotic activity in fetal liver tissue, and liver cell damage increases with prolongation of its duration [15]. Again in a study conducted in mice, it was shown that an increase in tissue temperature caused disruption of mitochondrial metabolism in embryonic tissue and formation of excessive reactive oxygen species [16]. Of course, it may not be very correct to interpret animal experiments for humans. For this reason, studies on humans would make a great contribution to this issue. Ultrasound is a pressure wave with a frequency, a form of energy [17]. When ultrasound waves are distributed towards the tissue, energy is absorbed by the tissue and transforms into heat depending on the frequency and density [18]. These thermal effects can change the balance between chemical reactions and consequently damage the surrounding tissue [19-21]. Studies have evaluated the effects of hyperthermia and in vivo temperature measurements induced by pulsed ultrasound [22-24]. For example, in an in vitro study, it was stated that the application of Doppler ultrasound caused a temperature increase of 1℃ in a short time such as one minute and a temperature increase of 2.4℃ in five minutes. And it has been reported that this temperature increase can damage the tissue [25]. Moreover, it has been stated that the potential risk will be higher when it is considered that this tissue is the first-trimester fetus,in which cell division is the fastest and the blood flow is less developed so that the heat cannot be dissipated sufficiently [2, 26]. It has also been reported that while there is a 1℃ temperature increase in routine ultrasound scanning in the first trimester, it causes a 1.5℃ temperature increase in pulsed Doppler use [27]. The fact that a temperature increase more than 1.5℃ above the normal value has been proposed as a universal threshold [28], and the teratogenic effect of the thermal increase has been demonstrated in animal experiments and many controlled human studies [29,30], support the conclusion that the fragile/vulnerable embryo in the first trimester may be damaged by this temperature increase.
The effects of Doppler ultrasound on the fetus may be immediate or may occur in the years after birth. As a matter of fact, as with the fetal programming hypothesis, in the basic physiology of the intrauterine environment ultrasound may lead to an irreversible path later on and it is argued that it may occur at a certain stage of life [6]. It is also thought that there could be fetal programming due to the thermal and mechanical effects of Doppler ultrasound [6]. An example is an epidemiological study showing that right handedness occurs more frequently in men exposed to intrauterine Doppler ultrasound [31]. In a study conducted on mice, it was found that apoptosis occurred in fetal myocardial cells exposed to Doppler ultrasound, but in the same study, no finding of this apoptosis was found in myocardial cells on the 10th day of the neonatal period. Therefore it was suggested that the effects of Doppler ultrasound on the fetus can be salvaged [32]. The intrauterine demonstration of this damage is evidence that Doppler affects the fetus. Although this effect cannot be demonstrated after birth, it does not mean that it will not cause another increase in risk in later years of life. In our study, we did not find any negative effects of Doppler ultrasound on early or late pregnancy. In animal studies, exposure times as long as 5 minutes can be used to evaluate the effects of Doppler ultrasound. However, the most important limitation of our study was that it is not appropriate to work with such different and long duration Doppler applications on humans for ethical reasons. The limitations of our study were that it was a study with a retrospective design and that there was no follow-up period that included advanced life stages. As defended in the fetal programming hypothesis, epidemiological studies with different designs are needed to show whether Doppler ultrasound causes an increase in risk in the future. The strength of our study was that Doppler ultrasonography performed with safety principles statistically demonstrated with a sufficient number of cases that it had no negative effect in the short term. Separate evaluation of the pregnancy results evaluated by more than one Doppler ultrasound provided a better understanding of the effects of Doppler ultrasound.
In conclusion, in our study no negative effect of Doppler ultrasound on fetal cardiac activity and obstetric-neonatal results was observed. We may have to face uncertainties regarding ultrasound safety in the coming years. There is no such thing as zero risk, and no detectable harm is not synonymous with no harm. Therefore, according to the ISUOG safety statement, Doppler examination of fetal vessels is not recommended in early pregnancy without a clinical indication [4]. Compliance with ISUOG's security indexes and the ALARA principle seems to be the most appropriate approach to minimize the possible risks in the existing infrastructure.
References
- Torloni MR, Vedmedovska N, Merialdi M, et al. (2009) Safety of ultrasonography in pregnancy: WHO systematic review of the literature and meta-analysis. Ultrasound Obstet Gynecol 33: 599-608.
- Abramowicz JS, Kossoff G, Marsal K, et al. (2003) Safety Statement, 2000 (reconfirmed 2003) International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). Ultrasound Obstet Gynecol 21: 100.
- Hershkovitz R, Sheiner E, Mazor M (2002) Ultrasound in obstetrics: A review of safety. Eur J Obstet Gynecol Reprod Biol 101: 15-18.
- Salvesen K, Lees C, Abramowicz J, et al. (2011) ISUOG statement on the safe use of Doppler in the 11 to 13 +6-week fetal ultrasound examination. Ultrasound Obstet Gynecol 37: 628.
- Stroux LA-O, Redman CW, Georgieva AA-O, et al. (2017) Doppler-based fetal heart rate analysis markers for the detection of early intrauterine growth restriction. Acta Obstet Gynecol Scand 96: 1322-1329.
- Aiken CE, Lees CC (2012) Long-term effects of in utero Doppler ultrasound scanning--a developmental programming perspective. Med Hypotheses 78: 539-541.
- WFUMB (2013) WFUMB/ISUOG statement on the safe use of Doppler ultrasound during 11-14 week scans (or earlier in pregnancy). Ultrasound Med Biol 39: 373.
- Society SGotMU (2009) Guidelines for the safe use of diagnostic ultrasound equipment. In: Society TBMU e.
- Henderson J, Willson K, Jago JR, et al. (1995) A survey of the acoustic outputs of diagnostic ultrasound equipment in current clinical use. Ultrasound Med Biol 21: 699-705.
- Meltzer RS (1996) Food and Drug Administration ultrasound device regulation: The output display standard, the "mechanical index," and ultrasound safety. J Am Soc Echocardiogr 9: 216-220.
- ter Haar GR, Abramowicz JS, Akiyama I, et al. (2013) Do we need to restrict the use of Doppler ultrasound in the first trimester of pregnancy? Ultrasound Med Biol 39: 374-380.
- Volpe P, De Robertis V, Campobasso G, et al. (2012) Diagnosis of congenital heart disease by early and second-trimester fetal echocardiography. J Ultrasound Med 31: 563-568.
- Nicolaides KH, Brizot ML, Snijders RJ (1994) Fetal nuchal translucency: Ultrasound screening for fetal trisomy in the first trimester of pregnancy. Br J Obstet Gynaecol 101: 782-786.
- Hyett J, Perdu M, Sharland G, et al. (1999) Using fetal nuchal translucency to screen for major congenital cardiac defects at 10-14 weeks of gestation: population based cohort study. Bmj 318: 81-85.
- Pellicer B, Herraiz S, Táboas E, et al. (2011) Ultrasound bioeffects in rats: Quantification of cellular damage in the fetal liver after pulsed Doppler imaging. Ultrasound Obstet Gynecol 37: 643-648.
- Ozawa M, Hirabayashi M, Kanai Y (2002) Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or invitro. Reproduction 124: 683-689.
- Abramowicz JS, Kremkau FW, Merz E (2012) Obstetrical ultrasound: Can the fetus hear the wave and feel the heat? Ultraschall Med 33: 215-217.
- Miller DL (2008) Safety assurance in obstetrical ultrasound. Semin Ultrasound CT MR 29: 156-164.
- Meizner I (2012) What do doctors understand regarding ultrasound safety during pregnancy? Harefuah 151: 234-236, 52.
- Church CC, Miller MW (2007) Quantification of risk from fetal exposure to diagnostic ultrasound. Prog Biophys Mol Biol 93: 331-353.
- Houston LE, Odibo AO, Macones GA (2009) The safety of obstetrical ultrasound: a review. Prenat Diagn 29: 1204-1212.
- Bosward KL, Barnett SB, Wood AK, et al. (1993) Heating of guinea-pig fetal brain during exposure to pulsed ultrasound. Ultrasound Med Biol 19: 415-424.
- Tarantal AF, O'Brien WD, Hendrickx AG (1993) Evaluation of the bioeffects of prenatal ultrasound exposure in the cynomolgus macaque (Macaca fascicularis): III. Developmental and hematologic studies. Teratology 47: 159-170.
- Duggan PM, Liggins GC, Barnett SB (1995) Ultrasonic heating of the brain of the fetal sheep in utero. Ultrasound Med Biol 21: 553-560.
- Helmy S, Bader Y, Koch M, et al. (2015) Measurement of thermal effects of doppler ultrasound: An invitro study. PLoS One 10: e0135717.
- Barnett SB, Maulik D (2001) Guidelines and recommendations for safe use of Doppler ultrasound in perinatal applications. J Matern Fetal Med 10: 75-84.
- Bly SH, Vlahovich S, Mabee PR, et al. (1992) Computed estimates of maximum temperature elevations in fetal tissues during transabdominal pulsed Doppler examinations. Ultrasound Med Biol 18: 389-397.
- Edwards MJ (1986) Hyperthermia as a teratogen: A review of experimental studies and their clinical significance. Teratog Carcinog Mutagen 6: 563-582.
- Edwards MJ, Saunders RD, Shiota K (2003) Effects of heat on embryos and foetuses. Int J Hyperthermia 19: 295-324.
- NCRP (National Council on Radiation Protection and Measurements). Exposure Criteria for Medical Diagnostic Ultrasound: II. Criteria Based on All Known Mechanisms. Report No - 140.
- Salvesen K (2011) Ultrasound in pregnancy and non-right handedness: Meta-analysis of randomized trials. Ultrasound Obstet Gynecol 38: 267-271.
- Jia H, Duan Y, Cao T, et al. (2005) Immediate and long-term effects of color Doppler ultrasound on myocardial cell apoptosis of fetal rats. Echocardiography 22: 415-420.
Corresponding Author
Aysun Tekeli Taşkömür, Amasya University Faculty of Medicine, Department of Gynecology and Obstetrics, Amasya, Turkey, Tel: 0 553-479-5680, Fax: 0 358-212-0001.
Copyright
© 2022 Taşkömür AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.