Letrozole versus Gonadotropins for Ovulation Induction in Clomiphene Citrate Resistance: A Randomized Controlled Study
Abstract
Background: Clomiphene citrate resistance (CCR) occur in 15 - 40% in women with PCOS. Several studies have shown that letrozole is superior to CC regarding side effects, ovulation, and pregnancy rates. Letrozole or gonadotropins use for ovulation induction in CCR is not well established which is superior and most safe.
Aims: To compare between letrozole and gonadotropin in induction of ovulation in clomiphene citrate resistant cases.
Design and Settings: This randomized clinical trial was carried at Gynecology and Obstetrics Department, Tanta University from 1, March 2019 till 31, October 2019.
Materials and Methods: Seventy infertile anovulatory patients with CCR were randomly allocated into 2 groups, group I (letrozole group) and group II (Gonadotropins group). Ovulation rate, endometrial thickness and pregnancy rates were assessed.
Statistical Tests: Med Calc 8.2.1 Tests of significance (Kruskal-Wallis, Wilcoxon's, Chi square, logistic regression analysis, and Spearman's correlation.
Results: There was highly significant number of pregnancies as evidenced by positive pregnancy test and intrauterine gestational sacs in letrozole group than in gonadotropin group (12 cases or 48 % versus 5 cases or 20 % respectively). The difference between letrozole group & gonadotropin group was statistically significant, also rate of pregnancy per cycle was (14.6 % versus 5.6%) the difference was also statistically significant.
Conclusion: Letrozole was superior to gonadotropin in clomiphene citrate resistant cases regarding ovulation and pregnancy rates with no effect on endometrial thickness.
Keywords
Letrozole, Ovulation induction, Clomiphene citrate resistance, Gonadotropins, Ovulation rate, Pregnancy rate
Introduction
Clomiphene Citrate (CC) has been used as first line ovulation induction agent for 40 years ago [1,2]. It is selective estrogen receptor modulator that stimulates endogenous FSH production and secretion by interrupting estrogen feedback to the hypothalamus and pituitary [3,4].
If ovulation not achieved by conventional dose (50 mg), dose is increased by 50 mg per day till we reach maximum dose (150 mg) [5]. Common indication of an ovulatory response are biphasic pattern on basal body temperature charting and serum progesterone measurement in luteal phase of more than 10 nmol/L if tested 6-8 days before the onset of menses [6]. Some cases don't respond to CC maximal dose for 3 cycles which is considered CCR. Clomiphene citrate resistance is seen in approximately 15-40% in women with PCOS [7].
Many studies investigated CCR by N-acetyl cysteine [8], adding prednisolone [9] or the use of letrozole and gonadotropins [10] to manage CCR. Letrozole is an aromatase inhibitor and is considered superior to CC regarding ovulation (62%) and pregnancy rates (14.7%) in CCR. Letrozole has many advantages over CC being rapidly eliminated from circulation (short half-life), induces monofollicular growth, lower preovulatory estradiol (E2) level without effect on endometrial thickness or endometrial receptivity nor cervical mucus [11].
Injectable gonadotropins are very expensive and require frequent monitoring with ultrasound assessment to minimize the risks from excessive follicular growth and development, other drawbacks of gonadotropins are cost, multiple pregnancy and ovarian hypersimulation [3].
The current study is conducted to compare between letrozole and gonadotropin in induction of ovulation and pregnancy rates in clomiphene citrate resistant cases.
Subjects and Method
Study setting and duration
This randomized clinical trial has been carried out at Gynecology and Obstetrics Department, Tanta University during the period from March 1, 2019 until October 31, 2019.
Patients
This study included 70 patients enrolled according inclusion and exclusion criteria. The inclusion criteria were (a) Infertility lasting one year or more in presence of regular intercourse, (b) Patients' age between 20-35years (c) Normal semen analysis according to WHO 2010 (d) Patent fallopian tubes (e) Normal prolactin and TSH. (e) Anovulatory cycles confirmed by mid-luteal progesterone ≤ 3 ng/ml and (f) Clomiphene citrate resistance for 3 cycles of 150 mg. Exclusion Criteria were (a) Any hormonal disturbances eg. hyperprolactinemia, (b) Immunological causes of infertility, (c) Coital errors, (d) Metabolic disorders and (e) Poor patient compliance.
Sample size calculation
The minimum sample size calculated using Epi info program version 7 for unmatched case control study; 80% power and 95% Confidence interval was 32 for each group. For better accuracy and validity of results the sample size was increased by 10%= 3 in each group. The total number of cases was 70 and 35 for each group.
Randomization and allocation
It was a prospective quesi-randomization. Based on the attendance order, patients with odd numbers were considered group I (Letrozole group) and those with even numbers were considered group II (Gonadotropin group). Allocation was equal 1:1.
Intervention
Group I (Letrozole group n = 35): These patients were treated with letrozole (Femara®, Novartis New York, NY, USA) in a dose 2.5 mg (one tablet daily) orally began on the 3rd day to the 7th day of the cycle. If ovulation is not achieved dose is increased by 2.5 mg in next cycle till 3 cycles. If hyperstimulation occurred, the cycle was cancelled and the patients were excluded from the study.
Group II (Gonadotropin group n = 35): These patients were given urofollitropin (fostimon®, IBSA, Lugano, Switzerland) in a dose of 75 IU/mL I.M from day 3rd to day 7th of the cycle beginning by one ampoule per day and the dose had been modulated according to response. If hyperstimulation occurred, the cycle was cancelled and the patients were excluded from the study.
Methods
Proper history taking including age, gravidity, parity, infertility duration, history of laparoscopic surgery, previous induction of ovulation in the last 6 months. Examination was done to assess weight, height (BMI), signs of androgen excess, thyroid gland and breast examination.
Serum FSH, LH, testosterone level, prolactin level and thyroid stimulating hormone on day 3 of spontaneous menstrual cycle were done. Serum Progesterone (P4) was done on day 22 (midluteal) to confirm ovulation if ≥ 10 ng/ml.
Basal transvaginal U/S on day 3 of the cycle to detect criteria of PCOS and count number of antral follicles in both ovaries and to exclude of any basal ovarian cyst. Transvaginal ultrasound was also used to follow follicular growth and endometrial thickness starting on day 8 and then every other day till assuming good ovulatory response when one or more follicles is ≥ 18 mm and endometrial thickness ≥ 6 mm.
Human Chorionic Gonadotropin (choriomon®, IBSA, Lugano, Switzerland) 10.000IU/I.M was administered when good ovulatory response was assumed. The intercourse was advised on the day of HCG injection and every other day for 4 days after injection HCG.
Number and size of Dominant follicles, endometrial thickness were recorded. Clinical pregnancy was detected by serum pregnancy test and by presence of intrauterine gestational sac with fetal pulsation.
Outcome measures
Primary outcomes was ovulation rate. Secondary outcomes were pregnancy rate.
Trial registration and ethical issues
Before starting the study, an approval from The Ethical Committee of Faculty of Medicine, Tanta university was obtained (IRB 32332/05/19).The trial was also registered on clinicaltrials.gov with the following code: NCT04834791 and is available on the following link: https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S000AUZ2&selectaction=Edit&uid=U000404W&ts=19&cx=-w6xabr
Explanation of the procedure to all women participating in the study. A written consent was taken from all patients before starting the study with counseling about risk and benefit of treatment.
Data management and statistical analysis
Data entry, processing and statistical analysis was carried out using MedCalc version 18.2.1 (MedCalc, Ostend, Belgium). Tests of significance (Kruskal-Wallis, Wilcoxon's, Chi square, logistic regression analysis, and Spearman's correlation) were used. Data were presented and suitable analysis was done according to the type of data (parametric and non-parametric) obtained for each variable. P-values less than 0.05 (5%) was statistically significant. Descriptive statistics including Mean, Standard Deviation (± SD) and range for parametric numerical data, while Median and Inter-Quartile Range (IQR) for non-parametric numerical data. Frequency and percentage of non-numerical data. Analytical statistics include Kruskal-Wallis test was used to assess the statistical significance of the difference of a non-parametric variable between more than two study groups.
Results
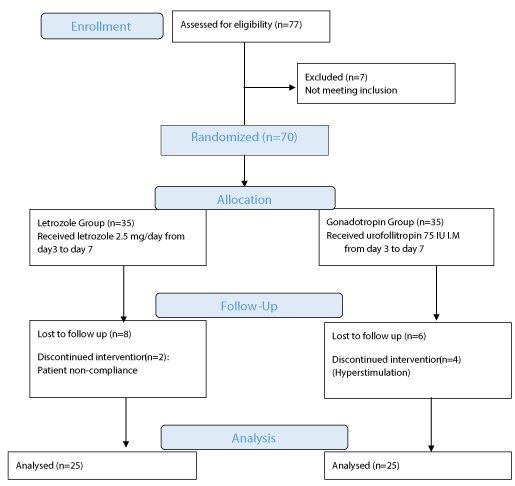
Seventy seven cases were assessed for eligibility from outpatient clinic of Tanta University. Seven cases were excluded with 70 cases randomized into 2 groups; 35 patients in each group as shown in CONSORT flow chart figure 1.
Demographic data of enrolled patients were displayed in table 1 where patients in both groups were matched regarding age, gravidity and parity. BMI was nearly similar in both groups with mean BMI 26.04 ± 4.33 in letrozole group compared to 27.01 ± 4.59 with no significant difference between both groups. Duration of infertility, basal hormonal profile were not significantly different in both groups.
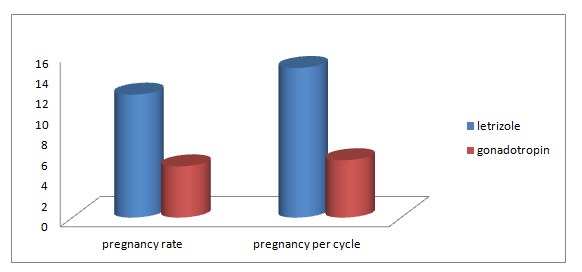
The results of stimulation were shown in table 2 where the number of mature follicles developed in letrozole group were 1.9 ± 0.3 compared to 1.2 ± 0.4 in gonadotropin group with significant p.value = < 0.001. Similarly endometrial thickness was significantly different in both groups with 8.93 ± 2.3 mm in letrozole group compared to 5.60 ± 1.63 mm in gonadotropin group, p.value = < 0.001. Pregnancy rate was shown in figure 2, where the rate was 48.0% in letrozole group and 20.0% in gonadotropin group with significant difference, p.value = 0.036.
Discussion
There are few limited adjunctive therapies on management of Clomiphene Citrate Resistance (CCR) that can be tried before jumping to laparoscopic ovarian drilling or IVF/ICS. These therapies include bromocriptine (if hyperpolactinaemia is present), Metformin (for insulin resistance), oral contraceptives (to suppress LH), low dose gonadotropins (to enhance ovulation) and extended doses of CC. However, their efficacy is limited to and many patients do not respond to each drug alone [12].
In the last few years, different studies approved that aromatase inhibitors (letrozole) is a good, effective and safe treatment modality in infertility and ovulation induction in PCOS. Moreover, use of letrozole has been recommended for patients who ovulate with clomiphene but have a thin endometrium [13].
In the present study there is no significant difference between the studied groups regarding demographic data and baseline biochemical data. These are similar to Harira, et al. [14] who reported no significant statistical difference between both groups, regarding their demographic criteria including age, BMI, duration and type of infertility, clinical (menstrual pattern) and laboratory variables.
In the current study, the number of mature follicles ≥ 18 mm was significantly higher in letrozole group. The mean number of dominant follicles (≥ 18 mm) in the letrozole and gonadotropin Group was statistically different (Gonadotropin group 1.1 ± 0.4 vs. letrozole 1.9 ± 0.3, P < 0.001).
These findings are supported by Yerushalmi, et al. [15] who reported that the mean number of mature follicles ≥ 18 mm on the day of HCG administration was 1.21 (range 1-2) where 19 patients (79%) developed one mature follicle and 5 patients (21%) developed 2 mature follicles. This limited number of mature follicles (range 1-2) with Letrozole will decrease the risk of multiple pregnancy and Ovarian Hyper Stimulation Syndrome (OHSS). Therefore, Letrozole treatment does not need intensive monitoring compared to CC and gonadotropins.
In this study, the mean mid-cycle trilaminar layer of endometrial thickness in the letrozole group was 8.93 ± 2.38 mm compared to 5.6 ± 1.63 mm in gonadotropin group that was statistically significant (P < 0.001).
In contrary to our results study of Xi [11] as they found that the number of developing follicles (≥14 mm follicles) and cycle cancellation rate due to ovarian hyper response were lowest in the letrozole + HMG groups, but the difference was not significant. The ovulation rate was highest in letrozole + HMG group, but the difference was not significant. No statistically significant differences were found for the endometrial thickness on the day of HCG administration and for the cancellation rate due to no response.
In the current study, there was highly significant number of pregnancies as evidenced by positive pregnancy test and intrauterine gestational sacs in letrozole group more than gonadotropin group (12 cases or 48% versus 5 cases or 20% respectively). The difference between letrozole group & gonadotropin group was statistically significant, also rate of pregnancy per cycle was (14.6% versus 5.6%) the difference was also statistically significant.
Our results are supported by study of Ganesh, et al. [16] who reported that the pregnancy rate in Group A was found to be significantly higher as compared to Group B (p < 0.0001) where group A received letrozole, and group B received CC with two doses RFSH). This is in good agreement with reports of other studies which conclude that letrozole has a better ovulation and pregnancy rate as compared to CC in patients with PCOS. An ovulation rate of 54.6% and pregnancy rate of 25% with letrozole induction in CC-resistant women with PCOS was reported by Harira [14]. They observed the ovulation rate to be 79.3% and the pregnancy rate 23.39% with letrozole.
Baysoy [17] has reported a similar observation like ours where the clinical pregnancy rate per cycle in the letrozole and HMG group was found to be comparable. There are, however, significantly higher pregnancy rates reported by Dahan [18] with gonadotropin (HMG/FSH) stimulation than IUI cycles stimulated with letrozole.
Nevertheless, it is suggested that ovulation induction with letrozole is associated with a satisfactory pregnancy rate as it is more cost-effective, simple and convenient to patients when compared to gonadotropins.
A study of Mousavi [19] found that the decreased risk of multiple gestation associated with letrozole ovulation induction when compared to treatment with gonadotropins would also have decreased societal and hospital costs during the infant's first 5 years. Because letrozole is taken orally, requires less monitoring than gonadotropins, and has a low rate of multiple gestation, it may represent a reasonable alternative to CC. However, true CC failures should be counseled as to the significantly higher pregnancy rates noted with gonadotropins when compared to letrozole [19].
Conclusion
Letrozole has higher effectiveness, lower adverse effects and low cost, Letrozole can be used instead of gonadotropins in patients resistant to clomiphene. Moreover, Letrozole induces mono-follicular development; so, the risk of multiple pregnancies is reduced. In addition, it is rapidly eliminated from the body; thus, lower risk of fetal anomaly. Further studies on large geographical scale and on larger sample size to emphasize our conclusion.
Acknowledgment
None.
Conflicts of Interest
None.
References
- Kafy S, Tulandi T (2007) New advances in ovulation induction. Current Opinion in Obstetrics and Gynecology. Curr Opin Obstet Gynecol 19: 248-252.?
- Roy KK, Baruah J, Singla S, et al. (2012) A prospective randomized trial comparing the efficacy of Letrozole and Clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. J Hum Reprod Sci 5: 20-25.
- Vause TDR, Cheung AP, Sierra S, et al. (2010) Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can 32: 495-502. ?
- Wu CH, Winkel CA (1989) The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril 52: 564-568.
- Practice Committee of the American Society for Reproductive Medicine (2013) Use of clomiphene citrate in infertile women: A committee opinion. Fertil Steril 100: 341-348.?
- Kim MJ, Byeon JY, Kim YH, et al. (2018) Effect of the CYP2D6* 10 allele on the pharmacokinetics of clomiphene and its active metabolites. Arch Pharm Res 41: 347-353.?
- Brown J, Farquhar C, Beck J, et al. (2009) Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst Rev 7: CD002249.
- Millea PJ (2009) N-acetylcysteine: Multiple clinical applications. Am Fam Physician 80: 265-269.
- Al-Shaikh SF, Fadhil I (2010) Prednisolone with clomiphene citrate in ovulation induction in clomiphene citrateresistant polycystic ovary syndrome. Med J Babylon 7: 571-578.
- Harira MM (2018) Combined Letrozole and Clomiphene versus Letrozole with low dose gonadotropin protocol for ovulation induction in infertile clomiphene-resistant women with polycystic ovary syndrome: Comparative study. Evidence Based Women's Health Journal 8: 266-272.
- Xi W, Liu S, Mao H, et al. (2015) Use of letrozole and clomiphene citrate combined with gonadotropins in clomiphene-resistant infertile women with polycystic ovary syndrome: a prospective study. Drug Des Devel Ther 9: 6001-6008.
- Elnashar A, Abdelmageed E, Fayed M, et al. (2006) Clomiphene citrate and dexamethazone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Hum Reprod 21: 1805-1808.
- Samara N, Casper RF (2018) Aromatase inhibitors. In: Infertility in Women with Polycystic Ovary Syndrome, 119-133.
- Harira MM (2018) Combined Letrozole and Clomiphene versus Letrozole with low dose gonadotropin protocol for ovulation induction in infertile clomiphene-resistant women with polycystic ovary syndrome: Comparative study. Journal of Evidence-Based Women's Health 8: 266-272.
- Yerushalmi G, Hourvitz A (2012) Using the IVM/IVF model for characterization and validation of the human ovulatory cascade. Gynecological, 91.
- Ganesh A, Goswami SK, Chattopadhyay R, et al. (2009) Comparison of letrozole with continuous gonadotropins and clomiphene-gonadotropin combination for ovulation induction in 1387 PCOS women after clomiphene citrate failure: a randomized prospective clinical trial. J Assist Reprod Genet 26: 19-24.?
- Baysoy A, Serdaroglu H, Jamal H, et al. (2006) Letrozole versus human menopausal gonadotrophin in women undergoing intrauterine insemination. Reprod Biomed Online 13: 208-212.
- Dahan MH, Quintero RB, Urban R (2008) A comparison of letrozole to gonadotropins for ovulation induction in subjects with advanced maternal age: a retrospective pilot study. Fertil Steril 90: 1226-1228.
- Mousavi SA, Masoumi SZ, Keramat A, et al. (2013) Assessment of questionnaires measuring quality of life in infertile couples: A systematic review. J Reprod Infertil 14: 110-119.
Corresponding Author
Ayman Shehata Dawood, Assistant Professor, Department of Obstetrics and Gynecology, Faculty of Medicine, Tanta University, Tanta, Egypt, Postal Code: 31111; Tel: +2010-2097-2067.
Copyright
© 2021 Dawood AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.