Can We Safely Stop Administering Anti-D Immune Globulin to First Trimester Rh-Negative Mothers Undergoing Abortion? A Systematic Review and Meta-Analysis
Abstract
Introduction: Fetal red blood cells can express the D-antigen as early as 52 days after the last menstrual period. However, there is no convincing evidence for the benefit of using anti-D immune globulin (RhIG) in the first trimester. The aim of the current review is therefore to determine whether administration of RhIG to women undergoing first trimester spontaneous or induced abortions prevents subsequent sensitization.
Methods: We searched Medline, Embase.com, Cochrane Central Register of Controlled Trials (CCRCT) via EBM Reviews (Ovid), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). Two reviewers performed the study selection, critical appraisal, and data extraction independently. Quality of evidence for major outcomes was assessed using the Grading of Recommendations, Assessment, development, and evaluation (GRADE).
Results: Four studies met the inclusion criteria with total of 444 participants. The fixed effect meta-analysis showed that there is 0.41 times less antibody formation in women who received RhIG compared to those who did not receive (OR = 0.41, CI, 0.05-3.66, P-value 0.43). Women who took anti-D were also 0.75 times less likely to be sensitized in subsequent pregnancy compared to women who received RhIG (OR = 0.75, CI 0.07-8.42, P-value 0.82). However, none of the studies reported on Increased fetal surveillance or fetal transfusion in subsequent pregnancy, neonatal morbidity, maternal adverse event and cost of treatment.
Conclusions: The evidence of RhIG provision following first trimester abortion to prevent antibody formation and sensitization in a subsequent pregnancy is statistically insignificant and very low quality. The practice of administering RhIG after first trimester abortion is based on expert opinion and largely extrapolated from fetomaternal hemorrhage in late pregnancy. However, the evidence indicates that the fetomataernal hemorrhage in the first trimester is not enough to cause sensitization. Therefore, the recommendation to administer RhIG in the first trimester should depend on effectiveness, resource availability, cost, values, preferences, equity, acceptability and feasibility of RhIG provision.
Keywords
Abortion, Rhogam, Rhig, First trimester, Early pregnancy, Rh sensitization
Background
There is no convincing evidence for the benefit of using anti-D immune globulin (RhIG) in the first trimester [1]. The World Health Organization (WHO) recommends that the determination of Rh status and the offer of RhIG prophylaxis are not considered prerequisites for early first trimester (≤ 63 days) medical abortion [2]. According to the North America Abortion Federation (NAF), it is reasonable to forgo Rh testing and RhIG for women having any type of abortion before 8 weeks from the last menstrual period [3]. American College of Obstetrics and Gynecology (ACOG), Royal College of Obstetrics and Gynecology (RCOG) and Society of Family Planning (SFP) consider Rh testing as a standard of care and RhIG should be administered if indicated to all non-sensitized RhD-negative women within 72 hours following abortion [4-6]. The earlier arguments in favor of RhIG administration at earlier gestational ages comes from fetomaternal hemorrhage studies [7,8]. But the presence of fetal RBCs in the maternal circulation does not necessarily equate with subsequent sensitization [9,10] and the fact that most fetal cells are in the placenta, it is reasonable to conclude the numbers of fetal cells available to enter maternal circulation in the first trimester are inadequate to cause sensitization. Hollenbach et al also described a cohort of patients receiving a surgical abortion between 6 to 22 weeks. Two-thirds of the subjects demonstrated fetal cells in the maternal circulation after instrumentation [11], but the majority also demonstrated this prior to the abortion procedure, raising the possibility of insensible cell exchange resulting in the presence of fetal cells in the circulation. Visscher RD and Visscher HC conducted a randomized controlled study to determine Rh-sensitization with or without RhID [12]. This study found zero chance of sensitization with or without administration of RhIG in subjects having abortions at early gestations.
Given these evolving data, many countries have stopped providing RhIG after first trimester abortion. For example, in the Netherlands, the policy for over 20 years has been not to administer RHIG for Rh-negative women having spontaneous abortions under 10 weeks' gestation or surgical or medical induced abortions under 7 weeks' gestation [13]. In the United Kingdom, the 2012 National Institute for Health and Care Excellence (NICE) guidelines recommended not giving RhIG for spontaneous or induced abortion at any gestational age in the first trimester [14]. In contrast, the standard in Canada is universal provision of RhIG for all Rh-negative abortion cases [15]. A study comparing the risk of sensitization in the Netherlands, where RhIG is not routinely given, to that in Canada, with its policy of universal provision, found a similar prevalence of clinically significant perinatal antibodies among women in both countries [16].
Previous systematic reviews conducted in 2001, 2006 and 2013 reported that there is no evidence that administration of RhIG prevents Rh-sensitization [1,17,18], hence the evidence is based on expert opinion and extrapolation of fetomaternal hemorrhage late in pregnancy. A preliminary search of PROSPERO, MEDLINE, the Cochrane Database of Systematic Reviews, and the JBI Database of Systematic Reviews and Implementation Reports was conducted and no current or on-going systematic reviews on the topic were identified. The aim of this review is therefore to determine whether administration of RhIG administration to women undergoing first trimester abortions prevents subsequent sensitization. Abortion in this review refers to both induced and spontaneous abortion.
Review Objectives
The review sought to determine whether administration of RhIG to women undergoing first trimester abortions prevents subsequent sensitization.
Methods
This systematic review was prepared using PRISMA reporting guidelines (Supplementary Table 1) for systematic reviews [19]. The review was conducted per the Cochrane handbook for a systematic review of interventions [20]. During the conduct of the review, we considered the following inclusion criteria:
Participants
Unsensitized Rh negative individuals seeking abortion ≤ 12 weeks (undergoing either medical or surgical abortion).
Intervention
Routine administration of RhIG.
Comparison
No routine administration of RhIG.
Outcomes
Primary outcomes considered were:
• Rate of iso-immunization in subsequent pregnancy.
• Rate of antibody formation detected six-month after initial pregnancy by indirect combs test.
Secondary outcomes considered were:
• The need for increased fetal surveillance or fetal transfusion in subsequent pregnancy.
• Neonatal morbidity characterized by jaundice, neonatal anemia, erythroblastosis or bilirubin encephalopathy in subsequent pregnancies.
• Maternal adverse events of anti-D administration including anaphylaxis.
• Cost of treatment.
Types of studies
This review considered randomized controlled trials (RCTs) and comparative observational studies published without restricting language and date of publication.
Search strategy
The search strategy developed for Medline, Embase.com, Cochrane Central Register of Controlled Trials (CCRCT) via EBM Reviews (Ovid), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en) was used to locate studies. The reference list of all studies selected for critical appraisal was screened for additional studies. Text words and Mesh terms were used to develop search strategy. We used the following search strategy for Medline: "rh sensitisation" [tw] OR "rh isoimmunization" [MeSH Terms] OR ("rh" [tw] AND "isoimmunization" [tw]) AND "abortion, induced "[MeSH Terms] OR ("abortion" [tw] AND "induced" [tw]) OR "induced abortion" [tw] AND ("pregnancy trimester, first" [MeSH Terms] OR ("pregnancy" [tw] AND "trimester" [tw] AND "first" [tw]) OR "first pregnancy trimester" [tw] AND "anti d" [tw]) OR ("rho(d) immune globulin" [MeSH Terms] OR ("rho(d)" [tw] AND "immune" [tw] AND "globulin" [tw]) OR "rho(d) immune globulin" [tw] OR "rhogam" [tw]). We searched data basis on August 25/2020.
Study selection
Following the search, all identified citations were collated and uploaded into Covidence [21] and duplicates were removed. Titles and abstracts were then screened by two independent reviewers [LT, TT] for assessment against the inclusion criteria for the review. The full text of selected citations was assessed in detail against the inclusion criteria by two independent reviewers. All disagreements that arose were resolved through discussion Potentially relevant studies were retrieved, and their citation details were imported into the Rev man 5.3.
Assessment of methodological quality
The methodological quality of the eligible studies was assessed by two independent reviewers using the guidelines in the Cochrane reviewers’ handbook [20]. All disagreements that arose were resolved through discussion. All studies regardless of the results of their methodological quality underwent data extraction, and the results of critical appraisal were reported in narrative form and tables.
Data extraction and synthesis
Data were extracted into Rev Man version 5.3 for analysis. The relevant information such as population characteristics, authors, study setting, study design, publication year, interventions, and summary of the findings was extracted. Studies were pooled in a statistical meta-analysis using Rev Man version 5.3. Effect sizes were expressed as odds ratios (for dichotomous data), and their 95% confidence intervals were calculated for analysis. Heterogeneity was assessed statistically using the Tau2 and I2 tests. I2 tests above 50% were considered as indicative of significant heterogeneity. Additionally, the heterogeneity among studies was assessed in terms of the type of abortion (i.e induced or spontaneous and medical or surgical abortion). We did not conduct any sensitivity or subgroup analysis because of the limited number of studies included in the analysis. We also used a fixed-effects model for the meta-analysis because of the small number of included studies [22]. In future updates with additional studies, we will do a sensitivity analysis and subgroup analysis in terms of clinical types of abortion and medical versus surgical methods used for evacuation.
The quality of evidence was assessed using a software package developed by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) [23].
Results
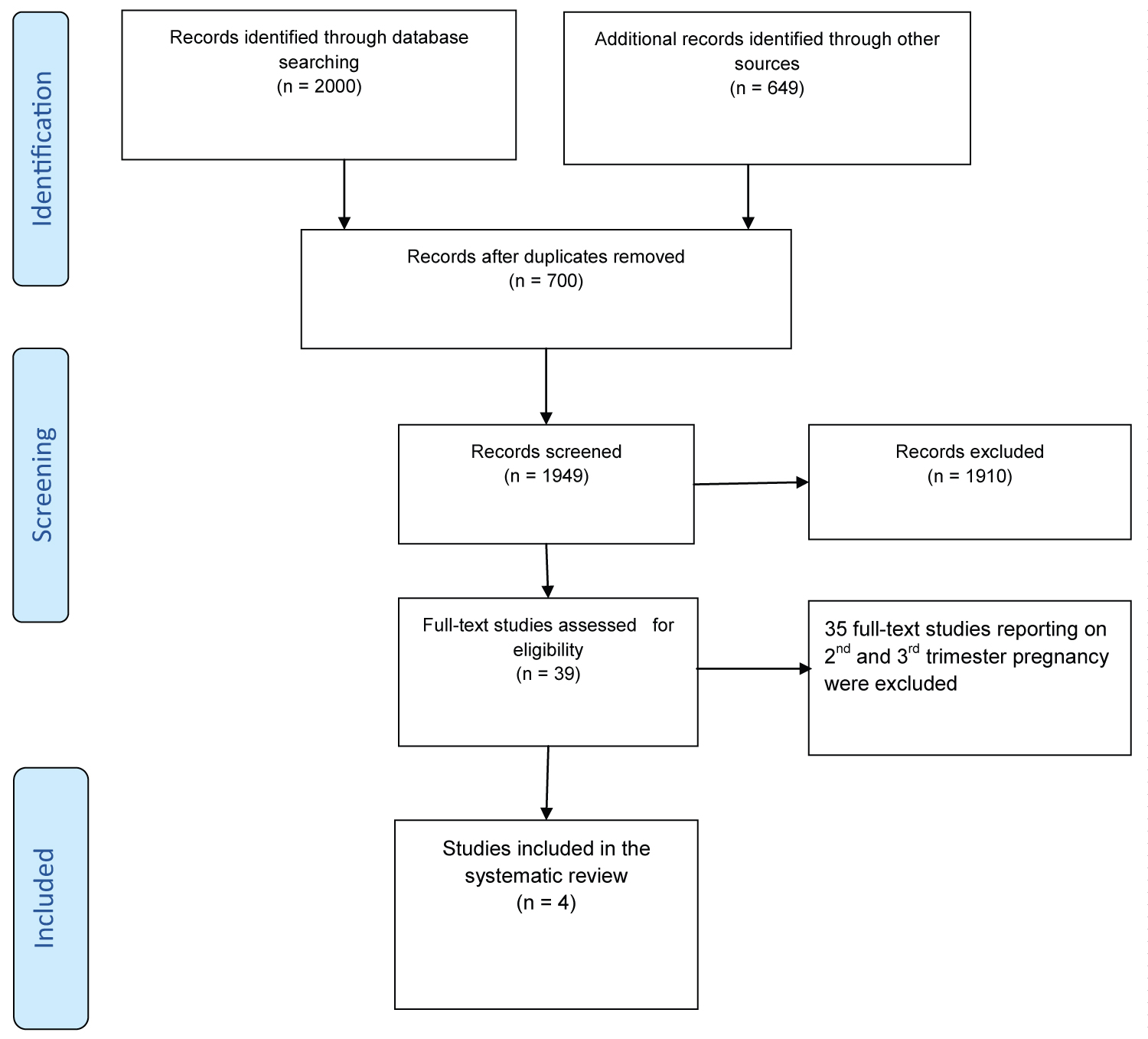
The search yielded a total of 2649 records. After removing duplicates, 427 documents were retained for further examination. After screening the titles and abstracts, 79 papers were retained for full-text review. Based on pre-defined inclusion criteria, four studies were included for the critical appraisal (Figure 1).
Characteristics of included studies
Four studies fulfilled the inclusion criteria and were included in the current review [12,24-26]. The Viscer, et al. study was a double-blind, placebo-controlled study in 8 to 24 week pregnancies conducted in the USA [12]. This study was included because most participants were between 8-16 weeks’ gestation, which includes our focus of ≤ 12 weeks. The Simonovits, et al. is a prospective observational study of Rh-negative women having first trimester abortions conducted in Hungary [24]. Gavin, et al. and Goldman, et al. were non-randomized clinical trials conducted in the USA and Israel, respectively [25,26]. Both studies reported on first and second trimester abortion, but for the current review, we extracted data for ≤ 12 weeks’ gestation abortion only. All studies compared the RhIG administration to either placebo or no administration of RhIG. Three of the studies reported on the outcome of antibody development 4-6 month after the antecedent abortion [12,25,26]. Two studies reported on the outcome of isoimmunization in a subsequent pregnancy [12,24]. No single studies reported on secondary outcomes of interest for the current review. See Table 1, detailed characteristics of the included studies are included as supporting information at the end of the manuscript.
The methodological quality of the included studies
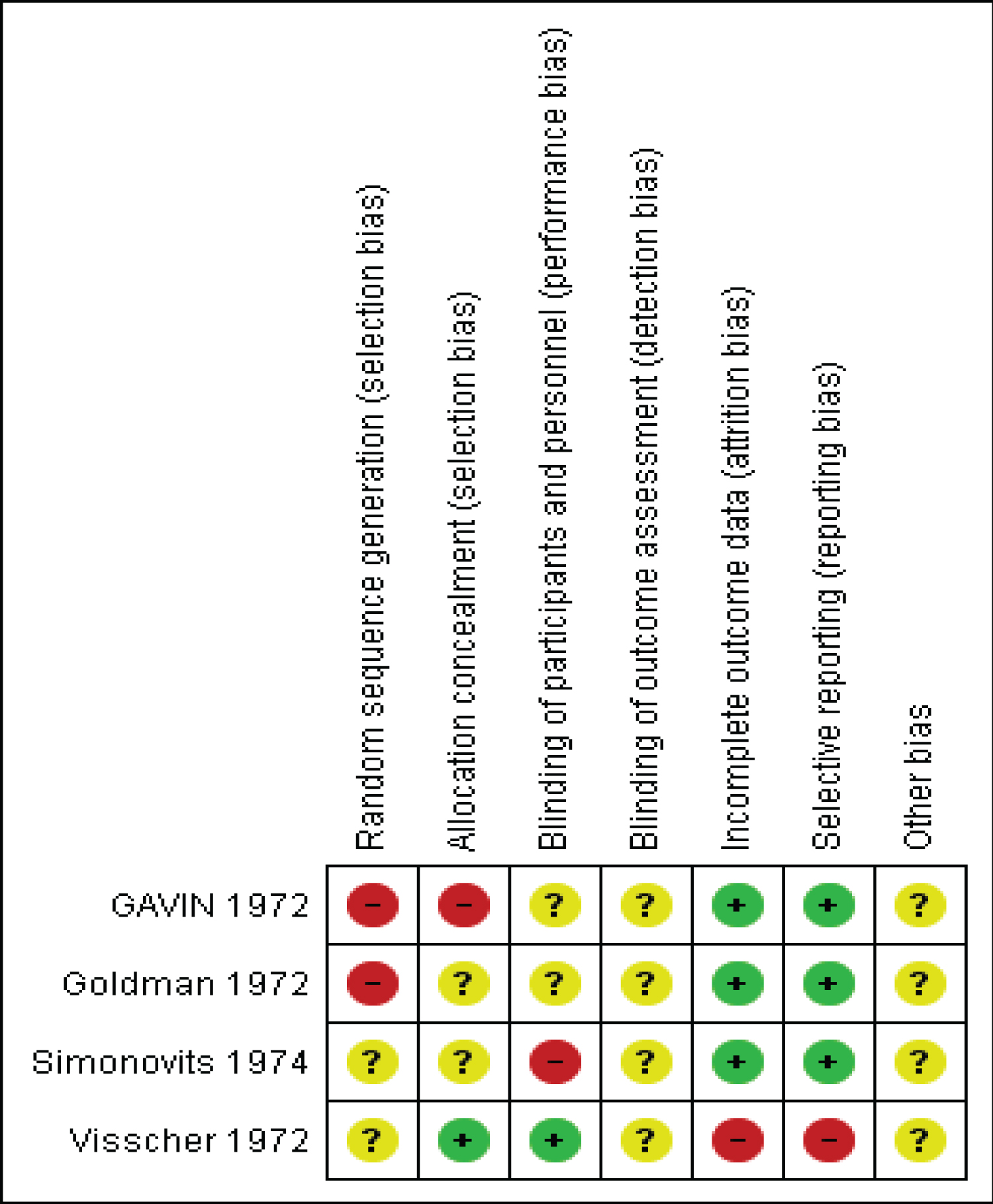
All four studies were at higher risk of bias as they were not randomized or the method of randomization was not reported, unclear or unreported, treatment allocation was not concealed, confounders not controlled and there was unclear or un-reported blinding of participants and outcome assessment (Figure 2).
Review findings
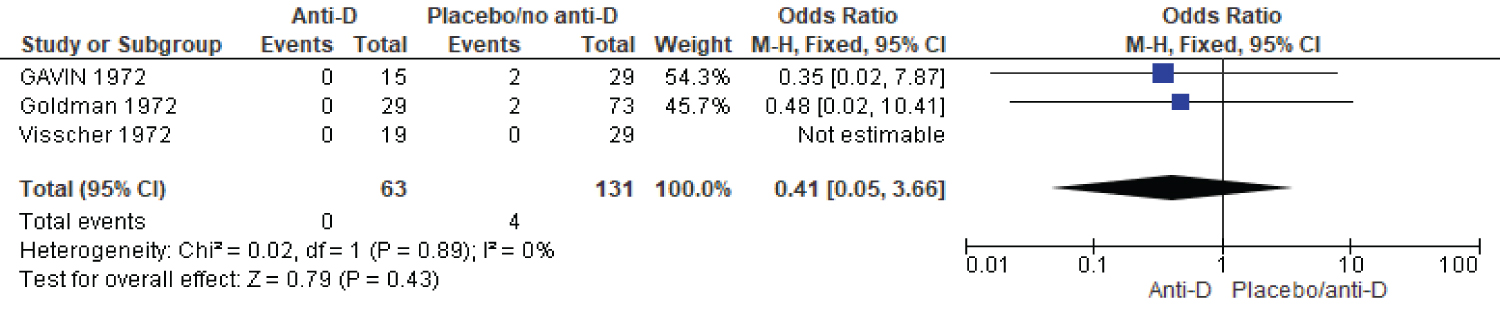
Three studies reported on subsequent antibody development as an indicator of sensitization. The fixed effect meta-analysis showed that there is 0.41 times less anti-body formation in women who received antibody compared to those who did not receive (three studies, 194 participants, OR = 0.41, CI, 0.05-3.66, P-value 0.43) (Figure 3).
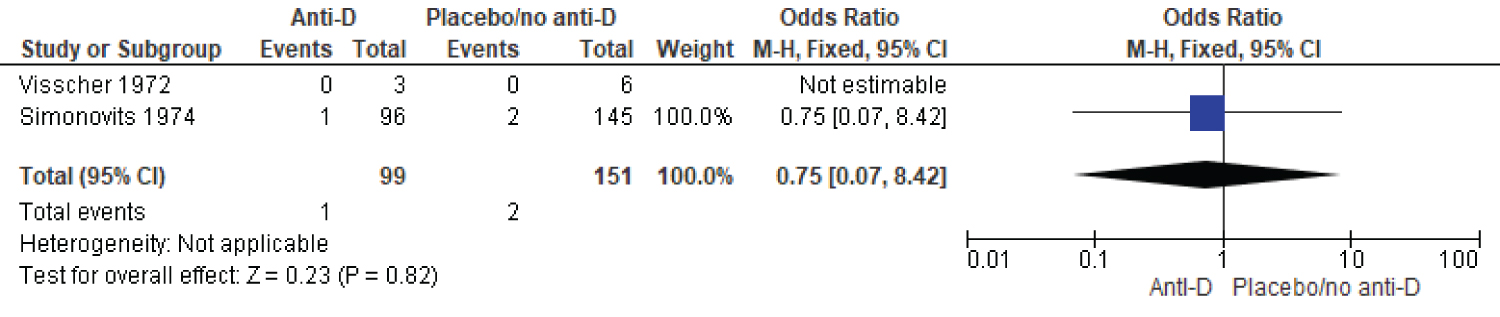
Two studies reported on iso-immunization in subsequent pregnancy. The fixed effect meta-analysis showed women who took RhIG were 0.75 times less likely to be sensitized in subsequent pregnancy compared to women who received RhIG (two studies, 250 participants, OR = 0.75, CI 0.07-8.42, P-value 0.82) (Figure 4).
Grading was done using online Gradepro. The two outcomes iso-immunization in subsequent pregnancy and subsequent antibody formation were assigned very low quality of evidence (Table 2).
Discussion
The current review looked at studies reporting on whether RhIG administration prevents or reduces the risk of sensitization among Rh negative women undergoing first trimester abortion. We identified four studies fulfilling inclusion criteria, all published between 1972-74. All four studies were at higher risk of bias as they were not randomized or the method of randomization was not reported, unclear or unreported, treatment allocation was concealed, and there was unclear or unreported blinding of participants and outcome assessment.
There is no statistically significant evidence of less antibody formation 4-6 months after an antecedent abortion in Rh negative women who took RhIG compared to those who did not. Similarly, there is no statistically significant decrease in sensitization in subsequent pregnancies in women who did or did not receive RhIG after an index first trimester abortion. The overall quality of these data is very low, and the confidence intervals were wide and crossed the line of no effect.
The current review of the literature search has been comprehensive, though the studies are limited and old. None of the studies reported on the cost of treatments or side effects, reducing the provision of contextually appropriate recommendations.
Conclusions
Implications for practice
The evidence of RhIG provision following first trimester abortion to prevent antibody formation and sensitization in subsequent pregnancy is statistically insignificant and very low quality. The practice of administering RhIG after first trimester abortion is based on expert opinion and is largely extrapolated from data on fetomaternal hemorrhage in late pregnancy. However, the evidence indicates that there is not adequate fetomaternal hemorrhage in first trimester to cause sensitization [9-11,27]. Therefore, the recommendation to administer RhIG in the first trimester should depend on resource availability, cost, values, preferences, equity, acceptability and feasibility.
Implications for research
The evidence about the impact of RhIG following first trimester abortion on subsequent sensitization are old, very limited and of very low quality. Consequently, we recommend well designed randomized control trial and the generation of quality evidence to inform current practice.
Acknowledgments
N/A.
Funding
This systematic review project did not get any funding support from any organization.
Conflicts of Interest
The authors declare no conflict of interest in this review.
Authors Contribution
Conceptualization
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Data curation
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Formal analysis
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Funding acquisition
NA.
Investigation
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Methodology
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Project administration
Lemi Belay Tolu. Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Resources
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Software
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Supervision
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
Validation
Lemi Belay Tolu, Tesfaye H. Tufa, Ferid A. Abubeker, Mekdes Daba, Sarah Prager.
References
- Jabara S, Barnhart KT (2003) Is Rh immune globulin needed in early first-trimester abortion? A review. Am J Obstet Gynecol 188: 623-627.
- WHO (2012) Safe abortion: Technical and policy guidance for health systems.
- Mark A, Foster AM, Grossman D, et al. (2019) Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: A recommendation from the National Abortion Federation's Clinical Policies Committee. Contraception 99: 265-266.
- https://www.rcog.org.uk/globalassets/documents/guidelines/abortion-guideline_web_1.pdf
- (2014) Practice bulletin no. 143: Medical management of first-trimester abortion. Obstet Gynecol 123: 676-692.
- Committee on Practice Bulletins – Gynecology, The Society of Family Planning (2020) Medication abortion up to 70 days of gestation.
- Leong M, Duby S, Kinch RA (1979) Fetal-maternal transfusion following early abortion. Obstet Gynecol 54: 424-426.
- Bergström H, Nilsson LA, Nilsson L, et al. (1967) Demonstration of Rh antigens in a 38-day-old fetus. Am J Otbstet Gynecol 99: 130-133.
- Horvath S, Tsao P, Huang ZY, et al. (2020) The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception 102: 1-6.
- Fiala C, Fux M, Danielsson KG (2003) Rh-prophylaxis in early abortion. Acta Obstet Gynecol Scand 82: 892-903.
- Hollenbach SJ, Cochran M, Harrington A (2019) “Provoked” feto-maternal hemorrhage may represent insensible cell exchange in pregnancies from 6 to 22 weeks gestational age. Contraception 100: 142-146.
- Visscher RD, Visscher HC (1972) Do Rh-negative women with an early spontaneous abortion need Rh immune prophylaxis? Am J Obstet Gynecol 113: 158-165.
- Dutch Association of Abortion Specialists (2018) Guideline for the treatment of women undergoing a termination of pregnancy.
- National Institute for Health and Care Excellence (NICE) (2012) Ectopic pregnancy and miscarriage: Diagnosis and initial management.
- Fung KFK, Eason E (2018) No. 133-prevention of Rh alloimmunization. J Obstet Gynaecol Can 40: e1-e10.
- Wiebe ER, Campbell M, Aiken AR, et al. (2019) Can we safely stop testing for Rh status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception 1: 100001.
- Blaine Hannafin, Frank Lovecchio, Paul Blackburn (2006) Do Rh-negative women with first trimester spontaneous abortions need Rh immune globulin? Am J Emerg Med 24: 487-489.
- Karanth L, Jaafar SH, Kanagasabai S, et al. (2013) Anti‐D administration after spontaneous miscarriage for preventing Rhesus alloimmunisation. Cochrane Database of Syst Rev.
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: e1000097.
- Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons.
- Covidence - Better systematic review management.
- Tufanaru C, Munn Z, Stephenson M, et al. (2015) Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 13: 196-207.
- GRADEpro G (2015) GRADEpro guideline development tool [software]. McMaster University 435.
- Simonovits I, Bajtai G, Kellner R, et al. (1974) Immunization of RhO(D)-negative secundigravidae whose first pregnancy was terminated by induced abortion. Haematologia (Budap) 8: 291-298.
- Goldman JA, Eckerling B (1972) RH immunization in spontaneous abortion. Acta Eur Fertil 3: 253-254.
- Gavin PS (1972) Rhesus sensitization in abortion. Obstet Gynecol 39: 37-40.
- Davidsohn I, Masaitis L, Stern K (1956) Experimental studies on Rh immunization. Am J Clin Pathol 26: 833-843 .
- Goldman JA, Eckerling B (1972) Prevention of Rh immunization after abortion with Anti-Rh (D)-immunoglobulin. Obstet Gynecol 40: 366-370.
Corresponding Author
Lemi Belay Tolu, Saint Paul's Hospital Millennium Medical College, Addis Ababa, Ethiopia
Copyright
© 2021 Tolu LB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.