The Elusive Magical Solution: The Relationship between Vitamin D and Fibroids
Abstract
Uterine fibroids are benign tumors of the smooth muscles of the uterus that are the most common indication of hysterectomy in the America with over $34.4 billion annual cost. Risk factors include age, hypertension, diabetes, polycystic ovarian syndrome, black race, early age of menarche, nulliparity, obesity and a genetic factor comes in place.
Their pathophysiology has not been fully understood; genetic and hormonal factors, particularly sex steroids, seem to play a role. Many treatments for fibroid have been proposed both medical and surgical. However, the perfect medical treatment is still elusive.
Vitamin D has been in the spotlight recently for many of its anti-cancerous effects. It was thus reasonable to suggest it might play a role in the pathophysiology of uterine fibroids Women who had fibroids seemed to not have sufficient serum levels of calcidiol. Overall, in vivo, it reduced tumor sizes when used as a drug. 0.5 μg/kg/d was sufficient to produce these results. It seemed to reduce the level of matrix metallo proteinases 2 and 9 and decrease COMT expression.
Based on these findings, it is recommended to start clinical trials for using vitamin D in the treatment of fibroids as a safe, long-term medical therapy. Toxicity can be avoided by having short term correction of deficiency then having a maintenance dose. Both vitamin D and its analogs can be used and it has been shown to have a synergistic effect with ulipristal acetate against fibroids.
Keywords
Fibroid, Vitamin D, Bleeding
List of Abbreviations
UF: Uterine Fibroid; FIGO: International Federation of Obstetrics and Gynecology; TGIF: Transforming Growth Interacting Factor; UPA: Ulipristal Acetate; RT-PCR: Reverse-Transcription Polymerase Chain Reaction; MMP: Matrix Metalloproteinase; EMA: European Medicines Agency; UVB: Ultraviolet B; HuLM: Human Uterine Leiomyoma; CDK: Cyclin-Dependent Kinase; TIMP: Tissue Inhibitor of Metalloproteinase
Aim of Work
The aim of this literature review is to determine whether there is a relationship between fibroids and vitamin D deficiency, and if established, whether vitamin D could be used as a treatment modality for uterine fibroids.
Introduction
Leimyomas, also known as myomas or uterine fibroids (UF), are benign tumors of the smooth muscles of the uterus. UFs are the most common benign tumor in the uterus in child-bearing age [1]. They are the single most common indication of hysterectomy in the United States, and have been for almost 55 years [2]. The cost of UFs to health-care systems is difficult to measure, as many cases would go undiagnosed, but estimates have put the cost of UFs to be above $34.4 billion per year [3].
The most common presentation of UFs is asymptomatic, and are usually discovered with Ultrasound. Only one fourth to one third of patients are symptomatic. The experienced symptoms include pelvic pain, severe bleeding that can lead up to anemia as well as reproductive disturbances [1].
Several risk factors have been identified for UFs, including age, hypertension, diabetes, polycystic ovarian syndrome, black race, early age of menarche, nulliparity and obesity. There is also a genetic factor, since non-random cytogenetic abnormalities have been found in about 40% of the tumors examined by Flake, et al. [4]. It has also been well-established in several reports that UFs are hormonally mediated, with prolonged elevated estrogen and progesterone levels being identified as a definite risk factor for UFs development. First degree relatives seem to have more than twice the likelihood of developing fibroids and nearly 6 times the likelihood if the cases have an early onset. Monozygotic twins were twice as likely to both get hysterectomy when compared to dizygotic twins [5].
Regarding race as a risk factor in particular, several devoted studies have been made in this regard and have found that black race is the most important risk factor for developing fibroids. Black women have significantly more chances to develop UFs for the same age and socio-economic class, more likely to develop UFs at an earlier age, more likely to have more severe symptoms, including severe bleeding and anemia. They were also more likely to report affected quality of life with symptoms affecting physical activities, relationships and causing work absenteeism. They were also 2.4 times more likely to have hysterectomy and 6.8 times more likely to have myomectomy sparing the uterus [6-8].
Fibroids
Classification
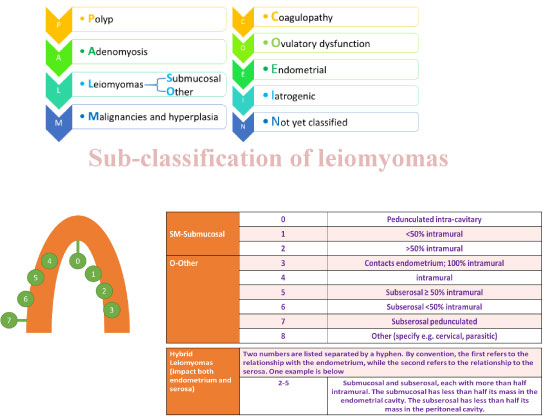
Abnormal uterine bleeding (AUB) has been researched extensively and The International Federation of Gynecology and Obstetrics (FIGO) has decided to adopt a new classification system in 2010 termed PALM COIEN for describing the causes of AUB, including fibroids. These are shown in Figure 1. This is an acronym comprising polyps, adenomyosis, leiomyomas, malignancy (and hyperplasia), Coagulopathy, Ovarian dysfunction, Endometrial, Itatrogenic and Not yet classified causes [9].
Pathophysiology
The pathophysiology of the development of UFs is not completely understood but several etiological factors seem to contribute.
There appears to be a genetic factor as 40-50% of patients show karyotypical chromosomal abnormalities [10]. Meanwhile, mutations of the MED12 have been shown in over 70% of UFs [11,12].
The generally held principle is that these genetic factors would then interact with the sex hormones. Estrogen and progesterone have repeatedly been implicated in the development of UFs. They promote cell division, upregulate growth factors and their receptors, downregulate apoptotic factors or up regulate their antagonists and interact with other hormones. Moreover, estrogen and progesterone influence each other as well as prolactin signaling which all influence the nuclear receptors for these hormones. Estrogen is thought to upregulate IGF-1, EGFR, TGF-beta1, TGF-beta3 and PDGF, while also down-regulating p53, increasing expression of the anti-apoptotic factor PCP4 and antagonizing PPAR-gamma signaling, all of which stop the natural apoptosis of tumor cells. Progesterone is believed to up-regulate EGF, TGF-beta1 and TGF-beta3. Progesterone also up-regulates Bcl-2 expression and down-regulates TNF-alpha, thus helping cancer cells to avoid apoptosis. On the other hand, Progesterone can downregulate IGF-1 and thus oppose growth. UF is characterized by an increase in expression of transforming growth interacting factor (TGIF) when compared with normal myometrium. TGIF is a potential repressor of TGF-β pathways in myometrial cells [13].
Moreover, as with the mechanism of endometriosis, Aromatase and 17 beta-hydroxysteroid dehydrogenase are abnormally more frequently expressed in fibroids, converting circulating androgens into estradiol. From this line of thinking, Aromatase inhibitors are currently being considered for treatment. The rationale is that at an optimum dose, they can completely inhibit estrogen production in the fibroid without affecting systemic estrogenic effects by estrogen from the ovaries [14,15]. In African-American women, Aromatase over expression is particularly pronounced [16,17].
UFs can expand by slow cellular proliferation, coupled with production of a large amount of extracellular matrix and angiogenesis [18,19].
Current treatments
Current forms of treatment of UFs vary from medical to surgical therapies.
Medical therapies in general constitute symptomatic relief and medical treatments targeting the UFs. Symptomatic medications include NSAIDs for reducing pain, or iron supplements to correct anemia due to blood loss. Medications targeting UFs include levonorgestrel intrauterine devices, cabergoline, ullipristal acetate, danazol, GnRH analogues, progesterone antagonists and aromatase inhibitors [20,21].
Levonorgestrel intrauterine devices releasing pregestagen locally and thus having few side effects, are originally used as a contraceptive device. Although not originally intended to be used for the treatment of fibroids, some studies such as Kailasam, et al. have reported good results with significant reduction in the size of the UFs [22,23].
Cabergoline, a dopamine D2 receptor agonist, has been shown to effectively reduce the size of the UFs as well as reduce symptoms [24]. The mechanism of action responsible for how cabergoline shrinks fibroids is unclear and it seems as though combining cabergoline with aromatase inhibitor did not make a significant change to the effects of reduction of the size of UFs [25].
Ulipristal acetate (UPA), originally used as an emergency contraception is a synthetic selective progesterone receptor modulator (SPRM). Plenty of research has supported its use for reducing the size of UFs before undergoing surgery or even alone without surgery. UPA was also reported to reduce symptoms, including bleeding, and improve quality of life [26-30]. Long-term use of UPA has shown up to 70% reduction in size of tumors and linked to increased action of matrix metaloprotinases (MMP) [31]. On the 9th of February, 2018, the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) posted an announcement that all women currently on UPA should have regular liver testing and no new patients should be started on UPA while the committee completes a review of the benefits and risks of the medication as some patients who were under the treatment for UFs using UPA have had liver injury up to failure requiring transplantation [32].
Danazol, a synthetic isoxazole derivative, has been stated as an effective treatment to reduce the volume of UFs and decrease symptoms. However, its dose-dependent side effects might be problematic. These side effects include hirsutism, acne, irreversible deepening of the voice, adverse lipid profile, weight gain, hot flashes and mood changes [20,21].
Gonadotropin-releasing hormone analogues may lead to temporary reduction in size of fibroids, thus making them useful as pre-surgical medical treatment but cannot be used alone. Their continued use beyond six months is not advised due to the risk of osteoporosis and other postmenopausal symptoms. GnRH antagonists seem to avoid the initial flare-up of the GnRH analogues but no other advantages have been observed [20,21].
Progesterone antagonists such as mifepristone have been shown in some studies to reduce symptoms due UFs but alarming histological changes may deter from their use in a clinical setting [33,34].
As a general rule, any medication that would significantly alter the metabolism of progesterone and estrogen would have a detrimental effect on reproduction and thus, its use would not be advisable [20,21].
Aromatase inhibitors were compared to GnRH agonists in a randomized controlled, clinical trial and found to be more effective in reducing the size of UFs, and this was the only trial that fit the criteria for a 2013 cochrane review. However, it has been critiqued as having been unblinded and not reporting the volume reduction in a manner that allowed odds ratio calculation. This led to the conclusion that there is not enough evidence to support the use of Aromatase inhibitors for treatment of UFs [35,36]. In 2017, Sayyah-Melli, et al. showed that aromatase inhibitors do reduce the volume of UFs and reduce symptoms and adding cabergoline made no significant difference, as stated before [25].
Therefore, each of these medical therapies has its own points against it. The ideal medical therapy for UFs would preferably be an oral, long-term tablet taken as rarely as possible which would have little to no side effects and would quickly control symptoms and reduce the size of UF and all the while, not affect fertility. Of all the medications listed above, none would fulfil these criteria and therefore, the search for the ideal medical treatment continues.
Surgical treatment might include uterine artery embolization, hysterectomy, myomectomy (which can be hysteroscopic, laporoscopic or laporotomic), endometrial ablation, radiofrequency ablation and Magnetic resonance guided focused ultrasound. Although a big debate exists as to which is the best and each of these options has advantages over others and has its own indications, they are not the main focus of this literature review and as such, will not be discussed in further detail [37-41].
Vitamin D
Metabolism
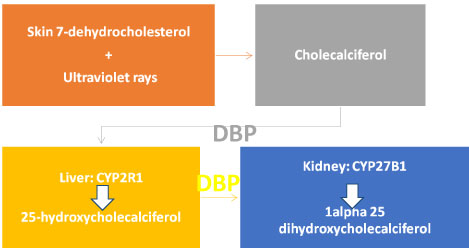
Vitamin D3 (cholecalciferol) is taken in the diet (from fortified dairy products and fish oils) or is synthesized in the skin from 7-dehydrocholesterol by ultraviolet irradiation. The vitamin D produced by 7-dehydrocholesterol depends on the intensity of UV irradiation, which varies with season and latitude, but is typically having a wavelength of 280-320 nm, with this conversion being interrupted by sunscreen and clothing [42].
Cholecalciferol is still not active biologically and must be activated to carry out its physiological functions. It is transported to the liver bound to vitamin D binding protein (DBP), which is solely responsible for transport of vitamin D and its metabolites. In the liver, vitamin D is converted to 25-hydroxyl cholecalciferol (25(OH)D3), by being hydroxylated at C-25 by one or more cytochrome P450 vitamin D 25-hydroxylases (such as CYP2R1 (the main enzyme), CYP2D11, and CYP2D25) [42]. A patient with symptoms of vitamin D deficiency, confirmed by lab was found to have a homozygous transition mutation of the CYP2R1 gene, thus suggesting that it is the main enzyme [43].
25-hydroxycholecalciferol is the major circulating form of vitamin D in the blood. After it is formed, it is transported by DBP to the kidney for the next step of hydroxylation. It enters the cells by endocytosis by binding with megalin, a low-density lipoprotein receptor. Once in the kidney, the 25(OH) Vit D3 is hydroxylated by CYP27B1 in the proximal convoluted tubule of the nephrons, to add a hydroxyl group to the first carbon atom in the α-ring Figure 2 [42,44].
Relationship with cancers
Vitamin D has been linked to several cancers in the past, with particular attention given to colorectal cancer, breast cancer and skin cancers.
For colorectal cancer, higher intake of calcium and vitamin D has been associated with a reduced risk of colorectal cancer in epidemiologic studies and polyp recurrence in polyp-prevention trials. The evidence to date suggests that daily intake of 1000-2000 IU/day of vitamin D3 could reduce the incidence of colorectal with minimal risk, as this would maintain the serum level to be above 33 ng/ml. A 50% lower risk of colorectal cancer was associated with a serum 25(OH)D level ≥ 33 ng/mL, compared to ≤ 12 ng/mL [45-49].
For breast cancer, inadequate photosynthesis or oral intake of Vitamin D are associated with high incidence and mortality rates of breast cancer in ecological and observational studies. Further studies have confirmed this with RCTs and meta-analyses [50]. Chen, et al. have found that there is a 45% reduction in the breast cancer incidence from the highest to the lowest quantile of circulating plasma levels of active 25 hydroxy-vitamin D3 in 25 studies [51].
For skin cancers, it is well established that ultraviolet B (UVB) radiation is the major causative agent of nonmelanoma skin cancer (NMSC), while simultaneously, UVB, as mentioned in the previous section, is necessary for formation of cholecalciferol from 7-dehydrocholesterol. Thus a paradox is created regarding the amount of sunlight exposure that is beneficial and whether supplementation of vitamin D might be helpful or harmful [52]. Moan, et al. have recommended that increased exposure to sunlight is overall beneficial and that vitamin D levels would help improve the prognosis of melanomas [53].
Vitamin D and Fibroid
An association in vitro
Based on these findings, given that the exact pathogenesis of fibroids is not fully understood and that hormones play an important role, and given that vitamin D plays an important role in several cancer types, it seems that Vitamin D may play an important role in the pathogenesis of UFs and might even have potential as a treatment, given that the search for a perfect medical treatment for fibroids is still on-going.
To answer this question, first were the in vitro studies that needed to show that either lower vitamin D levels were associated with increased fibroid risk or that vitamin D would have a positive effect that could potentially have an anti-fibroid action [54].
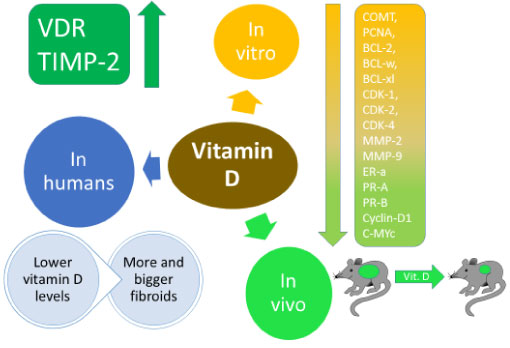
Bläuer, et al. demonstrated this in 2009, where in their biology lab, six women with fibroids undergoing hysterectomy had samples obtain for myometrial and leimyomas and the effect of vitamin D derivatives on them was measured and compared with controls. 0.1 Nm of Calcitriol (1, 25 (OH)2 D3) inhibited the growth of both myometrial and leiomyoma cells by 12% more than the control. 100 nM of calcitriol inhibited growth by 62%. At 500 nM and 1000 nM less inhibition of growth was seen with only 50%. Meanwhile proliferation was stimulated by the lower levels of vitamin D. This showed that vitamin D had the potential as both a risk factor and a treatment modality [55].
Sharan, et al. in 2011 demonstrated the possible mechanisms that vitamin D could reduce the size of the UFs, while simultaneously confirming that vitamin D could inhibit the growth of UF cells. human uterine leiomyoma (HuLM) were treated with vitamin D and the results compared with controls after 120 hours. Higher concentrations of vitamin D inhibited proliferation more. Then using Western blotting, proliferating cell nuclear antigen (PCNA), BCL-2, BCL-w, cyclin-dependent kinase (CDK) 1, and catechol-O-methyltransferase (COMT) levels were analyzed. All were down-regulated by vitamin D COMT mRNA measured by quantitative reverse-transcription polymerase chain reaction (RT-PCR) showed less expression of COMT with vitamin D treatment. The enzyme activity of COMT was also shown to be decreased. To confirm the findings, endogenous COMT expression was silenced and the effect of vitamin D disappeared. This showed that vitamin D may act mainly by inhibiting the expression and activity of COMT [56].
Furthermore, Halder, et al. have demonstrated in 2013 that matrix metalo proteinases MMP 1, 3, 9, 13 and 14 were significantly reduced in a dose dependent manner. Furthermore, pro-MMP-2, active MMP-2, pro-MMP-9 and protein levels of MMP-2 and MMP-9 were all significantly reduced. Vitamin D receptor (VDR) and Tissue inhibitor of metaloproteinase (TIMP-2) were upregulated in a dose dependent manner [57].
Brakta, et al. reviewed the literature in 2015 and confirmed these findings of ours that there is plenty of supportive evidence for in vitro studies that prove that lower vitamin D levels were significantly associated with an increased risk of vitamin D [58].
In vivo
Animal models needed to show that vitamin D would reduce the size of fibroids.
Halder, et al. demonstrated in 2010 that vitamin D in Eker rats had a highly effective anti-tumor activity in reducing the size of tumors compared with the control group given only vehicle. The results were significant. Proliferation promoting genes such as PCNA, cyclin D1, c-MYc, CDK1, CDK2 and CDK4 were suppressed. Antiapoptotic genes (BCL2 and BCL-xl), Estrogen receptor ER-a, and Progesterone receptors PR-A and PR-B were also suppressed Furthermore, rats that had inflammation, edema and fluid retention were completely resolved by the Vitamin D. Comparison between the treated group and the control group for liver and calcium toxicity showed no significant difference. This further proved that in vivo, vitamin D had the potential to be a therapeutic agent [59].
In another study by the same team, Halder, et al. in 2014 the efficacy of paricalcitol, which an analog of calcitriol, with calcitriol and a placebo on UFs. Paricalcitol has a lower hypercalcemic effect and is a potential activator of VDR. Both calcitriol and paricalcitol significantly reduced the UF size in nude mice [60].
In humans
The epidemiological studies needed to prove that women who had fibroids had less vitamin D levels, and that these results were statistically significant after removal of other known risk factors.
Baird, et al. reported this in 2013. In the 1996-1999 period, the National Institute of Environmental Health Sciences Uterine Fibroid Study enrolled randomly selected 35-49 year-old members of an urban health plan. They determined whether they had UFs or not by ultrasound scans. 620 black women and 416 white women had UFs. Radioimmunoassay of (25(OH)D) was used to determine their vitamin D levels and they were asked about their sun exposure. Associations were evaluated with logistic regression, controlling for potential confounders. The prevalence of vitamin D deficiency was high, as 90% of blacks and 50% of whites did not have levels above 20 ng/ml. Women with sufficient levels of vitamin D and had a sun exposure of more than one hour per day were less likely to get UFs. Sufficient vitamin D levels meant 32% less chance of having UFs, after controlling for other cofounders [61].
Sabry, et al. in 2013 made a cross sectional observational study where 154 women were recruited, 50 were fibroid-free and 104 had fibroids measuring at least 2 cm3 in volume calculated by the prolate ellipse formula (a × b × c × 0.523), where a is height, b is width, and c is depth. Vitamin D status was determined by radioimmunoassay of 25 (OH)D3. Lower vitamin D levels were significantly associated with having UFs, with higher levels significantly linked to smaller volumes. This seemed even more pronounced in blacks than whites. This showed yet again that vitamin D insufficiency is a risk factor for the development of UFs, and seemed to show that black race reacts more severely to vitamin D deficiency by developing larger fibroids [62].
Paffoni, et al. also showed similar findings in 2013, where 128 women with UFs and 256 controls were chosen. The controls were always within 1 year of age of the women who had the UFs. Women who had the UFs were diagnosed by transvaginal ultrasound and were selected if the fibroid had a mean diameter ≥ 10 mm. Women with UFs had significantly lower vitamin D levels than the controls, and the percentage of women with vitamin D deficiency was significantly higher in the group with UFs when compared to the control [63].
Summary
Figure 3.
Conclusion
Vitamin D deficiency is one of the causative factors of development of UFs, and vitamin D or its analogues can be used as a future safe, long-term, medical treatment of UFs. The issue facing this long-term treatment is the adjustment of the dosage because of fear of toxicity, which would cause hypercalcemia and functional hypoparathyroidism, which would adversely affect bones, leading to bone aches and fractures.
The Endocrine Society practice guidelines on the treatment of vitamin D deficiency recommend target level of serum 25 (OH)D of 30 ng/ml. This is achieved by an 8-week initial dose of 6000 IU/d for adults followed by a maintenance dose of 1500-2000 IU/d. This protocol should prevent toxicity. This therapy is very cheap compared to alternatives, with the initial 8-week regiment costing just $16 and the maintenance costing $32 annually [64].
Ali, et al. in 2017 have demonstrated that vitamin D could actually synergize the antiproliferative, apoptotic, antifibrotic and anti-inflammatory effects of ulipristal acetate against UFs [65].
The next step recommended is to carry out clinical trials to establish medical treatment guidelines with the usage of vitamin D or its analogs with or without the addition of other medical treatments to establish the best course of treatment.
References
- Walker CL, Stewart EA (2005) Uterine fibroids: The elephant in the room. Science 308: 1589-1592.
- Pokras R, Hufnagel VG (1988) Hysterectomy in the United States, 1965-84. Am J Public Health 78: 852-853.
- Cardozo ER, Clark AD, Banks NK, et al. (2012) The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 206: 211.e1-219.e1.
- Flake GP, Andersen J, Dixon D (2003) Etiology and pathogenesis of uterine leiomyomas: A review. Environ Health Perspect 111: 1037-1054.
- Hodge JC, Morton CC (2007) Genetic heterogeneity among uterine leiomyomata: Insights into malignant progression. Hum Mol Genet 16: 7-13.
- Stewart EA, Nicholson WK, Bradley L, et al. (2013) The burden of uterine fibroids for African-American women: Results of a national survey. J Women's Health 22: 807-816.
- Laughlin SK, Stewart EA (2011) Uterine leiomyomas: Individualizing the approach to a heterogeneous condition. Obstet Gynecol 117: 396-403.
- Stewart EA, Cookson CL, Gandolfo RA, et al. (2017) Epidemiology of uterine fibroids: A systematic review. BJOG 124: 1501-1512.
- Munro MG, Critchley HO, Broder MS, et al. (2011) FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 113: 3-13.
- Kaur H, Gulati A (2014) Pathophysiology of fibroids. Indian Journal of Pathology and Oncology 1: 10-13.
- Markowski DN, Bartnitzke S, Löning T, et al. (2012) MED12 mutations in uterine fibroids--their relationship to cytogenetic subgroups. Int J Cancer 131: 1528-1536.
- Mäkinen N, Mehine M, Tolvanen J, et al. (2011) MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 334: 252-255.
- Maruo T, Ohara N, Wang J, et al. (2004) Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update 10: 207-220.
- Yen-Ping Ho J, Man WC, Wen Y, et al. (2010) Transforming growth interacting factor expression in leiomyoma compared with myometrium. Fertil Steril 94: 1078-1083.
- Shozu M, Murakami K, Inoue M (2004) Aromatase and leiomyoma of the uterus. In: Seminars in reproductive medicine. Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA, Vol 22, 51-60.
- Ishikawa H, Reierstad S, Demura M, et al. (2009) High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab 94: 1752-1756.
- Moravek MB, Yin P, Ono M, et al. (2014) Ovarian steroids, stem cells and uterine leiomyoma: Therapeutic implications. Hum Reprod Update 21: 1-12.
- Fletcher NM, Saed MG, Abu-Soud HM, et al. (2013) Uterine fibroids are characterized by an impaired antioxidant cellular system: Potential role of hypoxia in the pathophysiology of uterine fibroids. J Assist Reprod Genet 30: 969-974.
- Fleischer R, Weston GC, Vollenhoven BJ, et al. (2008) Pathophysiology of fibroid disease: Angiogenesis and regulation of smooth muscle proliferation. Best Pract Res Clin Obstet Gynaecol 22: 603-614.
- Sankaran S, Manyonda IT (2008) Medical management of fibroids. Best Pract Res Clin Obstet Gynaecol 22: 655-676.
- RM Moroni, CS Vieira, RA Ferriani, et al. (2014) Pharmacological treatment of uterine fibroids. Ann Med Health Sci Res 4: S185-S192.
- Zapata LB, Whiteman MK, Tepper NK, et al. (2010) Intrauterine device use among women with uterine fibroids: A systematic review. Contraception 82: 41-55.
- Kailasam C, Cahill D (2008) Review of the safety, efficacy and patient acceptability of the levonorgestrel-releasing intrauterine system. Patient Prefer Adherence 2: 293-302.
- Vahdat M, Kashanian M, Ghaziani N, et al. (2016) Evaluation of the effects of cabergoline (Dostinex) on women with symptomatic myomatous uterus: A randomized trial. Eur J Obstet Gynecol Reprod Biol 206: 74-78.
- Sayyah-Melli M, Mobasseri M, Gharabaghi PM, et al. (2017) Comparing the effect of aromatase inhibitor (letrozole) + cabergoline (Dostinex) and letrozole alone on uterine myoma regression, a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol 210: 257-264.
- Donnez J, Tatarchuk TF, Bouchard P, et al. (2012) Ulipristal acetate versus placebo for fibroid treatment before Surgery. N Engl J Med 366: 409-420.
- Talaulikar VS, Manyonda IT (2012) Ulipristal acetate: A novel option for the medical management of symptomatic uterine fibroids. Adv Ther 29: 655-663.
- Pérez-López FR (2015) Ulipristal acetate in the management of symptomatic uterine fibroids: Facts and pending issues. Climacteric 18: 177-181.
- Levens ED, Potlog-Nahari C, Armstrong AY, et al. (2008) CDB-2914 for Uterine leiomyomata treatment: A randomized controlled trial. Obstet Gynecol 111: 1129-1136.
- Moroni RM, Vieira CS, Ferriani RA, et al. (2015) Presentation and treatment of uterine leiomyoma in adolescence: A systematic review. BMC Womens Health 15: 4.
- Courtoy GE, Henriet P, Marbaix E, et al. (2018) Matrix metalloproteinase activity correlates with uterine myoma volume reduction after ulipristal acetate treatment. J Clin Endocrinol Metab 103: 1566-1573.
- (2018) Women taking Esmya for uterine fibroids to have regular liver tests while EMA review is ongoing.
- Tristan M, Orozco LJ, Steed A, et al. (2012) Mifepristone for uterine fibroids. Cochrane Database Syst Rev 8: CD007687.
- Malartic C, Morel O, Akerman G, et al. (2008) La mifépristone dans la prise en charge des fibromes utérins. Gynécologie Obstétrique & Fertilité 36: 668-674.
- Parsanezhad ME, Azmoon M, Alborzi S, et al. (2010) A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril 93: 192-198.
- Song H, Lu D, Navaratnam K, et al. (2013) Aromatase inhibitors for uterine fibroids. Cochrane Database Syst Rev 10: CD009505.
- Gupta JK, Sinha A, Lumsden MA, et al. (2014) Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 12: CD005073.
- Stewart EA, Rabinovici J, Tempany CM, et al. (2006) Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 85: 22-29.
- Rabinovici J, David M, Fukunishi H, et al. (2010) Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril 93: 199-209.
- Edwards RD, Moss JG, Lumsden MA, et al. (2007) Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med 356: 360-370.
- Moss JG, Cooper KG, Khaund A, et al. (2011) Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG 118: 936-944.
- Christakos S, Ajibade DV, Dhawan P, et al. (2010) Vitamin D: Metabolism. Endocrinol Metab Clin North Am 39: 243-253.
- Cheng JB, Levine MA, Bell NH, et al. (2004) Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 101: 7711-7715.
- Flood A, Peters U, Chatterjee N, et al. (2005) Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol Biomarkers Prev 14: 126-132.
- McCullough ML, Robertson AS, Rodriguez C, et al. (2003) Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control 14: 1-12.
- Terry P, Baron JA, Bergkvist L, et al. (2002) Dietary calcium and vitamin D intake and risk of colorectal cancer: A prospective cohort study in women. Nutr Cancer 43: 39-46.
- Gorham ED, Garland CF, Garland FC, et al. (2007) Optimal vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am J Prev Med 32: 210-216.
- Wactawski-Wende J, Kotchen JM, Anderson GL, et al. (2006) Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354: 684-696.
- Garland CF, Gorham ED, Mohr SB, et al. (2007) Vitamin D and prevention of breast cancer: Pooled analysis. J Steroid Biochem Mol Biol 103: 708-711.
- Chen P, Hu P, Xie D, et al. (2010) Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat 121: 469-477.
- Burns EM, Elmets CA, Yusuf N (2015) Vitamin D and skin cancer. Photochemistry and Photobiology 91: 201-209.
- Moan J, Porojnicu AC, Dahlback A, et al. (2008) Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci U S A 105: 668-673.
- Luk J, Torrealday S, Neal Perry G, et al. (2012) Relevance of vitamin D in reproduction. Hum Reprod 27: 3015-3027.
- Bläuer M, Rovio PH, Ylikomi T, et al. (2009) Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril 91: 1919-1925.
- Sharan C, Halder SK, Thota C, et al. (2011) Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril 95: 247-253.
- Halder SK, Osteen KG, Al-Hendy A (2013) Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and-9 in human uterine fibroid cells. Hum Reprod 28: 2407-2416.
- Brakta S, Diamond JS, Al-Hendy A, et al. (2015) Role of vitamin D in uterine fibroid biology. Fertil Steril 104: 698-706.
- Halder SK, Sharan C, Al-Hendy A (2010) Vitamin D treatment induces dramatic shrinkage of uterine leiomyomas growth in the Eker rat model. Fertil Steril 94: S75-S76.
- Halder SK, Sharan C, Al-Hendy O, et al. (2014) Paricalcitol, a vitamin D receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod Sci 21: 1108-1119.
- Baird DD, Hill MC, Schectman JM, et al. (2013) Vitamin D and risk of uterine fibroids. Epidemiology 24: 447-453.
- Sabry M, Halder SK, Allah AS, et al. (2013) Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int J Womens Health 5: 93-100.
- Paffoni A, Somigliana E, Vigano' P, et al. (2013) Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab 98: E1374-E1378.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911-1930.
- Ali M, Laknaur A, Shaheen SM, et al. (2017) Vitamin D synergizes the antiproliferative, apoptotic, antifibrotic and anti-inflammatory effects of ulipristal acetate against human uterine fibroids. Fertil Steril 108: e66.
- Ali M, Al-Hendy A, Yang Q (2019) Vitamin D, a promising natural compound with anti-uterine fibroid characteristics. Fertil Steril 111: 268-269.
Corresponding Author
Sara Mohamed, Lecturer of Obstetrics and Gynecology, Mansoura Medical School, Egypt; Former Research Scholar, Augusta University, USA, Tel: +1-518-419-7301; +201005240203.
Copyright
© 2019 Mansi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.