Liver Trauma: Review of Management, and Patient Outcomes at Our Major Trauma Centre
Introduction
"Whenever you encounter massive bleeding, the first thing to remember is that it is not your blood..." Raphael Adar
The liver is the largest solid organ in the body, has two blood supplies and performs around 500 functions. It has an unrivalled capacity to regenerate in response to injury - a feature described in Greek mythology and one that has evolved in response to damage from the very toxins it is meant to clear. Its complexity however, can also be its downfall. It is one of the only organs whose functions cannot be artificially replicated and for this reason liver failure is often a fatal event. In spite of residing under the right costal margin, it is the most frequently injured organ during abdominal trauma [1,2] and despite advances in non-operative management [1,3,4], uncontrolled haemorrhage and delayed complications are associated with a mortality rate of 10-15%.
Over the past 40 years, there has been a shift from invasive operative management of liver trauma to a more "conservative" approach. This was driven largely by a rapid improvement in imaging techniques and the emergent use of CT scanners, which permitted the non-invasive serial assessment of trauma patients and their injuries [5,6].
The introduction of transarterial angioembolization in the 1970's offered a potential viable option for the treatment of acute arterial hepatic haemorrhage [7,8] and became the mainstay of therapeutic intervention in patients with hemodynamic stability by the late 90's [9-12]. Despite undoubtedly saving lives, the selection of patients for non-operative management is critical, not only because some have questioned its overuse [13] but also because failure and the subsequent need for surgery leads to a higher mortality rate [4].
The aim of this review is to discuss the management of hepatic trauma and associated vascular injuries from the perspective of one Europe's most active hepatobiliary and liver transplant centres. We will discuss how anatomical and physiological factors impact upon these injuries and discuss how lessons learnt from liver surgery and transplantation can improve the management of traumatic liver injury.
Applied Anatomy
The adult liver weighs approximately 2% of body weight and resides in the right upper quadrant under the diaphragm behind the costal margin and extends into the left upper abdomen. The superior surface is percussed as high as the 5th intercostal space - an important factor when dealing with blunt or penetrating trauma to the right hemithorax. The antero-inferior border is normally palpated on deep inspiration below the costal margin. The gallbladder is attached to the inferior surface, which in turn is closely related to the hepatic flexure and transverse colon, right kidney and adrenal gland, duodenum, pancreas and stomach. Also on the inferior surface, the inflow to the liver enters through the porta hepatis. The common hepatic artery from the coeliac trunk gives rise to the gastroduodenal artery and continues as the hepatic artery proper passing anteriorly over the portal vein and to the left of the common bile duct. These three main structures lie in the free edge of the lesser omentum behind which is the opening of the lesser sac. At the level of the hilum, the hepatic artery divides into right and left branches that run alongside the right and left branches of the portal vein. Atypical vascular anatomy is however common. Michels described 10 main variations in hepatic arterial supply based on the dissection of 200 cadaveric donors [14]. The most common include a middle hepatic artery arising from either the right or left hepatic (55%), a left hepatic arising from the left gastric artery (20%) and the right hepatic arising from the superior mesenteric artery (20%). Within a 10 cm2 area, the coeliac trunk and its branches, portal vein, IVC, pancreaticoduodenal arcade, superior mesenteric vessels and right renal pedicle converge. Trauma to this area can therefore be catastrophic. Vascular anatomy is important when considering the intervention of radiologists and will be discussed later.
The liver can be divided into 8 "Couinaud" segments [15]. The right lobe (segments 5-8) is usually 20% larger than the left (segments 1--4) although this can vary. The liver is anchored in the abdominal cavity by several peritoneal attachments and reflections that form 'ligaments'. The falciform ligament, the free-edge of which contains the ligamentum teres (the obliterated umbilical vein), attaches the anterosuperior surface of liver to the anterior abdominal wall and diaphragm. The liver is attached to the diaphragm posteriorly by two reflections of peritoneum, the anterior and posterior coronary ligaments which converge laterally to form the right and left triangular ligaments. In the centre of these points of attachment (posteriorly and to the right of the IVC) is the bare area of the liver - the only surface without a covering of peritoneum. There are also attachments to the right kidney, lesser curvature of the stomach and the first part of the duodenum.
Mechanisms of Injury
The relevance of these attachments is evident when considering the two principal mechanisms of blunt trauma, deceleration and crush injuries. The former occur in falls from height or road traffic collisions when high energy transfer causes movement of the liver relative to its diaphragmatic attachments [16]. This mechanism can produce lacerations in the hepatic parenchyma, most commonly between the right posterior section (segments 6 and 7) and the right anterior section (segments 5 and 8) and can also involve hepatic veins and the juxtahepatic vena cava. In contrast, crush injuries typically damage the central portion of the liver (segments 4, 5 and 8 - see Figure 1) or compress the liver between the lower ribs and spine injuring the caudate lobe (segment 1). Blunt trauma can also rupture the liver's capsule leading to haematoma formation.
Penetrating trauma is less common in the UK and Western Europe than in other countries such as the United States or South Africa [17-20]. Knife and gunshot wounds are the predominate cause, the latter of which create the greatest degree of parenchymal damage due to cavitation.
Classification of Liver Injury
The severity of liver trauma ranges from a minor capsular tear without parenchymal injury to hepatic avulsion. The American Association for the Surgery of Trauma adopted the classification of liver injury originally described in 1989 by Moore, et al. which was revised in 1994 (Table 1 and Table 2) [21,22]. For the purposes of this review, injuries have been divided into lacerations, haematomas and vascular injuries. The grade is usually calculated using information derived from imaging, operative findings or post-mortem. Multiple liver injuries warrant an increase of classification by one grade. Grades I and II represent 80-90% of cases and are usually managed conservatively [13]. Grades III to V may require surgical intervention, while grade VI injuries are regarded as fatal.
Initial Management and Triage of Patients
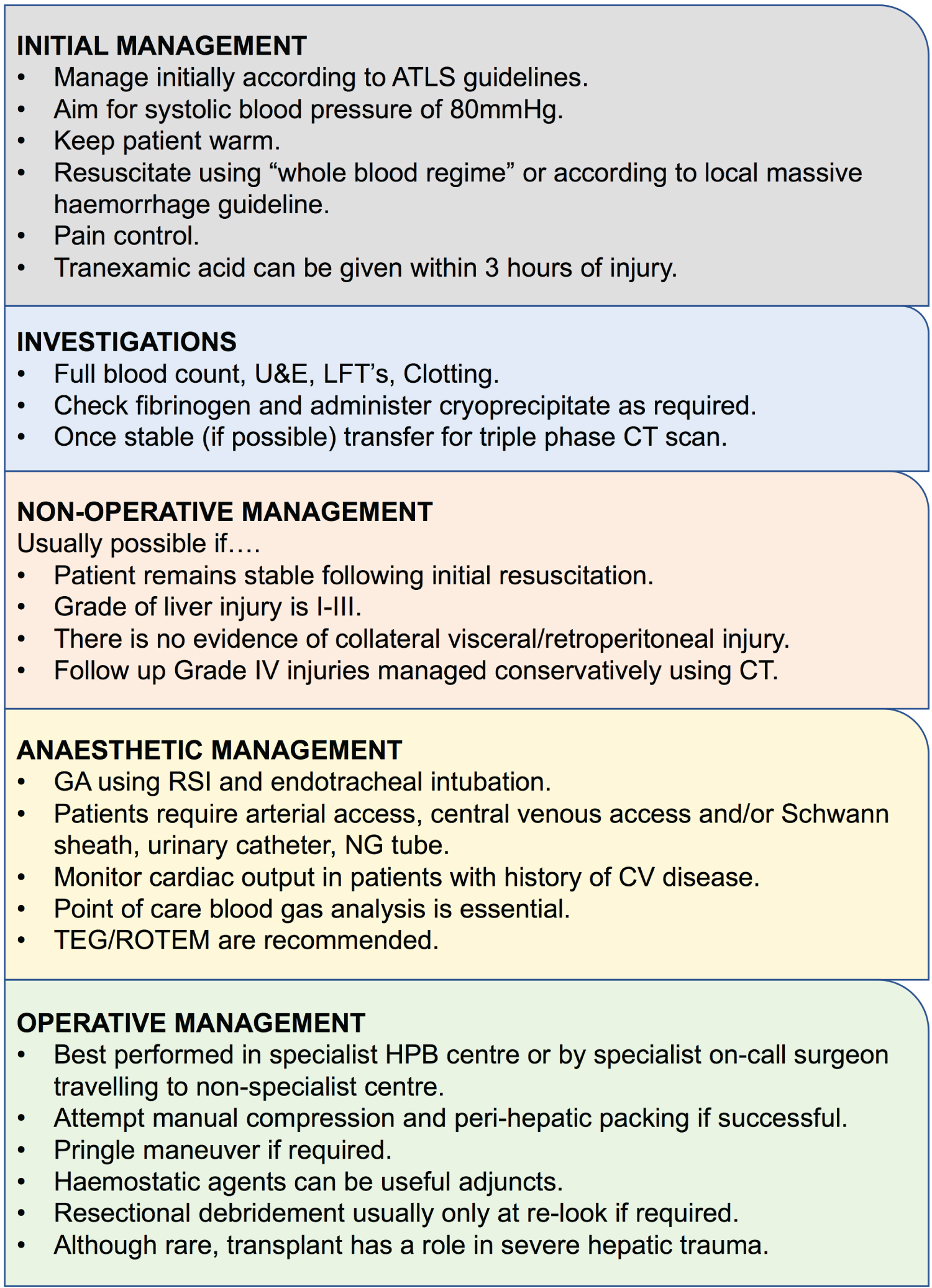
All patients who sustain trauma should be managed according to advanced trauma life support (ATLS) guidelines in an attempt to stabilise them prior to safely ascertaining the degree of injury suffered. Assuming the patient has suffered injury to their liver, it should be remembered that 80% of these cases are minor and can be managed non-operatively [1,23]. Action however, can be taken by clinicians when managing all patients with liver trauma, regardless of the likely course of management, which can reduce the risk of deterioration.
The liver receives 30% of cardiac output and therefore significant blood loss can ensue. Poorly managed haemorrhagic shock can trigger the lethal triad of progressive metabolic acidosis, coagulopathy and hypothermia, promptly followed by circulatory and multi-organ failure. Initial management of patients with the possibility of liver trauma and those who are actively bleeding should involve the definitive control of airway and ventilation which expedites safe transfer to CT and onward to possible angiography or the operating theatre, secure vascular access that permits rapid infusion and order blood products according to local protocols for massive haemorrhage. ATLS guidelines state that immediate infusion with up to 2 L of crystalloid should take place during the initial phases of assessment. Repeated administration of crystalloid to maintain blood pressure in haemorrhagic shock [24-26] should be avoided as excessive use can dilute the haematocrit and clotting factors which exacerbates bleeding prior to definitive control [27-29]. Cannon was the first to describe the deliberate hypotensive resuscitation of patients with abdominal trauma because, in his words, prior to definitive control "blood that is sorely needed may be lost" [30]. Since then, many have shown his sentiments were correct and permissive hypotension in conjunction with haemostatic resuscitation has been shown to improve survival in animal and human models of abdominal trauma with uncontrolled haemorrhage [31-34]. Fluid resuscitation therefore should be initiated to maintain a systolic blood pressure of close to 80 mmHg and given concerns over use of crystalloids, unstable patients should be resuscitated with a "whole blood" regime i.e. equal volumes of packed red cells, fresh frozen plasma and platelets. Plasma is generally required after loss of 30-40% of circulating volume (1.5-2 L) and platelets will be required shortly after. Rapid transfusion of blood products can trigger hypocalcaemia due to citrate toxicity and hyperkalaemia can occur as a result of release from lysed RBCs and ongoing metabolic acidosis. Fibrinogen levels should also be checked and cryoprecipitate administered as required. Hypothermia reduces platelet function, clotting cascade enzyme activity and induce fibronolysis [35,36]. Trauma patients who develop hypothermia require more blood products and have a higher mortality rate [37] - techniques such as the use of re-warming blankets and heat exchange rapid infusion pumps should therefore be employed to prevent patients developing it [38,39].
Computerised Tomography (CT)
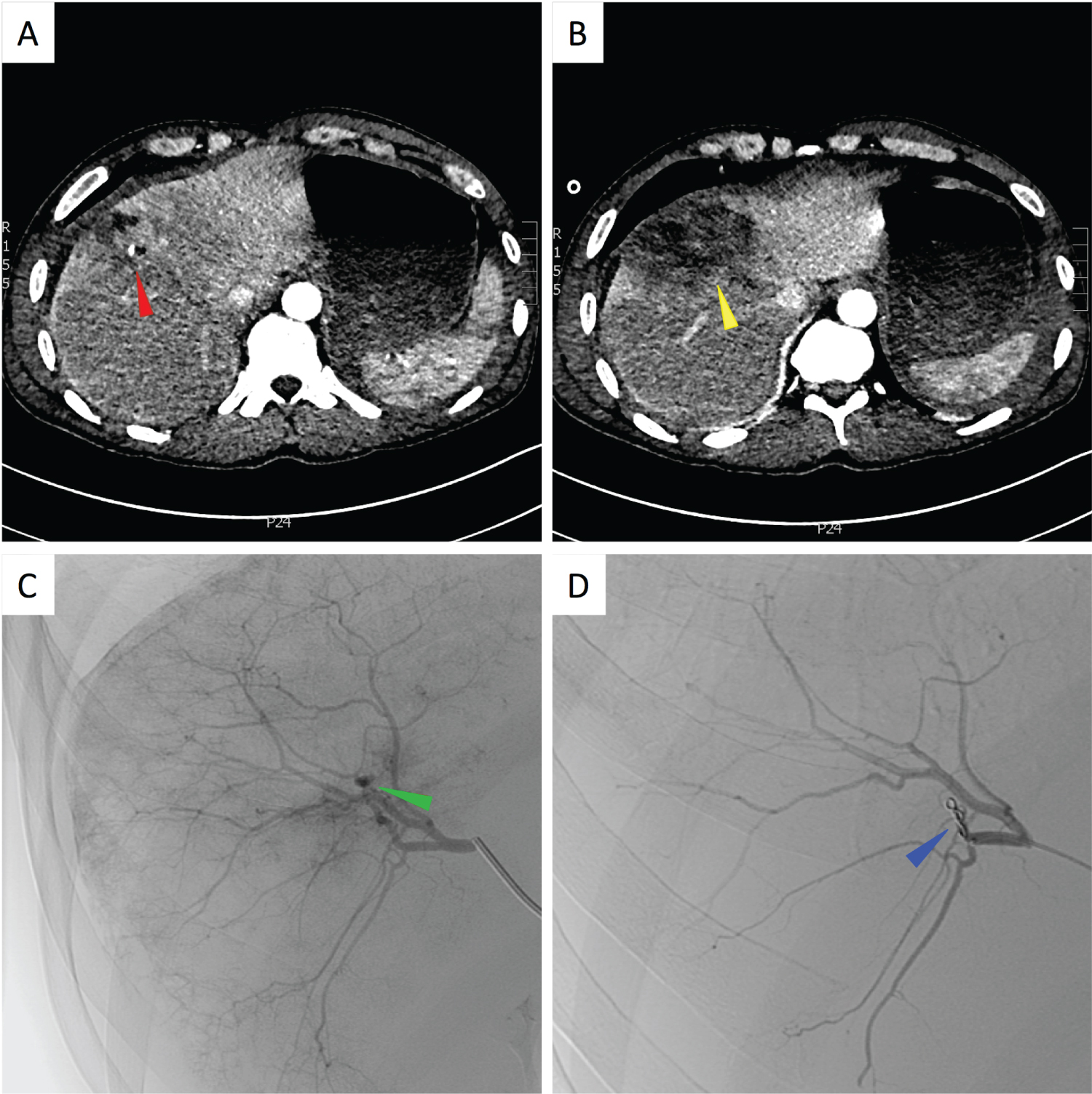
If a patient with suspected liver injury can be stabilised, they should be transferred to CT to undergo assessment of the injury [40,41]. Triple phase multi-slice CT can identify vascular injury, differentiate source (arterial, portal venous, venous) and has a reported sensitivity and specificity of 92-97% and 98.7% respectively [42]. Multi-plane reconstruction can also be performed to aide radiologist and surgeon. In addition to being able to categorise the injury, CT can be used to estimate volume of haemoperitoneum, localise the original source by pinpointing the "sentinel clot" [43] and even detect active extravasation [44]. The latter is a strong predictor of failure of non-operative management [45,46]. Haemoperitoneum volume has been used as a predictor of need for laparotomy [47-49] however due to discrepancies between CT and operative findings [50] and improvements in non-operative management, more onus is now on the haemodynamic stability of the patient. Concomitant injuries can also be identified on CT and would increase the likelihood of failure of a conservative approach [51]. Monitoring of liver injuries using serial CT's is controversial but is generally only recommended in grade IV or V injuries 7-10 days post injury [52] or when clinical signs dictate repeat investigations [53]. Figures 1A and Figure 1B show arterial phase CT images from a 47-year-old man who was hit by a car. The patient sustained a Grade IV injury of segments VIII and IVa with a blush of arterial contrast suggestive of active bleeding.
Non-Operative Management
Protocols can help categorise those patients who can be managed non-operatively [54]. The criteria for identifying suitable candidates for non-operative management are, however, constantly changing [55]. The evolution has been driven by several factors; the improvements in outcome observed following use of non-operative management in the paediatric population [56-58], the fact spontaneous hemostasis occurs in approximately 50% of patients, the liver's tremendous capacity to regenerate and the vast improvements in radiological modalities. This approach has not only improved outcomes in patients with grade III and IV injuries by up to 23.5% [59] but it has even been used effectively in certain patients with penetrating liver trauma [60,61]. Important criteria include haemodynamic stability after resuscitation, absence of signs of visceral or retroperitoneal injuries that necessitate surgery, and the presence of a multidisciplinary team that are experienced in dealing with liver trauma [17,47,62-67]. It is true that the failure rate of non-operative management is higher in those patients with injuries grade IV or worse [68]. The need however, for surgical intervention is rarely due to complications arising from the liver injury such as delayed haemorrhage, it is usually due to a collateral injury to the spleen or kidney [51]. Independent risk factors for failure on non-operative management include patient age, the need for blood products and active haemorrhage on CT [23,51,69]. Patients with grade IV injuries or worse should be followed up closely using CT to detect any evidence of secondary complications or re-bleeding that may require surgical or radiological intervention [70].
Interventional Radiology in Liver Trauma
A crucial adjunct in the management of patients is radiological intervention [71]. Angiography for liver trauma was first described in 1973 [72,73] with subsequent selective arterial embolization in 1977 [74]. Poletti, et al. developed CT-based criteria including; injury grade, presence of arterial injury or pseudoaneurysm and venous involvement to select patients who should undergo angiography with or without embolization [75]. Angioembolisation has many advantages; it is minimally invasive, it can reach vessels that are difficult to access surgically [76], it is effective in up to 90% of cases [77,78], has a low risk of re-bleeding and can be used in addition to surgical intervention either before or after surgery [79-81]. Johnson, et al. went further and argued that hepatic angiography should be used in all patients following damage-control laparotomy and packing for high grade injuries (> grade III) [82].
Liver-related techniques used in the trauma setting include; hepatic artery embolization or infusion, and portal or systemic vein embolization. Arterial bleeding can cause haemobilia, haematoma or haemoperitoneum and can be difficult to manage surgically due to the rich collateral supply within the liver. Once the source is identified, selective catheterisation and vessel isolation using a coaxial catheter can isolate the vessel prior to embolization using prothrombotic metal coils. Gaining proximal and distal control, the so-called "sandwich technique", overcomes the problem of distal reconstitution due to collateral supply. Crossing the injury can be difficult in cases of vessel transection but is easier when isolating a traumatic pseudoaneurysm. Larger vessels usually require coils whereas particles, gelatin sponge or microcoils can be used for smaller vessels [83]. Complications after transcatheter arterial embolization (TAE) include fever, delayed haemorrhage, hepatic necrosis, sepsis, and biliary fistula detected either by CT or ultrasound [84,85]. Figures 1B and Figure 1C show selective images of the embolization procedure carried out in the patient previously described. A pseudoaneurysm was identified and the vessel embolised using gelfoam and coils.
Anaesthetic Management
If surgery is required, the patient will require a general anaesthetic with tracheal intubation and appropriate wide bored intra venous access. The theatre should have a rapid infusor including both a blood warming and cell salvage system as per the current Royal College of Anaesthetists guidelines [86]. In view of likely delayed gastric motility due to the recent trauma, rapid sequence induction (RSI) or modified RSI is recommended.
Invasive arterial monitoring enables better haemodynamic control and monitoring of peri-operative permissive hypotension and allows regular blood sampling. Central venous access is important for both central venous pressure (CVP) measurement and vasopressor/inotrope infusions but also large wide bored access can be established through the placement of a Swann sheath. Cardiac output monitoring is advised especially for patients with a past medical history of cardiovascular disease where trans-oesophageal echocardiography, pulmonary artery catheterization and arterial waveform based techniques have all been described. Placement of a nasogastric tube enables gastric drainage and post-operative enteral feeding if required. A urinary catheter is needed to assess urine output and aid in fluid resuscitation and maintenance of renal function. Temperature monitoring is essential as active warming of trauma patients reduces the risk of trauma induced coagulopathy and a core temperature of > 36.0 °C is advised [87]. Blood components should be warmed to at least 37.0 °C using an infusor. Permissive hypotensive haemostatic resuscitation (with limited crystalloid use) and ratio-based blood product administration should be implemented [88,89].
Near testing of arterial blood gases for Hb, lactate, base excess, calcium, potassium should be carried out to help guide resuscitation and peri-operative management. Blood sugar should also be closely monitored as massive liver trauma may disrupt glucose metabolism. A Clauss fibrinogen measurement is also important as it is more sensitive in predicting developing coagulopathy in trauma patients than prothrombin time and activated plasminogen TT. Fibrinogen can be replaced using either FFP or cryoprecipitate however fibrinogen concentrate administration is currently not licensed for use in trauma in the UK.
Point of care tests including thromboelastography (TEG) or rotational thromboelastometry (ROTEM) assess in vitro clot formation and stability, and are used in both liver transplant anaesthesia and in patients with major trauma. They can provide quick identification of bleeding due to trauma-induced coagulopathy (TIC) or hyperfibrinolysis (excessive clot lysis) which can result from massive transfusion in trauma patients [90,91]. Recent observational studies have shown that goal-directed therapy via TEG leads to decrease in use of blood products [92,93].
The CRASH-2 trial examined the use of tranexamic acid (TXA) in trauma patients and demonstrated that early use (< 3 hours since trauma occurred) of TXA reduced all-cause mortality without increasing the risk of vascular occlusive events [94]. Despite this result, several issues were associated with the trial. Only 5% of the patients died because of bleeding. It had no effect of the need for surgical intervention or blood transfusion. The MATTER study (Military Application of Tranexamic Acid for Trauma Emergency Resuscitation), was more focussed and evaluated military trauma patients who needed at least one unit of blood [95]. The relative reduction in mortality was 6.7% and those who received TXA required fewer blood products. In the CRASH-2 trial TXA patients received the same amount of blood as those who did not receive the drug. TXA administration for patients with isolated liver trauma has not been investigated, however due to the safety profile and cost-effectiveness of TXA, its early use in trauma patients has been widely adopted and is also used in selective liver transplant recipients with coagulopathy.
Operative Management
Detailed management strategies are beyond the scope of this paper, however there are several ways to approach the surgical management of liver trauma. Operative management is usually indicated in patients where initial resuscitation attempts have been futile and interventional radiological management is not available or appropriate. In an ideal world, patients such as this should undergo surgery in a specialist centre with multidisciplinary teams with experience in complex HPB surgery, but in reality is that this is not always the case. Patients who require surgery are often those in dire need and transfer to such a centre is often not possible. In this scenario the job of the surgical team is to control the bleeding without causing further complications. A compromise includes specialist on call liver surgeons travelling to non-specialist hospitals; a practice that has been used successfully in our centre.
The initial approach should be a long midline laparotomy which permits extension into the chest if required, blood and clots should be removed and all 4 quadrants of the abdomen should be packed. At this point the anaesthetist should be given time to attempt to resuscitate the patient with blood products in order to minimise the progression of the 'lethal triad' which will only lead to deterioration of the clinical scenario. The lower quadrant packs should be removed to check the bowel for enterotomies which should be quickly controlled. The spleen should be examined next and removed if damaged. The right upper quadrant packs can finally be removed to assess the degree of liver injury. Most minor bleeds or lacerations can be reduced by returning the liver lobes to their anatomical position i.e. towards the midline. Any manoeuvre that further distracts the injured lobe will only exacerbate bleeding. The assistant can maintain this compression whilst bringing the liver up towards the diaphragm.
If bleeding continues, compression of the structures in the free edge of the lesser omentum - a Pringle manoeuvre (either with the fingers or with an atraumatic vascular clamp whilst being careful not to damage the common bile duct) - can help decrease blood loss by reducing inflow. Under normal circumstances, a liver can tolerate such occlusion for up to 1 hour, however in trauma the prolonged ischemia may propagate the parenchymal damage. Equally, the resulting decrease in cardiac output of up to 10% and increase in left ventricular afterload of 20-30% in an otherwise unstable trauma patient may cause cardiovascular compromise [96]. If haemorrhage continues despite inflow occlusion, hepatic vein or vena cava injury should be suspected and hepatic outflow control may also be required. Total hepatic vascular occlusion would require control of both the infrahepatic and suprahepatic vena cava and even if this is achieved by an experienced surgeon, the 60% reduction in cardiac output would be poorly tolerated by the liver and the patient.
Peri-hepatic packing may be the most appropriate management option if compression has been successful, but must be done well to be effective without causing further complications such as failure to control haemorrhage, renal vein compression or abdominal compartment syndrome [97]. If available, caval pressure monitoring can be employed to allay fears. The technique should aim to mimic the actions of the assistant, bringing the parenchyma together using sequential dry packs and compressing the injury itself to tamponade the bleeding. Patients should return to theatre after 48 hours for pack removal and injury reassessment [98].
If bleeding continues despite manual compression of the injury, surgical haemostasis may be required. Haemostatic agents such as thrombin or fibrin aggregates can be useful adjuncts and the argon beam coagulator has advantage over diathermy of not sticking to the liver tissue. Liver sutures can be used to approximate the ruptured parenchyma. Sutures should be large diameter round bodied needles and gentle tension will be enough to treat the majority of haemorrhage. Due to the risks of cavitating haematomas, re-bleeding, bile leaks and ischaemia, the injury should be explored, however, wherever possible to identify the source of bleeding to enable definitive haemostasis and to rule out other issues that would otherwise need attention.
Resectional debridement, the removal of tissue along the demarcated lines of injury, is best performed at the re-look after initial packing. Anatomical resection as a primary procedure is not usually performed due to difficulties that can arise in the presence of coagulopathy, shock and coexistent visceral injury. It may be necessary however in cases that involve significant devascularisation, major venous injury or shattered parenchyma and as with most complex surgical approaches, the best results are obtained in high-volume centres with experienced teams [99-101].
Total hepatectomy and liver transplantation for trauma, either at the time of initial surgery or due to subsequent liver failure, is rare. A review of 34 cases in the literature between 1987 and 2012 by Patrono, et al. demonstrated an overall patient survival rate of 65% (79% since 2002). Indications were acute liver failure (59%), haemorrhage (26%), biliary fistula (6%), secondary biliary cirrhosis (6%) and total liver necrosis (3%). Seventy-nine percent (79%) were performed as a direct consequence of the injury or due to acute liver failure after the initial treatment and 74% were performed as a single-stage procedure (no significant anhepatic phase) [102]. A more recent review demonstrated similar results in 46 patients and concluded this surgical approach had its place in the management of severe hepatic trauma [103].
Liver Trauma at QEHB
In 2019 there were 60 patients admitted with trauma to the liver (Table 3). 43 patients (72%) were male with an age distribution as follows - 18% < 20 years, 48% 20-39 years, 25% 40-59 years and 8% 60 years and above. 68% of patients had multiple injuries and the overall mortality rate was 10%. All deaths were secondary to combinations of injuries and there were no deaths of patients with isolated liver injuries. Seven patients underwent surgical intervention (grade II injuries required other intrabdominal injuries to be assessed at laparotomy, grade II and above for isolated liver injuries in patients who were haemodynamically unstable), 4 patients (2 of which were isolated injuries, grade II and IV) underwent radiological angioembolisation. The need for intervention, either radiological or surgical, was associated with a doubling in the average length of stay for patients (polytrauma 27 to 55 days and isolated liver injuries 12 to 23 days).
Conclusion
In summary, the management of liver trauma has evolved greatly over several decades (Figure 2). Non-operative management is the mainstay of treatment for the majority of patients, however failure of non-operative management is associated with a high morbidity and mortality. Centralisation of trauma services means that most major trauma patients should be managed at high-volume centres with hepatobiliary experience however it is still important to understand the pitfalls associated with treating these patients, should you care for one at a smaller general hospital. A multi-disciplinary approach, through engaging with other specialties such as anaesthetics and radiology, is important in order to give these patients an optimal standard of care and the best chance of recovery.
References
- Richardson JD, Franklin GA, Lukan JK, et al. (2000) Evolution in the management of hepatic trauma: A 25-year perspective. Ann Surg 232: 324-330.
- Piper GL, Peitzman AB (2010) Current management of hepatic trauma. Surg Clin North Am 90: 775-785.
- Badger SA, Barclay R, Campbell P, et al. (2009) Management of liver trauma. World J Surg 3: 2522-2537.
- Stassen NA, Bhullar I, Cheng JD, et al. (2012) Nonoperative management of blunt hepatic injury: An Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 73: S288-S293.
- Druy EM, Rubin BE (1979) Computed tomography in the evaluation of abdominal trauma. J Comput Assist Tomogr 3: 40-44.
- Federle MP, Goldberg HI, Kaiser JA, et al. (1981) Evaluation of abdominal trauma by computed tomography. Radiology 138: 637-644.
- Rosch J, Dotter CT, Brown MJ (1972) Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology 102: 303-306.
- Bookstein JJ, Goldstein HM (1973) Successful management of postbiopsy arteriovenous fistula with selective arterial embolization. Radiology 109: 535-536.
- Hashimoto S, Hiramatsu K, Ido K, et al. (1990) Expanding role of emergency embolization in the management of severe blunt hepatic trauma. Cardiovasc Intervent Radiol 13: 193-199.
- Corr P, Beningfield SJ, Krige JE (1992) Selective hepatic artery embolization in complex liver injury. Injury 23: 347-349.
- Schwartz RA, Teitelbaum GP, Katz MD, et al. (1993) Effectiveness of transcatheter embolization in the control of hepatic vascular injuries. J Vasc Interv Radiol 4: 359-365.
- Harper HC 3rd, Maull KI (2000) Transcatheter arterial embolization in blunt hepatic trauma. South Med J 93: 663-665.
- Polanco PM, Brown JB, Puyana JC, et al. (2013) The swinging pendulum: A national perspective of nonoperative management in severe blunt liver injury. J Trauma Acute Care Surg 75: 590-595.
- Michels NA (1953) Variational anatomy of the hepatic, cystic, and retroduodenal arteries; a statistical analysis of their origin, distribution, and relations to the biliary ducts in two hundred bodies. AMA Arch Surg 66: 20-34.
- Couinaud C (1954) Liver lobes and segments: Notes on the anatomical architecture and surgery of the liver. Presse Med 62: 709-712.
- Parks RW, Chrysos E, Diamond T (1999) Management of liver trauma. Br J Surg 86: 1121-1135.
- Brammer RD, Bramhall SR, Mirza DF, et al. (2002) A 10-year experience of complex liver trauma. Br J Surg 89: 1532-1537.
- Talving P, Beckman M, Haggmark T, et al. (2003) Epidemiology of liver injuries. Scand J Surg 92: 192-194.
- Zafar SN, Rushing A, Haut ER, et al. (2012) Outcome of selective non-operative management of penetrating abdominal injuries from the North American National Trauma Database. Br J Surg 99: 155-164.
- Mnguni MN, Muckart DJ, Madiba TE (2012) Abdominal trauma in durban, South Africa: Factors influencing outcome. Int Surg 97: 161-168.
- Moore EE, Shackford SR, Pachter HL, et al. (1989) Organ injury scaling: Spleen, liver, and kidney. J Trauma 29: 1664-1666.
- Moore EE, Cogbill TH, Jurkovich GJ, et al. (1995) Organ injury scaling: Spleen and liver (1994 revision). J Trauma 38: 323-324.
- Fang JF, Wong YC, Lin BC, et al. (2006) The CT risk factors for the need of operative treatment in initially hemodynamically stable patients after blunt hepatic trauma. J Trauma 61: 547-553.
- Shires T, Coln D, Carrico J, et al. (1964) Fluid therapy in hemorrhagic shock. Arch Surg 88: 688-693.
- Baue AE, Tragus ET, Wolfson SK Jr., et al. (1967) Hemodynamic and metabolic effects of Ringer’s lactate solution in hemorrhagic shock. Ann Surg 166: 29-38.
- Traverso LW, Lee WP, Langford MJ (1986) Fluid resuscitation after an otherwise fatal hemorrhage: I. Crystalloid solutions. J Trauma 26: 168-175.
- Bickell WH, Bruttig SP, Millnamow GA, et al. (1991) The detrimental effects of intravenous crystalloid after aortotomy in swine. Surgery 110: 529-536.
- Bickell WH (1993) Are victims of injury sometimes victimized by attempts at fluid resuscitation? Ann Emerg Med 22: 225-226.
- Roberts I, Evans P, Bunn F, et al. (2001) Is the normalisation of blood pressure in bleeding trauma patients harmful? Lancet 357: 385-387.
- Cannon WB, Fraser J, Cowell EM (1918) The preventive treatment of wound shock. JAMA 70: 618-621.
- Bickell WH, Wall MJ Jr., Pepe PE, et al. (1994) Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med 331: 1105-1109.
- Stern SA, Dronen SC, Birrer P, et al. (1993) Effect of blood pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann Emerg Med 22: 155-163.
- Dronen SC, Stern SA, Wang X, et al. (1993) A comparison of the response of near-fatal acute hemorrhage models with and without a vascular injury to rapid volume expansion. Am J Emerg Med 11: 331-335.
- Li T, Zhu Y, Hu Y, et al. (2011) Ideal permissive hypotension to resuscitate uncontrolled hemorrhagic shock and the tolerance time in rats. Anesthesiology 114: 111-119.
- Watts DD, Trask A, Soeken K, et al. (1998) Hypothermic coagulopathy in trauma: Effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma 44: 846-854.
- DeLoughery TG (2004) Coagulation defects in trauma patients: Etiology, recognition, and therapy. Crit Care Clin 20: 13-24.
- Krishna G, Sleigh JW, Rahman H (1998) Physiological predictors of death in exsanguinating trauma patients undergoing conventional trauma surgery. Aust N Z J Surg 68: 826-829.
- Eddy VA, Morris JA Jr., Cullinane DC (2000) Hypothermia, coagulopathy, and acidosis. Surg Clin North Am 80: 845-854.
- Peng RY, Bongard FS (1999) Hypothermia in trauma patients. Journal of the American College of Surgeons 188: 685-696.
- Becker CD, Mentha G, Terrier F (1998) Blunt abdominal trauma in adults: Role of CT in the diagnosis and management of visceral injuries. Part 1: Liver and spleen. Eur Radiol 8: 553-562.
- Shanmuganathan K, Mirvis SE (1998) CT scan evaluation of blunt hepatic trauma. Radiol Clin North Am 6: 399-411.
- Hoff WS, Holevar M, Nagy KK, et al. (2002) Practice management guidelines for the evaluation of blunt abdominal trauma: The East practice management guidelines work group. J Trauma 53: 602-615.
- Lubner M, Menias C, Rucker C, et al. (2007) Blood in the belly: CT findings of hemoperitoneum. Radiographics 27: 109-125.
- Taourel P, Vernhet H, Suau A, et al. (2007) Vascular emergencies in liver trauma. Eur J Radiol 64: 73-82.
- Fang JF, Chen RJ, Wong YC, et al. (2000) Classification and treatment of pooling of contrast material on computed tomographic scan of blunt hepatic trauma. J Trauma 49: 1083-1088.
- Wong YC, Wang LJ, See LC, et al. (2003) Contrast material extravasation on contrast-enhanced helical computed tomographic scan of blunt abdominal trauma: Its significance on the choice, time, and outcome of treatment. J Trauma 54: 164-170.
- Meyer AA, Crass RA, Lim RC, et al. (1985) Selective nonoperative management of blunt liver injury using computed tomography. Arch Surg 120: 550-554.
- Farnell MB, Spencer MP, Thompson E, et al. (1988) Nonoperative management of blunt hepatic trauma in adults. Surgery 104: 748-756.
- Feliciano DV (1992) Continuing evolution in the approach to severe liver trauma. Ann Surg 216: 521-523.
- Poletti PA, Wintermark M, Schnyder P, et al. (2002) Traumatic injuries: Role of imaging in the management of the polytrauma victim (conservative expectation). Eur Radiol 12: 969-978.
- Velmahos GC, Toutouzas KG, Radin R, et al. (2003) Nonoperative treatment of blunt injury to solid abdominal organs: a prospective study. Arch Surg 138: 844-851.
- Yoon W, Jeong YY, Kim JK, et al. (2005) CT in blunt liver trauma. Radiographics 25: 87-104.
- Cox JC, Fabian TC, Maish GO, et al. (2005) Routine follow-up imaging is unnecessary in the management of blunt hepatic injury. J Trauma 59: 1175-1178.
- Schweizer W, Tanner S, Baer HU, et al. (1993) Management of traumatic liver injuries. The British Journal of Surgery 80: 86-88.
- Gruca Z, Rzepecki A, Wasowski J (1973) Injury of the common hepatic artery due to blunt abdominal trauma. Pol Tyg Lek 28: 909.
- Karp MP, Cooney DR, Pros GA, et al. (1983) The nonoperative management of pediatric hepatic trauma. J Pediatr Surg 18: 512-518.
- Cywes S, Rode H, Millar AJ (1985) Blunt liver trauma in children: Nonoperative management. J Pediatr Surg 20: 14-18.
- Cywes S, Bass DH, Rode H, et al. (1991) Blunt liver trauma in children. Injury 22: 310-314.
- Coimbra R, Hoyt DB, Engelhart S, et al. (2006) Nonoperative management reduces the overall mortality of grades 3 and 4 blunt liver injuries. Int Surg 91: 251-257.
- Omoshoro-Jones JA, Nicol AJ, Navsaria PH, et al. (2005) Selective non-operative management of liver gunshot injuries. Br J Surg 92: 890-895.
- DuBose J, Inaba K, Teixeira PGR, et al. (2007) Selective non-operative management of solid organ injury following abdominal gunshot wounds. Injury 38: 1084-1090.
- Feliciano DV, Mattox KL, Jordan GL, et al. (1986) Management of 1000 consecutive cases of hepatic trauma (1979-1984). Ann Surg 204: 438-445.
- Cogbill TH, Moore EE, Jurkovich GJ, et al. (1988) Severe hepatic trauma: A multi-center experience with 1,335 liver injuries. The Journal of trauma 28: 1433-1438.
- Meredith JW, Young JS, Bowling J, et al. (1994) Nonoperative management of blunt hepatic trauma: the exception or the rule? J Trauma 36: 529-534.
- Tugnoli G, Cinquantini F, Coniglio C, et al. (2015) Non-operative management of hemodynamically stable grade V liver trauma. J Emerg Trauma Shock 8: 239-240.
- Suen K, Skandarajah AR, Knowles B, et al. (2015) Changes in the management of liver trauma leading to reduced mortality: 15-year experience in a major trauma centre. ANZ J Surg 86: 894-899.
- Coccolini F, Montori G, Catena F, et al. (2015) Liver trauma: WSES position paper. World J Emerg Surg 10: 39.
- Malhotra AK, Fabian TC, Croce MA, et al. (2000) Blunt hepatic injury: A paradigm shift from operative to nonoperative management in the 1990s. Ann Surg 231: 804-813.
- Schroeppel TJ, Croce MA (2007) Diagnosis and management of blunt abdominal solid organ injury. Curr Opin Critical care 13: 399-404.
- Cuff RF, Cogbill TH, Lambert PJ (2000) Nonoperative management of blunt liver trauma: The value of follow-up abdominal computed tomography scans. Am Surg 66: 332-336.
- Carillo EH, Platz A, Miller FB, et al. (1998) Nonoperative management of blunt liver trauma. Br 85: 461-468.
- Levin DC, Watson RC, Sos TA, et al. (1973) Angiography in blunt hepatic trauma. Am J Roentgenol Radium Ther Nucl Med 119: 95-101.
- Nahum H, Levesque M (1973) Arteriography in hepatic trauma. Radiology 109: 557-563.
- Rubin BE, Katzen BT (1977) Selective hepatic artery embolization to control massive hepatic hemorrhage after trauma. AJR Am J Roentgenol 129: 253-256.
- Poletti PA, Mirvis SE, Shanmuganathan K, et al. (2000) CT criteria for management of blunt liver trauma: Correlation with angiographic and surgical findings. Radiology 216: 418-427.
- Hoffer EK, Borsa JJ, Bloch RD, et al. (1999) Endovascular techniques in the damage control setting. Radiographics 19: 1340-1348.
- Sclafani SJ, Shaftan GW, McAuley J, et al. (1984) Interventional radiology in the management of hepatic trauma. J Trauma 24: 256-262.
- Johnson JW, Gracias VH, Schwab CW, et al. (2001) Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma 51: 261-269.
- Burch JM (1997) New concepts in trauma. Am J Surg 173: 44-46.
- Denton JR, Moore EE, Coldwell DM (1997) Multimodality treatment for grade V hepatic injuries: Perihepatic packing, arterial embolization, and venous stenting. J Trauma 42: 964-967.
- Fandrich BL, Gnanadev DA, Jaecks R, et al. (1989) Selective hepatic artery embolization as an adjunct to liver packing in severe hepatic trauma: Case report. J Trauma 29: 1716-1718.
- Johnson JW, Gracias VH, Gupta R, et al. (2002) Hepatic angiography in patients undergoing damage control laparotomy. J Trauma 52: 1102-1106.
- Lopera JE (2010) Embolization in trauma: Principles and techniques. Semin Intervent Radiol 27: 14-28.
- Velmahos GC, Demetriades D, Chahwan S, et al. (1999) Angiographic embolization for arrest of bleeding after penetrating trauma to the abdomen. Am J Surg 178: 367-373.
- Hagiwara A, Yukioka T, Ohta S, et al. (1997) Nonsurgical management of patients with blunt hepatic injury: Efficacy of transcatheter arterial embolization. AJR Am J Roentgenol 169: 1151-1156.
- Anaesthetists RCo (2016) Guidance on the provision of anaesthesia services for trauma and orthopaedic surgery.
- Perlman R, Callum J, Laflamme C, et al. (2016) A recommended early goal-directed management guideline for the prevention of hypothermia-related transfusion, morbidity, and mortality in severely injured trauma patients. Crit Care 20: 107.
- MacLeod JB, Winkler AM, McCoy CC, et al. (2014) Early trauma induced coagulopathy (ETIC): Prevalence across the injury spectrum. Injury 45: 910-915.
- Simmons JW, Pittet JF, Pierce B (2014) Trauma-induced coagulopathy. Curr Anesthesiol Rep 4: 189-199.
- Cotton BA, Harvin JA, Kostousouv V, et al. (2012) Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg 73: 365-370.
- Da Luz LT, Nascimento B, Shankarakutty AK, et al. (2014) Effect of thromboelastography (TEG(R)) and rotational thromboelastometry (ROTEM(R)) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: Descriptive systematic review. Crit Care 18: 518.
- Johansson PI, Stensballe J, Ostrowski SR (2012) Current management of massive hemorrhage in trauma. Scand J Trauma Resusc Emerg Med 20: 47.
- Yin J, Zhao Z, Li Y, et al. (2014) Goal-directed transfusion protocol via thrombelastography in patients with abdominal trauma: A retrospective study. World J Emerg Surg 9: 28.
- Roberts I, Shakur H, Coats T, et al. (2013) The CRASH-2 trial: A randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 17: 1-79.
- Morrison JJ, Dubose JJ, Rasmussen TE, et al. (2012) Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) Study. Arch Surg 147: 113-119.
- Lentschener C, Ozier Y (2002) Anaesthesia for elective liver resection: Some points should be revisited. Eur J Anaesthesiol 19: 780-788.
- Aydin U, Yazici P, Zeytunlu M, et al. (2008) Is it more dangerous to perform inadequate packing? World J Emerg Surg 3: 1.
- Nicol AJ, Hommes M, Primrose R, et al. (2007) Packing for control of hemorrhage in major liver trauma. World J Surg 31: 569-574.
- Strong RW, Lynch SV, Wall DR, et al. (1998) Anatomic resection for severe liver trauma. Surgery 123: 251-257.
- Tsugawa K, Koyanagi N, Hashizume M, et al. (2002) Anatomic resection for severe blunt liver trauma in 100 patients: Significant differences between young and elderly. World J Surg 26: 544-549.
- Ariche A, Klein Y, Cohen A, et al. (2015) Major hepatectomy for complex liver trauma. Hepatobiliary Surg Nutr 4: 299-302.
- Patrono D, Brunati A, Romagnoli R, et al. (2013) Liver transplantation after severe hepatic trauma: A sustainable practice. A single-center experience and review of the literature. Clin Transplant 27: E528-E537.
- Ribeiro-Jr MA, Medrado MB, Rosa OM, et al. (2015) Liver transplantation after severe hepatic trauma: Current indications and results. Arq Bras Cir Dig 28: 286-289.
Corresponding Author
Edwards M, Department of Otolaryngology, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
Copyright
© 2022 Laing RW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.