Beneficial Effect of a Juice-Based Probiotic for Colon Health
Abstract
Background & Aims: The colon is considered "unhealthy" when chronic diseases, conditions and disorders (e.g., Irritable Bowel Syndrome, Inflammatory Bowel Disease such as Crohn's Disease and Ulcerative Colitis) are present. Also, digestive irregularities or discomfort happen when diarrhea or constipation is present. The causes of occasional diarrhea include microbial infection, antibiotic-associated diarrhea, traveler's diarrhea, medications and ozone therapy. Keeping colon microbiome healthy is a fundamental step in fighting against unhealthy colon diseases, conditions, disorders, irregularities and discomfort. One logical route is to replenish healthy colon microbiome with probiotics and/or synbiotics. We have developed and marketed the pioneering juice based probiotic/synbiotic dietary supplement Doctor's Biome Colon Health® (DBCH) for the following purposes: (1) To help relieve occasional diarrhea, gas and bloating; (2) To help reduce the risk of colon microbial infections; and (3) To support and replenish healthy colon microbiome. The aim of this study was to confirm the efficacy and show safety of Doctor's Biome Colon Health by users with a variety of health conditions.
Methods: In order to develop the optimum product for the intended purposes, we decided to use the optimum profile of probiotic bacteria and the optimum carrier for the chosen probiotic bacteria. We chose a blend of five strains of Bifidobacteria and ten strains of Lactobacilli from DuPont Nutrition & Biosciences. We also chose a proprietary blend of sterilized 100% organic green vegetable and fruit juices Furthermore, every batch of DBCH is tested by an independent, FDA-registered accredited microbiology lab to confirm absence of pathogenic microorganisms. Eighty (80) individuals with a variety of gastrointestinal conditions (occasional diarrhea, ozone therapy, GI disorders and irregularities) were enrolled in this postmarketing surveillance study. The participants were recommended to drink one bottle (2 fluid ounce) of DBCH daily for up to 8 weeks. They were also offered a self-administered questionnaire to write their name, address and phone number, gender, age group, and to score their satisfaction for safety and efficacy of DBCH.
Results: Hard copies of 80 self-administered questionnaires were collected, their data were transferred to a large table divided into the following variables: gender, age group, duration of use, score value (1 = Unacceptable, 7 = Excellent) and comments. The average scores for males and female participants are pretty close. Average scores after 3 weeks of use were between "very good" to "excellent" for both males and females. Several participants voluntarily wrote positive comments on how consumption of DBCH has affected their qualities of life.
Conclusions: The overall conclusion of this study is that Doctor's Biome Colon Health is highly effective and safe probiotic/synbiotic dietary supplement to help relieve occasional diarrhea, gas and bloating; to help reduce the risk of colon microbial infections; and to support and replenish healthy colon microbiome.
Keywords
Doctor's biome colon health, Juice based probiotics, Juice based synbiotics, Colon health dietary supplement
Introduction
The gut microbiome plays an increasingly important and complex role in human health. Research has shown that it strongly influences the development and outcome of chronic diseases ranging from metabolic disease, gastrointestinal diseases and disorders to colorectal cancer [1-5].
The burden on the healthcare system both in patient care and financially is enormous. It is estimated by the center for disease control (CDC) that over 14 million patients were diagnosed with ulcers and over 22 million patients were seen in physicians' offices in addition to over 8 million emergency room visits with a primary diagnosis of digestive system disease and over 3 million hospital admissions in 2018. The estimated cost for 2018 was over 136 billion USD, more than heart disease, trauma and mental health. These numbers have been and are expected to grow yearly [6-9].
There has been a great focus on the use of prebiotics, probiotics and synbiotics to help in prevention and treatment of digestive diseases and disorders.
Prebiotics were redefined at the 2008, 6th Meeting of the International Scientific Association of Probiotics and Prebiotics (ISAPP) as "a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health" [10,11].
The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) proposed a useful definition of Probiotics in 2014 as "live microorganisms that, when administered in adequate amounts, confer a health benefit on the host" [12,13].
The definition of Synbiotic (a combination prebiotic and probiotic) was updated by the ISAPP in 2020 to "a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host. Within this definition, 'host' microorganisms comprise both autochthonous (resident or colonizing the host) and allochthonous (externally applied, such as probiotics) microorganisms, either of which can be targets for the substrate contained in the synbiotic" [14]. Prebiotics, probiotics and synbiotics have shown mixed results in being beneficial in the prevention and treatment of digestive diseases and disorders [15-17]. The literature is replete with studies showing that the microbiota of the colon has shown a direct relationship to many medical conditions including but not limited to diabetes, weight gain, mood, autism, multiple sclerosis, in addition to the many various digestive diseases and disorders. Probiotics must survive in the acidic gastric environment if they are to reach the small intestine and colonize the host, thereby having the potential of imparting their benefits to the host. Selected strains of probiotics have been developed to have the traits that are believed to be important for surviving GI tract passage including, most importantly, tolerance to both the highly acidic conditions found in the stomach as well as the concentrations of bile salts found in the small intestine [18-20].
Various populations of gut bacteria create their beneficial effects mediated by Prebiotics. Through the fermentation of dietary fiber, they can produce Fructo-oligosaccharides (FOS) and Galacto-oligosaccharides (GOS), short-chain fatty acids, which through endogenous signals can play important roles in lipid homeostasis, improving immune functions and reducing inflammation [21,22].
Consuming prebiotics, especially in combination with various Lactobacilli and Bifidobacteria can improve immunity functions by increasing the population of beneficial protective microbiome. Human and animal studies have shown that prebiotics are able decrease the population of harmful bacteria in the gut [23-26].
Many human and animal studies have shown that some probiotics have had beneficial effects in the gut for Irritable Bowel Syndrome (IBD), Necrotizing Enterocolitis (NE), Clostridium difficile infection (CDI), Ulcerative Colitis (UC), and Crohn's Disease (CD) [9,27-45].
The colon is considered "unhealthy" when chronic diseases, conditions and disorders (e.g., Irritable Bowel Syndrome, Inflammatory Bowel Disease such as Crohn's Disease and Ulcerative Colitis) are present. Also, digestive irregularities or discomfort happen when diarrhea or constipation is present. The causes of occasional diarrhea include microbial infection, antibiotic-associated diarrhea, traveler's diarrhea, medications and ozone therapy. Keeping colon microbiome healthy is a fundamental step in fighting against unhealthy colon diseases, conditions, disorders, irregularities and discomfort. One logical route is to replenish healthy colon microbiome with probiotics and/or synbiotics.
Aim of the Study
We have developed and marketed the pioneering juice based probiotic dietary supplement Doctor's Biome Colon Health® = DBCH (Figure 1) for the following purposes: (1) To help relieve occasional diarrhea, gas and bloating; (2) To help reduce the risk of colon microbial infections; and (3) To support and replenish healthy colon microbiome. The aim of this postmarketing surveillance was to confirm safety and efficacy of the Doctor's Biome Colon Health® by users with a variety of health conditions. This study would also widen the breath and duration of our experience with this product.
Materials and Methods
Optimum product
In order to develop the optimum product for the intended purposes, we decided to use the optimum profile of probiotic bacteria and the optimum carrier for the chosen probiotic bacteria.
Optimum probiotics: Bifidobacteria and Lactobacilli are broadly recognized for their key roles in the human intestinal microflora throughout life. A high proportion of bifidobacteria and lactobacilli in the intestinal tract is considered beneficial to health [18-20]. Considering the results of published literature and reviewing commercially-available probiotic bacteria from credible global suppliers, we chose a blend of five strains of Bifidobacteria (Table 1) and ten strains of Lactobacilli (Table 1) from DuPont Nutrition & Biosciences. The chosen strains would survive the stomach acid 80% or more [46]. A minimum of 27 Billion Colony Forming Units (CFU) are infused in the organic juice at the time of manufacture. Laboratory tests show that within one week the numbers can rise up to 100 billion CFU as the bacteria are living, not in "suspended animation" hoping to survive the stomach acids and bile salts as with capsule, powder and tablet probiotics/synbiotics and come back to life.
Optimum Carrier: In search for an optimum carrier (also known as medium or excipient), we chose a proprietary blend of sterilized green vegetable and fruit juices consisting of 100% organic diluted mint juice, cucumber juice, lettuce juice, kale juice, celery juice, apple juice and lemon juice. A considerable amount of prebiotic (fiber) was left in the juice for the bacteria to consume. Sensory evaluation of DBCH (blend of probiotics in blend of organic juices) showed a pleasant taste of this product to consumers across the board. Furthermore, every batch of DBCH is tested by an independent, FDA-registered accredited microbiology lab to confirm absence of pathogenic microorganisms (such as Coliform, E. Coli O157:H7, Listeria monocytogenes, Salmonella, Shigella and Staph. aureus).
As a result of using the above optimum profile of probiotics and optimum carrier, we developed the pioneering, patent-pending organic juice-based probiotic dietary supplement Doctor's Biome Colon Health®. This product may be stored at room temperature for two months and at refrigerator temperature for three months.
Surveillance
Eighty (80) individuals with a variety of gastrointestinal conditions (occasional diarrhea, ozone therapy, GI disorders and irregularities) were enrolled in this postmarketing surveillance study. The participants were recommended to drink one bottle (2 fluid ounce) of Doctor's Biome Colon Health® dietary supplement daily for up to 8 weeks. They were also offered a self-administered questionnaire ("Colon Health Report") to write their name, address and phone number, gender, age group, and to score their satisfaction for safety and efficacy of DBCH (Table 2).
Results
Hard copies of 80 self-administered questionnaires ("Colon Health Report") were collected, their data were transferred to a large table divided into the following variables:
• Gender
• Age Group
• Duration of Use
• Score Value
• Comments
Profile of participants (gender and age) are shown in Table 3. Participants showed great cooperation by remarkable stay in the study (Table 4).
Scores for safety and efficacy
There was no reported adverse event (AE) or serious adverse event (SAE) reported by the participants. There were two mild complaints: A 51-70 years old female reported constipation after 3 weeks of use. However, her scores before and after that time (1, 2, 4 and 8 weeks of use) were 6 ("very good") and 7 ("excellent"). Also, a 51-70 woman scored "good" for two weeks but indicated that she had some heart burn.
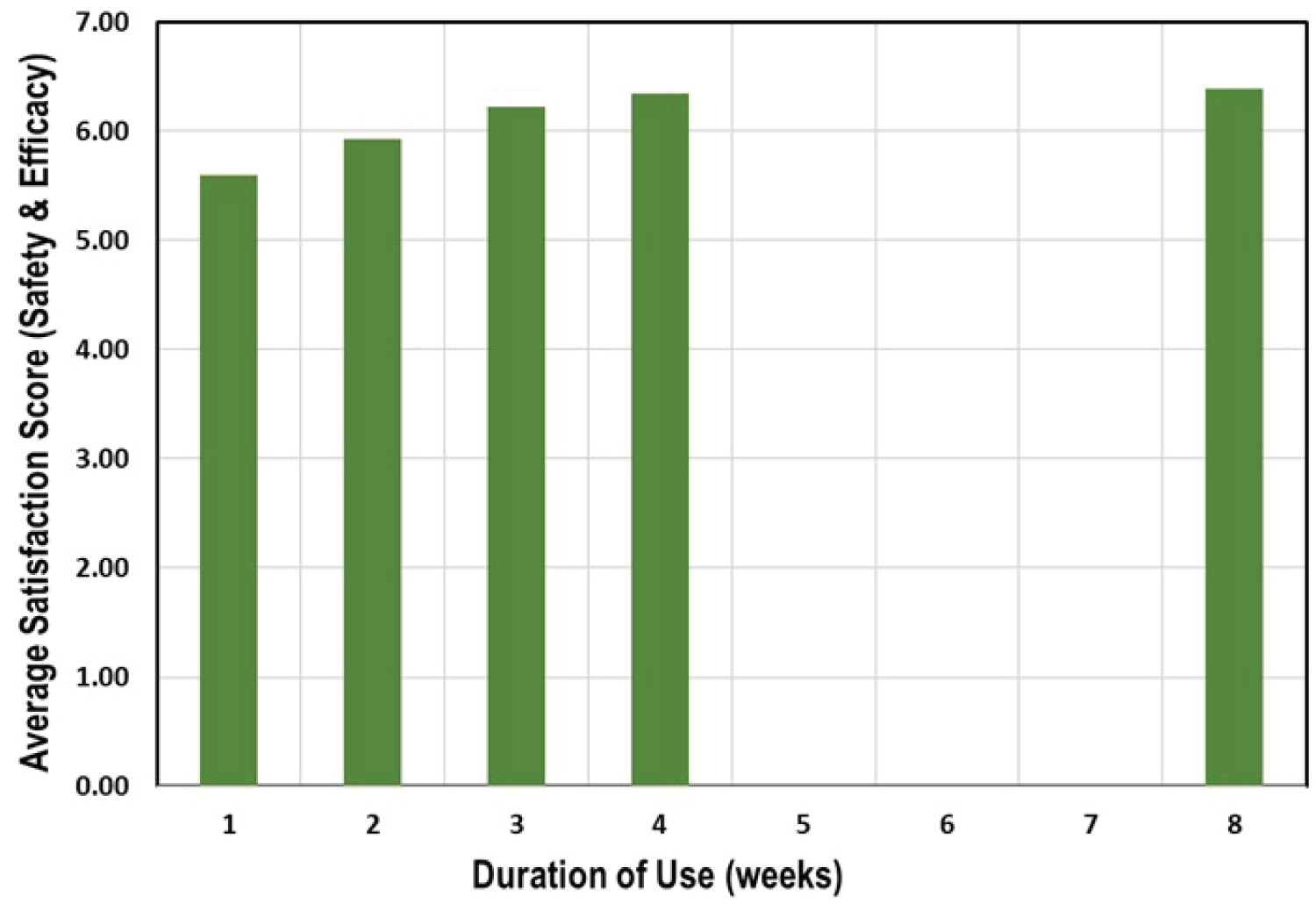
Average scores for safety and efficacy are presented in Table 5. As it can be seen, average scores for males and female participants are pretty close. After 1 week of use, the average score for males is 5.73 and for females is 5.52 (between "good" to "very good"). The scores for both males and females increase after 2 weeks of use, and after 3 weeks of use reached 6.24 for males and 6.21 for females (between "very good" to "excellent"). Average scores still increased for both genders after 4 weeks up to 8 weeks of use where scores reached 6.36 for males and 6.44 for females (between "very good" to "excellent").The total average scores are naturally between the scores of males and females (Figure 2).
Voluntary comments affecting quality of life
Several participants voluntarily wrote comments on their self-administered questionnaires about how consumption of Doctor's Biome Colon Health has affected their qualities of life:
• User#2 (female, >70 years): "P.S. it helped more than any other pres. Medicine."
• User#7 (female, 51-70 years): "I have had chronic diarrhea and gas for over 20 years. I used Immodium AD to treat it until it was so persistent that my gastroenterologist prescribed Lomotril. Using Doctor's Biome had rapid and favorable symptom relief. The taste is also pleasant. This is an excellent probiotic."
• User#18 (male, 32-50 years): "First time in my life that I am doing so well."
• User#27 (female 51-70 years): "I had improvement in GI function. No discomfort. I would like to continue using."
• User#33 (female 51-70 years): "I have been taking DB for about 6 weeks. I am quite pleased with the results. I have been pleasantly surprised to feel less gassy during the day and more comfortable, even with common perimenopausal symptoms. To my surprise it has curbed my appetite for a while after drinking the product in the morning."
• User#34 (female 31-50 years): "It makes me go to the bathroom regularly and helps make me feel less blocked."
• User#36 (female 51-69 years): "IBS with constipation gone. Weight loss with proper diet is going very well. Walking without fatigue."
• User#40 (female >70 years): "Had acid reflux and constipation for years. Acid reflux gone and normal BM constipation gone."
• User#42 (female 51-69 years): "I have had Crohn's disease for many years. I was amazed by the positive results I have experienced. Food digested more easily and my heartburn is gone. The product exceeded my expectations! More positive results as time goes on."
• User#43 (male 51-69 years): "I had very good improvement the only reason I did not give excellent was I still have occasional IBS, this probiotic is the best I have had thank you."
• User#48 (male 51-69 years): "My life would suffer greatly if I could no longer use this product. Not kidding. Only probiotic that has ever worked as effectively. Highest possible recommendation."
• User#49 (female 31-50 years): "This product is amazing. I've never been "regular" but this has made me less bloated and constipated. Well worth the price."
• User#50 (female 51-69 years): "I feel better than I have in years. Chronic IBS gone. Joint pain in hips, lower back and knees and my energy level up so I am able to walk without pain. Very grateful to be feeling overall wellness."
• User#70 (female >70 years): "No more gas and stomach pains."
• User#79 (male 51-70 years): "I'm seeing great results in a short period of time. I love this product!! Wow!! It's helped my diverticulitis."
• User#80 (female 31-50 years): "I am finally able to use the bathroom regularly since taking it. I've suffered from constipation for over 20 years. This is a God send!"
Discussion & Conclusion
As far as safety is concerned, no reported adverse event (AE) or serious adverse event (SAE) is not surprising because probiotics/synbiotics are considered to be "GRAS" or "generally accepted as safe" (this classification is given to products that are composed of ingredients that are natural or have been safely used for many years). The chosen probiotics have been widely used in foods and dietary supplements, and the chosen carrier is a blend of 100% organic vegetable and fruit juices.
As far as efficacy is concerned, the results showed that up to 2 weeks of use, the average score of 80 participants is between 5 to 6 ("good" to "very good") and after 3 weeks of use, the average score of 80 participants is between 6 to 7 ("very good" to "excellent").
The high effectiveness could be the result of simultaneous quantitative and qualitative effects: From a quantitative point of view, a general mechanism of microbial inhibition is through the "competitive exclusion principle" [47]. This means that when two species compete in a limited environment (e.g., colon) and compete for the same limited amount of nutrients, the species that has advantage over the others (e.g., larger numbers) will dominate the environment and lead to the exclusion of the weaker competitor. For example, in our study the daily probiotic use of 27 billion minimum at the time of manufacturing, live and active Colony Forming Units (CFU) has a quantitative advantage.
From a qualitative point of view, the chosen probiotics have released some bioactive compounds (e.g., acid, bacteriocin, etc.) into the juice that has contributed to inhibition of colonic microbial infection. One such compound is lactic acid which potentially can reduce the pH of the colon to an acidic range unfavorable for spore germination and/or growth of pathogens such as C. difficile. The observed results of this postmarketing surveillance study are consistent with our earlier published in-vitro results that DBCH completely inhibits Clostridium difficile [48].
The overall conclusion of this postmarketing surveillance is that Doctor's Biome Colon Health® is a highly effective and safe dietary supplement to help relieve occasional diarrhea, gas and bloating; to help reduce the risk of colon microbial infections; and to support and replenish healthy colon microbiome.
Conflict of Interest
Dr. Robins and Dr. Kamarei are partners in Doctor's Biome Company (Newgen 27 LLC).Dr. Kamarei was paid as a consultant.
References
- Kim SK, Guevarra RB, Kim YT, et al. (2019) Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol 29: 1335-1340.
- Elangovan A, Allegretti JR, Fischer M (2019) Microbiota modulation-based therapy for luminal GI disorders: Current applications of probiotics and fecal microbiota transplantation. Expert Opin Biol Ther 19: 1343-1355.
- Arienaki MG, Kopaei MR (2018) Probiotics are a good choice in remission of inflammatory bowel diseases: A meta-analysis and systematic review. J Cell Physiol 233: 2091-2103.
- Scarpato E, Russo M, Staiano A (2018) Probiotics in pediatric gastroenterology: Emerging indications: Inflammatory bowel diseases. J Clin Gastrornterol 2017: S7-S9.
- Patel R, DuPont HL (2015) New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis 60: S108-S121.
- Everhart JE (2008) The burden of digestive diseases in the United States.
- CDC (2018) Summary health statistics: National health interview survey.
- Canadian society for intestinal research: Quality of life.
- Peery AF, Crockett SD, Murphy CC, et al. (2019) Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology 156: 254-272.e11.
- Davari DD, Negahdaripour M, Karimzadeh I, et al. (2019) Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods 8: 92.
- Gibson GR, Scott KP, Rastall RA, et al. (2010) Dietary prebiotics: Current status and new definition. Food Science and Technology Bulletin 7: 1-19.
- Binda S, Hill C, Johansen E, et al. (2020) Criteria to qualify microorganisms as "probiotic" in foods and dietary supplements. Front Microbiol 11: 1662.
- FAO/WHO (2002) Guidelines for the Evaluation of Probiotics in Food. FAO, Paris, 1-11.
- Swanson KS, Gibson GR, Hutkins R, et al. (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17: 687-701.
- Jotham S, Niv Z, Eran S, et al. (2019) The pros, cons, and many unknowns of probiotics. Nat Med 25: 716-729.
- Thad W, Jacqueline S (2017) Probiotics for gastrointestinal conditions: A summary of the evidence. Am Fam Physician 96: 170-178.
- Derwa Y, Gracie DJ, Hamlin PJ, et al. (2017) Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 46: 389-400.
- Jin LZ, Ho YW, Abdullah N, et al. (1998) Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett Appl Microbiol 27: 183-185.
- Tannock GW (2004) A special fondness for lactobacilli. Appl Environ Microbiol 70: 3189-3194.
- Goldin BR, Gorbach SL, Saxelin M, et al. (1992) Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 37: 121-128.
- Louis P, Flint HJ, Michel C (2016) How to manipulate the microbiota: Prebiotics. Adv Exp Med Biol 902: 119-142.
- Macfarlane GT, Steed H, Macfarlane S (2008) Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol 104: 305-344.
- Steed H, Macfarlane S (2009) Mechanisms of prebiotic impact on health. Prebiotics and Probiotics Science and Technology, Springer, New York, NY, USA, 135-161.
- Klatt NR, Canary LA, Sun X, et al. (2013) Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest 123: 903-907.
- Langen LV, Mirjam A, Dieleman LA (2009) Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis 15: 454-462.
- Ronald DH, Benjamin AP, Hillary RM, et al. (2019) Gut microbiome: Profound implications for diet and disease. Nutirients 2019 11: 1613.
- Nicola P, Rita C, Edoardo F, et al. (2018) Gut dysbiosis and irritable bowel syndrome: The potential role of probiotics. J Infect 76: 111-120.
- Zhongyi Z, Baoning W, Liyuan M, et al. (2020) Long-term exposure to ceftriaxone sodium induces alteration of gut microbiota accompanied by abnormal behaviors in mice. Front Cell Infect Microbiol 10: 258.
- Mckenzie YA, Thompson J, Gulia P, et al. (2016) British Dietetic Association systematic review of systematic reviews and evidence-based practice guidelines for the use of probiotics in the management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet 29: 576-592.
- Eamonn MMQ (2015) Probiotics in irritable bowel syndrome: The science and the evidence. J Clin Gastroenterol 49: S60-S64.
- Lucinda AH, Noemi B (2017) Modulation of the gut microbiota: A focus on treatments for irritable bowel syndrome. Postgrad Med 129: 872-888.
- Yuichiro Y (2017) Gut microbiota in health and disease. Ann Nutr Metab 71: 242-246.
- Khan I, Ullah N, Zha L, et al. (2019) Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 8: 126.
- Takayuki Y, Takahiro S, Moeko K (2017) Dietary and enteral interventions for Crohn's disease. Curr Opin Biotechnol 44: 69-73.
- Lev L, Irit AB, Ofer BB (2016) Probiotics and prebiotics in Crohn's disease therapies. Best Pract Res Clin Gastroenterol 30: 81-88.
- Antonella G, Valentina T, Fatima C, et al. (2018) Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health 15: 1679.
- Akihiko O, Balfour RS (2020) Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig Dis Sci 65: 757-788.
- Pace F, Pace M, Quartarone G (2015) Probiotics in digestive diseases: Focus on Lactobacillus GG. Minerva Gastroenterol Dietol 61: 273-292.
- Kathene CJH, Thomas RA, Richard YW, et al. (2016) Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Adv Nutr 7: 928-937.
- Maria JSL, Carolina GL, Julio PD, et al. (2015) The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. Biomed Res Int 2015: 505878.
- Christopher AL, Nicholas AK, Tim R, et al. (2019) British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68: s1-s106.
- Fujimori S, Gudis K, Keigo M, et al. (2009) A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 25: 520-525.
- (2018) Gastroenterology: Journal scan/research.
- Zipporah IE, Lakhbir K, Morris G, et al. (2020) Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev.
- Lakhbir K, Morris G, Patricia AB, et al. (2020) Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 3: CD005573.
- Acid tolerance of bacteria used in Doctors Biome™ DCUSA. Inc. subsidiary of Dupont, Danisco, USA.
- Alexandru H, Dang HN (2020) The competitive exclusion principle in stochastic environments. J Math Biol 80: 1323-1351.
- Robins HF, Kamarei AR (2020) Complete inhibition of Clostridium difficile with a probiotic juice beverage. J Gastroenterol Res 4: 104-111.
Corresponding Author
Dr. Howard F Robins, Chief Medical Officer, Doctor's Biome (Newgen 27 LLC), 80 Davids Dr., Unit 2, Hauppauge, NY 11788, USA, Tel: (631) 240-3066, Cell: (516) 967-1009.
Copyright
© 2021 Robins HF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.