Responses of Antioxidants Enzymes to Oxidative Stress in the Floral Species Drimia Maritime
Abstract
In natural habitats, plants that encounter adverse growth conditions are preserved in natural reserves where rare or endangered species, such the rare plant species "Drimia maritima" are protected. However, abiotic stress, generated by air pollution near Bentael natural reserve - Lebanon, where "Drimia maritima" is widespread, is suspected to induce alteration in the biochemical status of the plant. The antioxidant potential was determined by measuring the activities variations of five different enzymes related to stress: Catalase (CAT), Superoxide Dismutase (SOD), Ascorbate peroxidase (APX), Guaiacol peroxidase (POX), and Glutathione reductase (GR). The study was conducted in Bentael natural reserve that was exposed to road works on its borders, within three various locations: location 1 directly localized on the road (P1), location 2, opposite to P1 (P2) and a control location in the middle of the reserve (Ctrl). All enzymes showed higher activities in P1 than in the other sites with proportional correlations for all the studied sites. The resulting outcome demonstrate that alteration in enzymes activities are significant element in plant responses to environmental derivatives abiotic stresses which appraise the danger of landscape activities on conserved areas' wild floral species.
Keywords
Abiotic Stress, Antioxidant Enzymes, Antioxidant potential, Reactive Oxygen Species
Introduction
"Drimia maritima"(L.) Baker, is a medicinal, ornamental plant native to especially the Mediterranean area and which belongs to the family Liliaceae. It was registered as rare species by the UNEP (United Nations Environment Program), and, in Lebanon, it is mostly found in natural reserves. Unfortunately, in a Mediterranean Reserve, specifically in Lebanon (Bentael natural reserve- Byblos), "Drimia maritima" is facing the danger of air pollution: a new road has been inaugurated in the south area of the reserve and it is threatening its ecosystem balance by creating an environmental stress on the fauna and flora.
Abiotic stress, major factor that affects productivity of plants, is known to stimulate the generation of "Reactive Oxygen Species" (ROS). Although these species are natural byproducts of any cell, stresses manage to disrupt the cellular homeostasis by accumulating ROS. Therefore, to identify the stress condition, the detection of ROS is necessary and hydrogen peroxide was chosen as indicator of stress effects.
Toxic hydrogen peroxide is a ROS that can act both as reductant and oxidant. This form is the most structure of ROS with a speedy capacity of diffusion across cell membranes. It instigates oxidative damage in leaf cells beginning with tissue injury and leading to senescence promotion after disruption of cell integrity and metabolic function. Its damages reach surrounding cells and initiate an antioxidative response.
One of the forms of protection against oxidative damage is enzymatic defenses. Several enzymes are known to act as antioxidants, the most important are superoxide dismutase (SOD, E.C. 1.15.1.1), catalase (CAT, E.C. 1.11.1.6), guaiacol peroxidase (POX, E.C. 1.11.1.7), glutathione reductase (GR, E.C. 1.8.1.7) and ascorbate peroxidase (APX, E.C. 1.11.1.11) [1]. These enzymes, present in practically all cellular compartments, act as scavengers of ROS. The activities of these enzymes can serve as indicator of stress tolerance.
Material and Methods
Hydrogen peroxide determination
According to [2], 400-500mg of fresh leaves were homogenized with 5mL trichloroacetic acid (0.1%) in an ice bath. After homogenization, separation of the phase by centrifugation was done for 15 minutes at 3000g. The supernatant was then collected. 0.5mL of the supernatant was mixed with 0.5mL of potassium phosphate buffer (10mM, pH 7) and 1 mL potassium iodide (1M). The absorbance was recorded at 390nm.
Enzymes' extraction
Fresh leaves were thoroughly grounded in an ice bath with potassium phosphate buffer (100mM, pH 7) containing poly vinylpyrrolidone (1%) and 0.2g quartz sand for until no fibrous residue can be seen. After grinding, centrifugation was realized with MPW-350R refrigerated laboratory centrifuge at 4000 rpm for 15 min and the supernatant was collected for enzyme's assays [3].
Enzyme's activity assay
Superoxide dismutase activity: SOD activity was recorded by the diminution in absorbance of superoxide nitro blue tetrazolium complex by the enzyme. 0.1 mL of enzyme extraction was incubated with 3 mL of reaction mixture (0.5mL of 13µM methionine, 0.5mL of 63µMnitro-blue tetrazolium (NBT), 0.5 ml of 1.3µM riboflavin, 0.5 ml of 0.05M sodium carbonate and 1mL distilled water) for 10 min at 25oC under fluorescent lamp illumination. Absorbance was read at 560nm [4].
Catalase activity: CAT activity was estimated according to [5]. 1 mL of the enzyme extract was added to 4 mL of the reaction mixture (1 mL of 0.01 M H2O2 and 3 mL of 0.1 M sodium phosphate buffer). Reaction is initiated by adding hydrogen peroxide, and stopped after adding 10mL of 2% H2SO4and incubation at 25oC for 5min. The titration of the was realized against 0.005 N potassium permanganate.
Ascorbate Peroxidase activity: According to [6], 10µL of the enzyme extract was added to 1 mL of reaction mix (100 mM tris-acetate buffer at pH 7.0, 2 mM ascorbic acid, and 2 mM of H2O2). Every 100s, the absorbance decrease was noted at 290 nm following the oxidation of ascorbate and calculated using extinction coefficient ε=2.8 mM-1.cm-1.
Peroxidase activity: Guaiacol oxidation method was used to determine peroxidase activity. 3 mL reaction mixture (10 mM potassium phosphate buffer (pH 7.0), 8 mM guaiacol) was added to 10μl enzyme extract. The reaction was initiated by adding 0.5 mL of 1% H2O2. The absorbance's increase due to guaiacol oxidation, was recorded every 30 s at 470nm [7]. The unit of peroxidase activity was expressed using the extinction coefficient ε=6.39 mM1cm-1.
Glutathione reductase activity: Glutathione reductase was assayed using the method described by [8]. The reaction was initiated by adding 0.1 mL of 2 mM oxidized glutathione (GSSG) into the mixture (1 mL of 0.2 M potassium phosphate buffer (pH=7.5), 0.1 mL of 2 mM NADPH, 0.1 mL enzyme extract and distilled water to make up a final volume of 2.9 mL). The increase in absorbance at 412 nm recorded over a period of 5 min at 25℃ on Thermo/Evolution600/160908 UV/VIS Spectrophotometer.
Results and Discussion
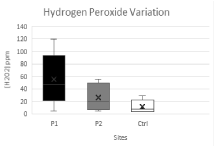
Hydrogen peroxide
Hydrogen peroxide levels in "Drimia maritima" leaves are drawn in Fig.1. A first remark to note is that the uppermost recorded levels of hydrogen peroxide are in P1. It is shown clearly a stark difference between P1, P2 and Ctrl. Hydrogen peroxide's level decreased from P1 to P2 to Ctrl, which indicated that oxidative stress is mainly established in P1.
Hydrogen peroxide has many essential roles in plant metabolism; it's involved in variety of reactions and signaling cascades necessary for all aspect of plant growth and integration of activities [9], but, at the same time, accumulation of hydrogen peroxide related to virtually any environmental stress is potentially damaging [10].
Superoxide dismutase
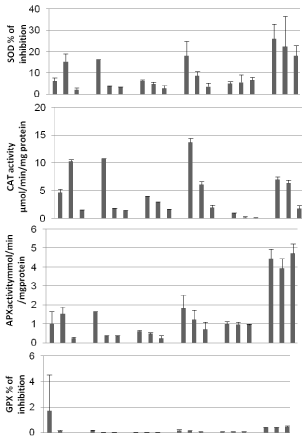
Plant is showing its highest SOD activity in P1 from both years except for May 2015 and June 2016. Ctrl is recording the lowermost activities side for all months except December 2016 year. In addition, SOD activity is increasing over the months from year to year. Airborne pollution stress had induced a gradual raise in the activity of SOD in "Drimia maritima" showing that the plant has established a strong antioxidant activity. Nonetheless, this raise in SOD activity is proven that airborne pollution stress is still affecting plants in the vicinity of road even after finishing road work activities. Superoxide dismutases constitute the first line of defense against ROS [11,12]. And because they are present in all subcellular locations, it is obvious that their activities increase with the establishment of oxidative stress.
Catalase
Catalase activity recorded its highest intensity in P1 sites all over the studied year except for May 2015 where P2 recorded the extreme activity. The increase of CAT activity is predicable according to the fact that SOD activity increased in the same manner. CAT has a high specificity for hydrogen peroxide [13], so its activity is related to the concentrations of the latter.
Ascorbate peroxidase
APX activity is an ascending order in P1and P2 all over the studied years except for May 2015. In Ctrl from December 2016, a sharp rise in activity was observed. APX is known to be anefficient regulator of ROS specially for being present in a diverse number of subcellular organelles [14].
Glutathione reductase
GR is showing its highest activity in P1 all over the months but a sharp rise in this activity was observed in December 2016. GR acts by recycling oxidized glutathione with the use of NADPH as co-factor [15].
Guaiacol peroxidase
GPX activity is higher in P1 in all years and months except for Ctrl in December 2016, this latter result concord with the one observed in SOD an APX activities, which suggest the presence of another kind of stress that could be from biotic origin.
In the presence of oxidative stress, the studied enzymes above (SOD, CAT, GR, APX, GPX) are considered the predominant ROS scavenging system in the plant. SOD is the only known enzyme to use a free radical as a substrate [16]; nonetheless, the reaction is followed by the generation of another ROS. The effectiveness of this system is in the increased activity simultaneously of CAT and peroxidases in order to scavenge hydrogen peroxide generated by SOD. Figure 2 confirm these postulations as it is noticed synchronized fluctuations of the levels of the three enzymes [17] (Table 1).
Multiple t-test were conducted to compare the studied parameters in the months for two years of study in June 2015, the enzymes presented significant differences In December 2015, the significance was restrained to hydrogen peroxide In the second year, the only enzyme that presented significant variances was catalase along with a difference in hydrogen peroxide As shown in the table above, the differences between the antioxidants and the stress markers indicated by their significance the presence of an intense stress (Table 2).
An analysis of variance (One way ANOVA) with tukey comparisons showed a significant difference of hydrogen peroxide between sites, months and years. The significant differences were observed in enzymes when comparisons were done between sites versus month and year.
Conclusion
In recent years, attention on the effect of anthropogenic activities on ecological communities has increased. Air quality is highly affected by human activities. Bentael nature reserve is facing a huge threat of road works on its borders [18]. The evaluation of the pollution used "Drimia maritima", a rare and sensitive species to pollution, to appraise the real danger of this threat, and specialized methods to estimate the oxidative stress generated from landscape activities [19]. The measurements of oxidative stress markers and scavengers in "Drimia maritima" demonstrated its fight against stress. The time-course evolution of Hydrogen peroxide (a Reactive Oxygen Species) contents revealed a high degree stress. Enzymatic antioxidants, presented at high values in P1, confirmed the results of the stress marker hydrogen peroxide and pointed the danger of these anthropogenic activities. Many solutions are described such as the implementation of weather stations that include pollution detectors, the establishment of biomonitoring programs and the most important proceedings that can be executed are the organization of Green belts around cities [18].
Acknowledgment
I would like to express my deepest and special gratitude to the CEO of the Lebanese Agricultural Research Institute Dr. Michel Afram, who contributed by financing, supporting and coordinating the project of the study.
References
- Abogadallah GM, Serag MM and Quick WP (2010) Fine and coarse regulation of reactive oxygen species in the salt tolerant mutants of barnyard grass and their wild-type parents under salt stress. Physiol Plant 138: 60-73.
- Karata I, Ozturk L, Demir Y, et al. (2014) Alterations in antioxidant enzyme activities and proline content in pea leaves under long-term drought stress. Toxicol Ind Health 30: 693-700.
- Rached kanouni M, Kehal L, Touaba C, et al. (2013) Change in Activity of Antioxidative Enzymes in Leaves of Acacia Retinodes , Biota Orientalis and Casuarina Equisetifolia Under Heat Stress Condition 9: 402-410.
- Zouari M, Ben Ahmed C, Elloumi N, et al. (2016) Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf 128: 195-205.
- Kiran CR, Rao DB, Sirisha N, et al. (2012) Impact of Germination on Biochemical and Antioxidant Enzymes of Ceiba pentandra (Kapok) Seeds. Am J Plant Sci 03: 1187-1192.
- Panda SK (2012) Assay Guided Comparison for Enzymatic and Non-Enzymatic Antioxidant Activities with Special Reference to Medicinal Plants. Intech.
- Konieczny R, Banas AK, Surówka E, et al. (2014) Pattern of antioxidant enzyme activities and hydrogen peroxide content during developmental stages of rhizogenesis from hypocotyl explants of Mesembryanthemum crystallinum L. Plant Cell Rep 33: 165-177.
- Abdelghani C, Mouna L, Salama A, et al. (2017) Electrolyte ions and glutathione enzymes as stress markers in Argania spinosa subjected to drought stress and recovery. African J Biotechnol 16: 10-21.
- Jubany-Marí T, Munné-Bosch S and Alegre L (2010) Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiol Biochem 48: 351-358.
- Cheeseman JM (2007) Hydrogen Peroxide and Plant Stress : A Challenging Relationship. Plant Stress 1: 4-15.
- Alscher RG, Erturk N and Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53: 1331-1341.
- Kar RK (2011) Plant responses to water stress: Role of reactive oxygen species. Plant Signal Behav 6: 1741-1745.
- Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2: 53.
- Pandey VP, Awasthi M, Singh S, et al. (2017) A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem Anal Biochem 06.
- Rellán-Álvarez R, Hernández LE, Abadía J, et al. (2006) Direct and simultaneous determination of reduced and oxidized glutathione and homoglutathione by liquid chromatography-electrospray/mass spectrometry in plant tissue extracts. Anal Biochem 356: 254-264.
- Pal P, Singh A (2019) In-vitro Antioxidant and Anti-inflammatory Activities of Quisqualis indica Linn. Leaves Extract. J Adv Biol Biotechnol 21: 1-13.
- Kavitha Krishna R, Krishnakumari S and Chandrakala S (2012) Evaluation of antioxidant properties of different parts of Amorphophallus commutatus, an endemic aroid of Western Ghats, South India. Int J Pharma Bio Sci 3: 443-455.
- Houri T, Khairallah Y, Zahab AAl, et al. (2020) Heavy Metals Accumulation Effects on The Photosynthetic Performance of Geophytes in Mediterranean Reserve. J King Saud Univ 32: 874-880.
- Khairallah Y, Houri T, Haddad G, et al. (2016) Can the damage caused by anthropogenic activities on Urginea maritima in Bentael natural reserve be a signal of health problems? Middle East Conference on Biomedical Engineering, MECBME. IEEE 6-9.
Corresponding Author
Yara R Khairallah, Lebanese Agricultural Research Institute, Fanar-Metn-Beirut-Lebanon P.O. Box 90-1965, Orcid: 0000-0002-9581-0780.
Copyright
© 2022 Khairallah YR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.