Fine Root Response of Poplar Trees Pollarded at the End of the Growing Season
Abstract
Aim: The effectiveness of wide-spaced-planted poplar trees (Populus spp.) for reducing shallow landslide occurrence relies mainly on the extensive root development of individual trees and root interlocking between neighbouring trees. This study aimed to determine the impact of pollarding at the end of the growing season on the fine root structure of poplar trees aged 11 years.
Methods: One group of three trees were pollarded (P) in early autumn 2012, and were compared with another group of three unpollarded (UP) trees. Changes in root dynamics after pollarding were determined by the soil core method at different times during one year.
Results: Fine root length and mass density was 60 to 80% lower in P than UP trees particularly during late spring 2012 and early autumn 2013, but only at 0-150 mm soil depth. One year after pollarding, fine root density of P trees was similar to pre-pollarding values.
Conclusions: Pollarding has its major impact in fine roots located in shallow soil, during the peak and the last stage of the growing season one year after pollarding.
Keywords
Populus spp, Fine roots, Shallow landslide, Pollarding, pastoral hill country, Tree pasture system
Introduction
Vulnerability to soil erosion of pastoral hill country in New Zealand increased dramatically following the replacement of forest by pasture in the late 19th and early 20th [1,2]. Soil erosion, represented principally by shallow mass movement erosion processes directly impacts pasture productivity through losses of productive soil and water holding capacity [1,3].
Populus (poplar) and Salix (willow) trees wide-space planted (10 to 15 m apart) in pastoral hill country of New Zealand have proved the most effective tool against erosion, reducing incidence and extent of shallow landslides after erosion-inducing storms by 70% to 95% [4,5]. Populus and Salix spp can be easily established from large unrooted poles with minimum protection (plastic sleeves) under stock grazing presence, they are fast growing, and they have extensive root systems. However, almost all poplars planted in pastoral hill country for slope stabilization have received negligible or no above-ground management [6,7]. Due to lack of management, many poplars have grown very large, resulting in excessive shading of understorey pasture. Large poplars are also prone to breakage of branches and toppling during strong winds, particularly where the soil water content is high, which are liabilities to farm infrastructure and livestock [8].
Managing poplars for tree size can be achieved by pruning or pollarding (complete removal of tree crown) [6,7]. In order to reduce the likelihood of unwanted epicormic shoot regrowth [9], insect attack or fungal wound infections, pruning and pollarding are recommended to be conducted late in the growing season from February to March (autumn) when soil water contents are often low compared with other times of the year [10].
Despite the benefits derived from managing tree size (timber, fodder, increase in light transmission to understorey pasture), canopy removal can cause intense changes in the tree photosynthate source-sink balance [11,12]. This balance might be particularly challenged when canopy removal occurs at the end of the growing season when carbohydrate energy reserves in the stem and roots are at their minimum [11,13,14]. Depending on the magnitude of the imbalance between source and sink photosynthates, structures remaining after pollarding, particularly the most labile such as fine roots, might die [15,16]. The contribution of fine roots to slope stabilization is substantial because they can comprise more than 90% of the total root length of poplar trees [17,18]. In this study the objective was to determine the immediate and short-term impact of pollarding in autumn on the fine root structure of mature poplar trees.
Materials and Methods
Site description
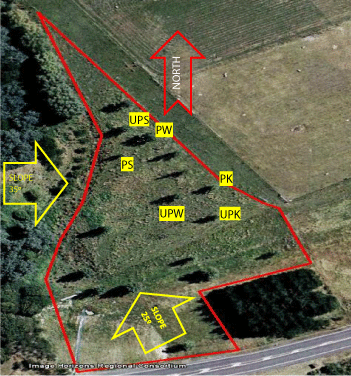
The study was located at the Massey University Equestrian Facility, which is near the Turitea Stream in Palmerston North in New Zealand (40.4°S, 175.6°E) (Figure 1). The site comprised flat terrain at the edge of a small valley bordered by slopes of 25° and 35° at the southern and western sides, respectively. At the bottom of the slopes there was a swampy zone between 2 and 3 m wide that provided moisture to the surrounding area. Parts of the study paddock were rush-infested (Juncus spp.), with water ponding a recurrent phenomenon after periods of heavy or prolonged rainfall. The soil is a Manawatu fine sandy loam, classified as a weathered fluvial recent soil [19], which corresponds to a Stagnic Fluvisol characteristics [20].
In 2001 2.5 m long poplar (Populus spp) poles of different clones were randomly planted for a tree density of 33 stems per hectare. Distance between trees involved in this study ranged from 10 to 30 m apart from each other.
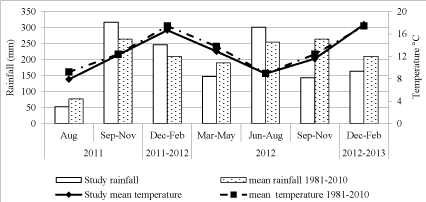
Rainfall and mean temperatures during the study are shown in (Figure 2). Rainfall in the 2011-2012 growing season (September-February) exceeded the long-term (1981-2010) average whereas in the 2012-2013 growing-season, rainfall was about 45% less than the long-term average [21].
Trees and experimental design
The diversity in clones and micro-environmental variation at the study site precluded using all available trees in the study (Figure 1). Avoiding trees on excessively wet or sloping land and the desire to minimise the range of clones involved in the study resulted in the selection of six trees, which were randomly allocated to two groups, pollarded (P) and unpollarded (UP). Each group had a representative tree of the three clones 'Kawa' (K) (Populus deltoides Marsh. × yunnanensis Dode); 'Shinsei' (S) (P. nigra L. × maximowiczii Henry.) and 'Weraiti' (W) (P. deltoides Marsh. × nigra L.) (Figure1). Immediately before pollarding, the six trees were measured for diameter at breast height (1.40 m; DBH) using a diametric tape and height using a clinometer. Trees in the P group were pollarded to 1.8 m above the ground in early autumn 2012 (March 25th) when they were still foliated. The total woody (non-leafy) dry biomass of P trees above pollarding height was measured (Table 1).Trees in the UP group remained unpollarded, as a control to compare the changes in the root system following pollarding. Initial differences in height and DBH between the two groups of trees were not significant (Table 1).
One year after study commencement DBH and height were measured again (Table 1). Resprouting activity in P trees was measured one year after pollarding by recording number and total basal diameter of shoots > 2 mm basal diameter. The length of the longest shoot was also estimated one year after pollarding (Table 1).
Root density dynamics after pollarding
To determine the root dynamics following pollarding, roots were recovered by the soil core sampling technique, using a steel corer (8 cm internal diameter) [22,23]. In order to determine the most appropriate coring depth, trenches were excavated in March 2012 to observe root distribution. Around two trees at the site ('Shinsei' and 'Weraiti'), additional to the experimental P and UP trees, three trenches (1.5 m length × 1.0 m depth × 1.2 m width) perpendicular to transects 120° apart from each other were dug at 2 m from the trunk. Roots protruding from the smoothed wall of each trench were counted over a 90 cm × 90 cm mesh steel frame with 15 cm × 15 cm squares. We found 97% and 98% of lateral and oblique roots were within 0 to 450 mm soil depth for the 'Weraiti' and 'Shinsei' clones, respectively, with more than 90% of the total roots being fine roots. Hence roots were sampled from soil cores collected in 150 mm increments down to 450 mm soil depth. Radial coring position from the tree trunk was based on the results of poplar root system excavations conducted by McIvor et al. . They found that roots 2-5 mm diameter comprised 14.1, 4.9, 11.2, 14.6, 20.7, 19.6 and 15.0% of the total length at distances from the trunk of 0-0.5, 0.5-1, 1-2, 2-4, 4-6, 6-8 and 8+ m respectively. Assuming most of fine roots branch from 2-5 mm diameter roots and to reduce the likelihood of encountering large roots ( > 20 mm diameter), and the intermeshing of roots from neighbouring trees, soil cores were taken at 2 m from the tree stem. Soil cores were collected in early autumn 2012 (March 23rd) (2 days before pollarding), late autumn (May 15th), late winter (August 27th), late spring (November 19th) and in early autumn 2013 (March 4th). At each sampling, to account for potential variability in root distribution around the tree, three sampling positions were located 120o apart from each other around the tree trunk. At the initial sampling, coring positions were located at 0, 120 and 240°, subsequent samplings were conducted 20° clockwise from the previous coring positions.
Soil cores were washed under a jet water tap and live roots were recovered using a 1 mm sieve. Live roots were distinguished from dead roots by colour, turgidity and tissue integrity using a dual illuminated (top and light box) desk stand 8× magnifier (Otzuka SKK-CL™). Blackness and loss of cortex turgidity or complete cortex and/or stele absence were used as criteria to classify and discard roots as dead. After recovery, the roots were preserved in 70% ethanol in plastic tubes until measured for length and mass.
For length measurement, roots were sorted into coarse roots (> 2 mm), small fine roots (sfr) (>1≤2mm) and very fine roots (vfR) ( ≤ 1 mm). This study focuses only on roots ≤ 2 mm diameter. Root diameter classification and length measurement were simultaneously conducted by using an optical scanner and Win RHIZO software [24]. The scanned roots were then oven dried at 75°C to constant weight and weighed for root mass estimation.
Statistical analysis
At each sampling, root density data for the three sampling positions around individual trees were averaged and expressed as per m3 of soil. All data were analysed as a repeated measures design with trees as subjects. The MIXED procedure of the SAS software 9.4 [25], was used with an autoregressive correlation structure to take account of the repeated measures. For root density dynamics, treatments (UP and P), sampling time and their interactions were considered in the model as fixed effects and separate analyses were run for each depth (0-150 mm, 150-300 mm and 300-450 mm) and for three different root diameter categories: overall fine roots (fR ≤2 mm), small fine roots (sfR 1-2 mm) and very fine roots (vfR ≤ 1 mm).A Shapiro-Wilk test was used to test for normal distribution of the data. Log natural transformation of data was only required for the length density of fine roots located at the 0-150 mm soil depth.
Results
Above ground tree responses
Initial DBH and height were not significantly different between treatments (Table 1). One year after pollarding, P trees had an 85% lower increase in DBH and a 3-fold greater increase in height growth than UP trees (Table 1). Unpollarded trees showed a DBH increment of 3.9 cm whereas P trees had a much reduced increment of 0.6 cm. The tallest tree at pollarding ('Weraiti') had the lowest height growth increment one year after pollarding, whereas the shortest tree ('Shinsei') at the same time had, along with the 'Kawa' tree, the greatest increments in height growth (Table 1).There was considerable variation between pollarded trees in their shoot regrowth characteristics, with 'Weraiti' having the least resprouting with respect to all attributes (Table 1).
Fine root length and mass density dynamics of poplar trees after pollarding
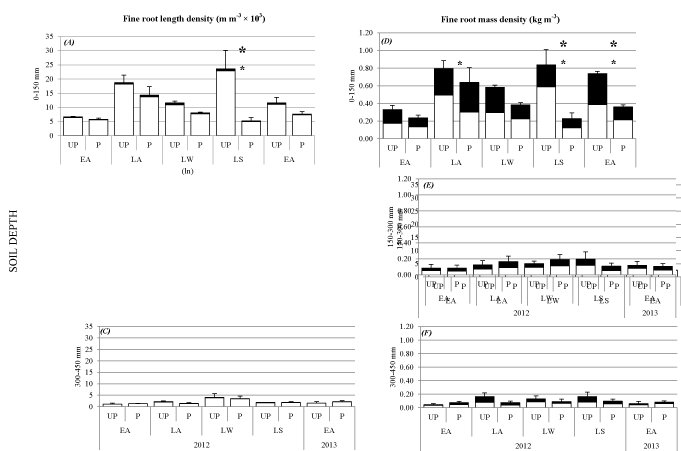
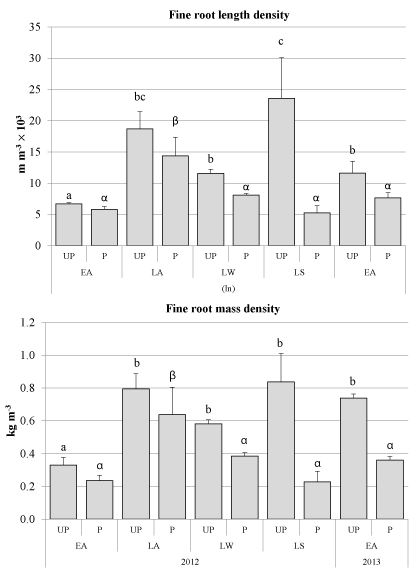
Responses to pollarding were found only at 0-150 mm soil depth with fRLD and vfRLD varying between UP and P trees within particular sampling times and within treatments between sampling times (P < 0.05). (Figures 3A) Below 150 mm soil depth, major differences in fRLD of P trees were not detected between sampling times and there were no significant differences in fRLD between P and UP treatments (Figures 3A-C).
Immediately after pollarding from early autumn to late autumn, at the 0-150 mm soil depth, both UP and P trees showed increases in fine root length and mass densities by more than 2-fold (P < 0.01) (Figure 4). Within this period of time and extending to late winter fRLD and vfRLD did not vary significantly between P and UP trees, but by late spring, fRLD and vfRLD for the P trees were about 80% less than for UP trees (P < 0.05; Figure 3A). By early autumn 2013, fRLD of UP trees had decreased since spring, resulting in similar densities in the UP and P treatments (Figure 3A).
The seasonal patterns for fRMD of P tree sat 0-150 mm soil depth followed those for fRLD (Figures 3A, D). However, in contrast to vfRLD, significant differences in vfRMD between P and UP treatments were detected sooner in late autumn 2012, two months after pollarding (Figures 3A, D). There after, fRMD and vfRMD did not vary between treatments until late spring 2012 and early autumn 2013 (Figure 3D). In late spring fRMD and vfRMD of P trees averaged about 75% less than for UP trees (P < 0.05; Figure 3D), and this pattern was also found in the following autumn but the relative difference between the treatments had reduced from 75% less to 50% less (P < 0.05; Figure 3D).
From 150 mm to 450 mm soil depth, fRMD did not vary significantly between the P and UP treatments at any of the sampling times (Figures 3E, F).
One year after pollarding, at 0-150 mm soil depth, fRLD and fRMD of P trees were similar to values recorded before the trees were pollarded (Figure 4). In contrast, in March 2013 (early autumn), fRLD and fRMD of UP trees were about 2-and 2.4-fold greater (P < 0.05) than in March of the previous year, respectively (Figure 4).
Discussion
Fine root density dynamics of poplar trees after pollarding
After conducting high intensity tree above-ground removal practices such as coppicing or as in this case, pollarding, the appearance and early growth of new photosynthetic tissue completely relies on resources stored in the tree remnant structures [11,13].
Reductions in fine root densities are frequently reported following complete tree canopy removal. An immediate reduction in fine root length and mass densities following late summer pollarding or coppicing was reported for tree willow [26], poplar [27] and Quercus [28] with very fine roots (< 1 mm diameter) experiencing the greatest decline, which was still apparent to 300 mm soil depth one year following treatment [27,28]. Jones et al. [29] reported that the main changes in fRLD of Prosopis juliflora and Acacia nilotica following pruning, occurred in the top 400 mm of soil 60 days after trees were pruned with Prosopis juliflora showing a reduction of 45% in fine root length density. In this study impact of pollarding was also only observed in the thinnest fine roots (< 1 mm diameter) and at a shallow soil above 150 mm depth.
Seasonal root dynamics observed in the pollarded poplar trees in the current study showed that impact of pollarding is not uniform throughout the year but it diminishes during late winter and enhances during the growing season in late spring. Spring-summer is a high demand resources season and available photosyntates must be likely channeled to restore tree above-ground structure in pollarded trees rather than to support their root appearance and extension.
Root tensile strength measured as force per root cross-sectional area is greater in fine than in coarse roots (>2 mm diameter), and therefore in soil shear planes perpendicular to the soil surface, more fine roots can withstand greater tension stresses than a few coarse roots [30]. Large contribution of fine roots to the total tree root length system estimated to be around 80 to 90% imposes a significant importance to this type of roots in the root soil binding and the control of shallow soil mass movements [17].
Out of the four sampling periods conducted at the current study after pollarding, fine root mass was affected in three of those, whereas fine root length once. When significant differences were found, fine and very fine root densities of P trees were about 40 to 20% that recorded in UP trees, particularly in late spring, when both root length and mass density were simultaneously compromised by pollarding. Spring in New Zealand is also one of the seasons with the greatest rainfalls during the year [31]. Additional to mechanical reinforcement, presence of high fine root densities across the slope contributes also to sustain the tree transpiration process working and maintain soil pores water pressure within margins that reduce the risk of shallow mass movements [32,33].
Practical implications on the size management study of poplar trees used for soil conservation purposes on hill pastoral land in New Zealand.
Impact of pollarding in the root structure of trees wide-space planted for soil erosion control on hill pastoral land in New Zealand, has recently started to be studied. Similar proportional reductions in fine root density as those reported in the current study were also observed by McIvor et al. [26] in pollarded willow trees. In their study, McIvor et al. [26] reported that one year after pollarding in late summer for fodder purposes, pollarded trees had around 50% and 60% less very fine root length and mass density respectively than unpollarded trees, and that similar densities to unpollarded trees were not recovered until 3 to 4 years after pollarding.
Impact on the coarse root structure (roots > 2 mm diameter)of Populus × euramericana 'Veronese' trees was still observed 8 years following pollarding when root length and mass structure of pollarded trees was 32% and 68% less, respectively, than that recorded in similar aged non-pollarded trees [34].
When establishing trees on hilly pastoral land for soil erosion control purposes, the main aim is they grow and develop a root structure that interlocks neighboring trees creating a soil protective root network, with like the steel mesh in the concrete. Under the environmental and soil conditions of a hill country site in New Zealand, McIvor et al. [18] estimated that in an ideal square pattern plantation where poplar poles are planted 10 m apart, it would take around nine and a half years for the trees to start interlocking with neighboring trees. Older and bigger trees would provide greater root densities and seemingly higher slope stability. However, older and bigger trees would also come with a considerable cost in pasture production reduction, along with a variety of problems associated with oversized trees such as breakage of limbs, toppling of entire trees and subsequent damage on infrastructure or streams and roads blockage [35].
The latest raises the question about which individuals the impact of pollarding on the root structure should be compared to. Against unpollarded trees that eventually will reach undesirable sizes? or against the root densities of the very same trees observed at least the year before being pollarded, as long as they have reached the soil protective root network? Proposing the latter as the answer, it is suggested a better understanding can be drawn from the pollarding impact if subsequent studies monitor the trees' seasonal root densities the year previous to pollarding along with different time lapses after pollarding.
Field work with mature trees in natural or semi natural stands usually imposes methodological constraints that are not always easy to control. This study had limited clonal representation so the data should be treated with some caution. On the other hand, pasture-poplar stands on hilly land with a variety of clones' presence, might be a strategy to take in to account when soil reinforcement is considered after tree canopy removal techniques are conducted. There is evidence showing that spatial response of the poplar trees fine root system after complete canopy removal, might be clonally driven [27]. Poplar clones showing differential impact in the vertical fine root structure might help to maintain reasonable fine root densities across the vertical soil structure from the top soil to deeper sections, which all together could contribute to maintain the soil integrity.
Conclusions
Within the first year following pollarding of the poplar trees there was a substantial reduction in their fine root length and mass. However, the impact was not uniform thorough the year after pollarding, but was particularly observed during the growing and heavy rainy season, which would compromise their ability to maintain the soil and counteract forces stressing the soil integrity. When pollarding is required at a particular pasture-poplar tree location, current technical recommendations advise that no more than a quarter or a third of the trees on the paddock are pollarded, and that the pollarded trees are distributed as much as possible across the slope. Results from this study align which such recommendations. It remains to be studied how long it would take for pollarded poplar trees to recover fine root densities similar to pre-pollarding levels.
Acknowledgements
We thank the technical staff of Massey University, Mark Osborne, Ian Furkert and Brian Best for their field and laboratory assistance.
References
- Basher L, Botha N, Dodd M, et al. (2008) Hill country erosion: a review of knowledge on erosion processes, mitigation options, social learning and their long-term effectiveness in the management of hill country erosion.
- McIvor I, Douglas G, Dymond J, et al. (2011) Pastoral Hill Slope Erosion in New Zealand and the Role of Poplar and Willow Trees in Its Reduction. Soil Erosion Issues in Agriculture.
- Mackay AD (2008) Impacts of intensification of pastoral agriculture on soils: Current and emerging challenges and implications for future land uses. N Z Vet Jrnl 56: 281-288.
- Hawley JG and Dymond JR (1988) How much do trees reduce landsliding. J Soil Water Conserv 43: 495-498.
- Douglas GB, McIvor IR, Manderson AK, et al. (2013a) Reducing shallow landslide occurrence in pastoral hill country using wide-spaced trees. Land Degrad Dev 24: 103-114.
- National Poplar and Willow Users Group (2007) Growing Poplar and Willow trees on Farms. National Poplar an Willow Users Group, New Zealand.
- McIvor I (2015) Trees for the farm: A decision support tool for farmers. Plant and Food research, Auckland, New Zealand.
- Douglas GB, Wall AJ, Dodd MB, et al. (2013b) Balancing pastoral and plantation forestry options in New Zealand and the role of agroforestry. 22nd International Grassland Congress, Proceedings 1003-1010.
- Desrochers A, Maurin V and Tarroux E (2015) Production and role of epicormic shoots in pruned hybrid poplar: effects of clone, pruning season and intensity. Ann For Sci 72: 425-434.
- New Zealand Poplar Commission (1995) Poplar Agroforestry: an introduction to using poplars for timber. Palmerston North.
- Kozlowski TT (1992) Carbohydrate sources and sinks in woody-plants. Bot Rev 58: 107-222.
- Chesney PE (2012) Shoot pruning and impact on functional equilibrium between shoots and roots in simultaneous agroforestry systems. Agroforestry for biodiversity and ecosystem services-science and practice 87-112.
- Dickmann DI and Pregitzer KS (1992) The structure and dynamics of woody plant root systems. Ecophysiology of short rotation forest crops. Elsevier Applied Science, Essex, England 95-123.
- Dickmann DI, Nguyen PV and Pregitzer KS (1996) Effects of irrigation and coppicing on above-ground growth, physiology, and fine-root dynamics of two field-grown hybrid poplar clones. For Ecol Manag 80: 163-174.
- Chesney P and Vasquez N (2007) Dynamics of non-structural carbohydrate reserves in pruned Erythrina poeppigiana and Gliricidia sepium trees. Agrof Syst 69: 89-105.
- Landhäusser S and Lieffers V (2012) Defoliation increases risk of carbon starvation in root systems of mature aspen. Trees 26: 653-661.
- Coleman MD, Dickson RE and Isebrands JG (2000) Contrasting fine-root production, survival and soil CO2 efflux in pine and poplar plantations. Plant Soil 225: 129-139.
- McIvor I, Douglas GB, Hurst SE, et al. (2008) Structural root growth of young Veronese poplars on erodible slopes in the southern North Island, New Zealand. Agrofor Syst 72: 75-86.
- Hewitt AE (2010) New Zealand soil classification. Manaaki Whenua-Landcare Research New Zealand Ltd. Lincoln, NZ.
- WRB (2014) World Reference Base for Soil Resources 2014, update 2015. FAO, Rome.
- Porteous A and Mullan B (2013) The 2012-13 drought: an assessment and historical perspective. MPI Technical Paper No: 2012/18. NIWA, M.f.P.I.b. (Ed.), Wellington, New Zealand p: 48.
- Böhm W (1979) Methods of studying root systems. Springer-Verlag, Berlin New York.
- Oliveira G, Van Noordwijk M, Gaze S, et al. (2000) Auger sampling, ingrowth cores and pinboard methods. Springer pp: 175-210.
- Regent Instruments Inc, 2012. WinRHIZO. Canada.
- SAS II (2014) Base SAS® 9.4 Procedures Guide: Statistical Procedures. SAS Institute Inc Cary, NC.
- McIvor I, Hedderley D and Mason K (2020) Effect of pollarding for fodder on fine root dynamics of soil conservation willow trees in New Zealand. Adv Environ Stud 4: 252-260.
- Lee DK (1994) Effects of coppicing on the growth and distribution of hybrid poplar roots. Bull Seoul Natl Univ Arbor 14: 30-38.
- Ma C, Zhang W, Wu M, et al. (2013) Effect of aboveground intervention on fine root mass, production, and turnover rate in a Chinese cork oak (Quercus variabilis Blume) forest. Plant Soil 368: 201-214.
- Jones M, Sinclair E and Grime VL (1998) Effect of tree species and crown pruning on root length and soil water content in semi-arid agroforestry. Plant Soil 201: 197-207.
- Stokes A, Atger C, Bengough AG, et al. (2009) Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 324: 1-30.
- NIWA (2010) Baseline Tables: Rainfall and temperature tercile tables based on the 30-year period 1971-2000. National Institute of Water and Atmospheric Research, Ministry for Primary Industries.
- Gray DH and Sotir RB (1996) Biotechnical and soil bioengineering slope stabilization: a practical guide for erosion control. John Wiley & Sons, New York.
- Reubens B, Poesen J, Danjon F, et al. (2007) The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: a review. Trees 21: 385-402.
- McIvor I, Douglas G, van den Dijssel C, et al. (2019) Pollarding wide-spaced poplar trees on pastoral hillslopes alters root development. Agrofor Syst 93: 2227-2241.
- National Poplar and Willow Users Group (2007) Growing Poplar and Willow trees on Farms.
Corresponding Author
Mauricio Maldonado, School of Agriculture and Environment, Massey University, Private Bag 11222 Palmerston North 4442, New Zealand.
Copyright
© 2022 Maldonado M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.