The Challenge of Global CO2 Reduction: The Potential of the Method Based on Seawater Electrolysis
Introduction
The atmospheric CO2 concentration dramatically increased in the last 300 years, due to fossil fuels consumption and deforestation. From approximately 280 ppm before the start of the industrial revolution, about a 140 ppm upsurge was estimated. This phenomenon is understood to be responsible for a rise in global temperature, which will lead to glaciers melting and sea levels rising. Consequently, the research focused on atmospheric carbon capture and storage is fundamental for achieving the goals of the Paris Agreement concerning climate change mitigation.
A primary strategy to face these problems is to reduce the consumption of fossil fuels, by introducing, as an example, electric engines and renewable energies. Nevertheless, another essential task is the sequestration of the existing CO2 excess in the atmosphere and its stable storage. From this point of view, for several years the most promising technique was the geological sequestration through injection and confinement of liquefied CO2 into selected deep underground rock formations (such as saline reservoirs and depleted oil/gas fields). However, the hazard of potential CO2 leakage is the main weak point related to this method. A more recent alternative was the geochemical sequestration, based on CO2 injection into minerals that may drive carbonation reactions, producing stable carbonate rocks and implying a negligible risk of return to the atmosphere [1,2].
In our opinion, geochemical sequestration can be efficiently used and optimized by exploiting both seawater electrolysis and the oceans' natural CO2 absorption feature.
Keywords
Carbon dioxide, Seawater, Electrolysis, Mineralization, Climate change
Oceans CO2 Absorption
The oceans store about 60 times more CO2 than the atmosphere, cover over 70% of the Earth's surface, and absorb approximately 25% of the anthropogenic CO2 emissions.
The CO2 assimilation is achieved both through biologically-mediated and chemically-mediated sequestration: the former includes the processes that regulate the inorganic carbon incorporation into organic matter (photosynthesis by phytoplankton) and the transport to the deep sea (the portion of organic carbon not converted back to CO2 via the food chain, sinks to seafloor sediments); the latter is based on the reaction of CO2 with seawater to form carbonic acid, that breaks into hydrogen ions and bicarbonate (a chemical form of carbon that does not easily escape the ocean).
Background to our proposal
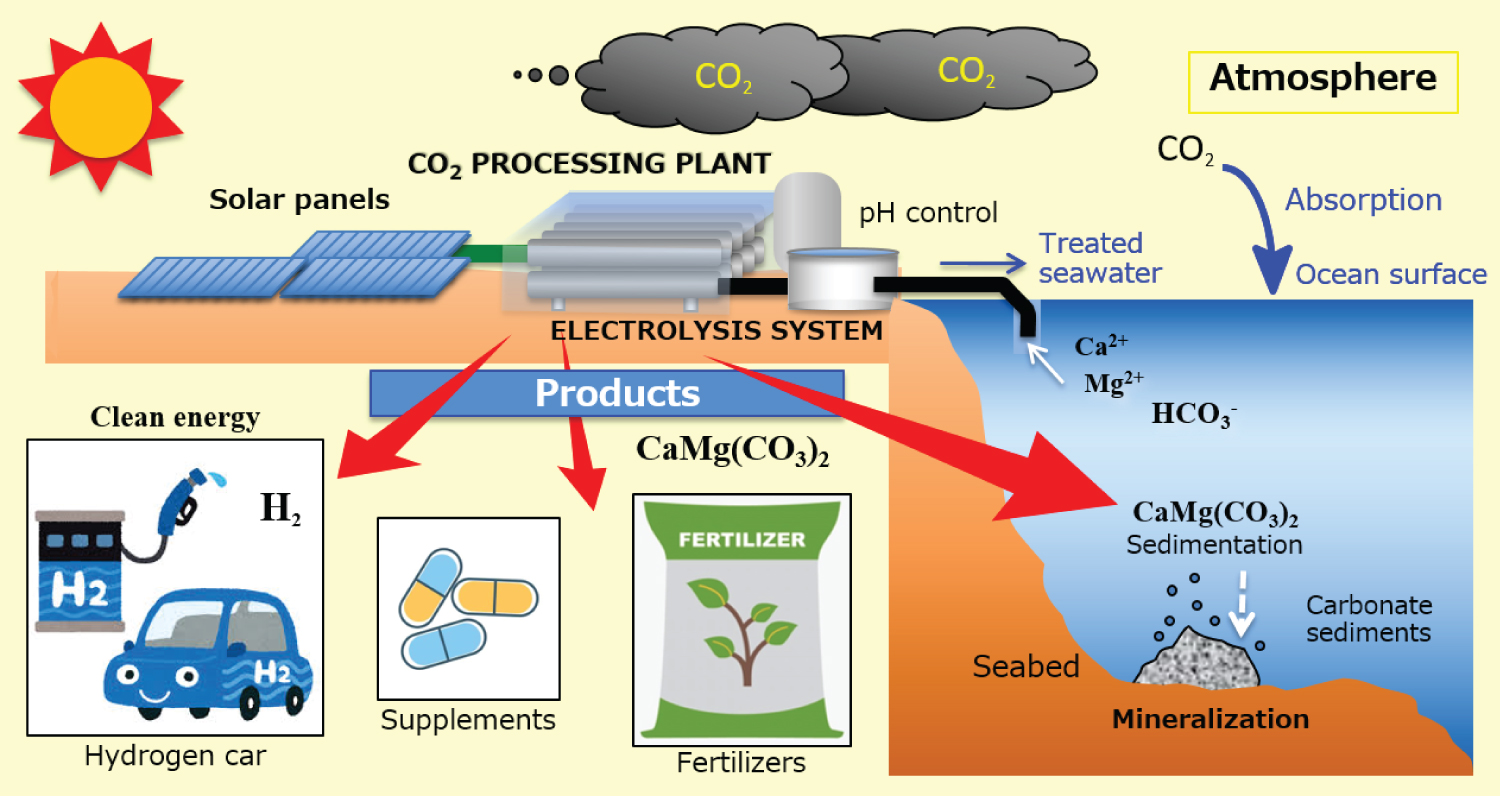
Our research group previously proposed, in the past, a method for reducing CO2 in the atmosphere based on the ocean's carbonate chemistry [3,4]. According to that scheme, when seawater is electrolyzed the dissolved CO2 (incorporated in the carbonate family ions) reacts with the calcium-magnesium components producing insoluble carbonate minerals CaMg(CO3 )2. If the process is carried out on the seawater surface layer the concentration of carbonate ions on the ocean's surface will decrease and the absorption of atmospheric CO2 should be enhanced. Moreover, seawater electrolysis produces hydrogen, which can be stored and used/sold as an energy resource. The electrolysis system must be performed using carbon-free energy (such as solar, to do not release CO2). A similar approach was recently proposed by other researchers, confirming the validity and feasibility of the idea [5].
However, it must be considered that if the produced carbonate minerals are left to sediment in the ocean's deep layer the seawater alkalinity will tend to decrease, the ability to absorb CO2 will be reduced, and effects on the marine environment may occur. Therefore, we propose to revise the process as follows.
Suggestions for an improved and efficient CO2 sequestration plant based on seawater electrolysis
The main part of our CO2 sequestration plant will be the electrolysis system, which could be built on the coast or as an offshore platform. The energy will be provided by a dedicated solar power system (Figure 1).
The insoluble carbonate minerals will be retrieved and reused for many purposes (such as the production of fertilizers or drugs/supplements, use in the construction industry, etc.) instead of letting them deposit on the seabed.
Due to the effect of increased CO2 concentration in the atmosphere and the abovementioned natural CO2 absorption by the ocean, the seawater pH showed a decrease of about 0.05 in the last 30 years. As a consequence, it is expected that the CO2 absorption rate will tend to decrease. Therefore, it will be important not only to pretreat/purify the input seawater (before the treatment), to produce water pure enough for electrolysis, but also to control the characteristics of the output seawater (after the treatment) and to check/restore the pH and the chemical components (Ca and Mg) depleted during the process.
The main reactions of the electrolysis process will be: on the cathode of the electrolytic cell will take place the reactions producing hydrogen (H2O + e- → ½H2 + OH-) and carbonate minerals (Ca2+ + Mg2+ + 2HCO3- + 2OH- → Ca-Mg(CO3)2 + 2H2O); on the anode, through the water-splitting reaction (H2O → 2H+ + ½O2 + 2e-), chloride ion oxidation reaction will be achieved (2Cl- → Cl2 → Hydrolysis → ClO-).
The ClO- ion may affect the marine ecosystem due to its potential effect on the biota (it has biocide property), and it is produced proportionally to the electrolysis rate. It can be eliminated using activated carbon or electrolytic reduction treatment.
As a result of small-scale experiments, no CO2 release occurs during the production of carbonate mineral precipitates. In addition, we verified that by mixing the cathode-treated seawater and the anode-treated seawater, water having a slightly alkaline pH compared to the input seawater is obtained. Therefore, it is also possible to contrast the acidification of seawater and to promote the absorption of atmospheric CO2.
To sum up, the main features of the proposed method are:
1) Seawater electrolysis can effectively sequestrate atmospheric CO2.
2) CO2 is converted into solid stable carbonate minerals.
3) Useful resources (minerals and hydrogen gas) can be generated and reused.
4) By using carbon-free electricity (solar or wind energy) new CO2 is not released.
5) It can evolve as a new type of business.
This proposal requires the examination and contribution from experts in many branches of environmental sciences. We will be glad to receive comments and opinions.
References
- Blondes MS, Merrill MD, Anderson ST, et al. (2018) Carbon Dioxide Mineralization Feasibility in the United States. USGS Scientific Investigations Report 2018-5079.
- Li Q, Liu G (2016) Risk Assessment of the Geological Storage of CO2: A Review. Geologic Carbon Sequestration. Springer 249-284.
- Tatenuma K (2008) A Proposal for Global Reduction Method of Atmospheric CO2 - Isolation of surplus CO2 from the biosphere and fix it to form an insoluble mineral onto a sea bottom. Carbon Dioxide Reduction Metallurgy 35-43.
- Tatenuma K, Suzuki J, Arai O, et al. (2003) Carbonate Production Behavior through Electrolysis Treatment of Seawater, and Its Effect. Bulletin of the Society of Sea Water Science 57: 103-112.
- La Plante EC, Simonetti DA, Wang J, et al. (2021) Saline water-based mineralization pathway for gigatonne-scale CO2 management. ACS Sustainable Chem Eng 9: 1073-1089.
Corresponding Author
Fabio Spaziani, Kaken Inc, 1044 Hori, Mito 310-0903, Ibaraki, Japan.
Copyright
© 2022 Tatenuma K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.