Genotoxic Evidences of Glyphosate and Chlorpyriphos on Eisenia fetida Coelomocytes
Abstract
The organophosphorus herbicide glyphosate (GLY) and the organophosphate insecticide chlorpyriphos (CPF) are key pesticides in modern management cultures worldwide. Sublethal toxicity of the commercial herbicide formulation Roundup® and the insecticide formulation Terfos® were evaluated on Eisenia fetida coelomocytes exposed under in vivo and ex vivo laboratory conditions. Induction of DNA single-strand breaks evaluated by the single cell gel electrophoresis assay and coelomocyte viability as well as alterations in coelomocyte trophic indexes were employed as endpoints for genotoxicity and cytotoxicity, respectively. Specimens were exposed at concentrations corresponding to recommended pesticide field application rate, and endpoints were evaluated after 7 and 14 days of treatment (in vivo exposure). In addition, coelomocytes were exposed to aqueous leachate of pesticide-contaminated soils during 1 h (ex vivo exposure). Earthworms exposed to Roundup® and Terfos® showed an increased frequency of DNA damage. Also, a decrease of coelomocyte viability and decrease of trophic indexes were observed in all treatments. The results demonstrate that either GLY- and CPF-based formulations exerted genotoxic as well as cytotoxic effects in coelomocytes of E. fetida exposed in vivo and ex vivo.
Keywords
Commercial formulations, DNA damage, Herbicides, Insecticides, Single cell gel electrophoresis assay, Sublethal effects
Introduction
The widely use of pesticides in modern agricultural enables increased crop yields. However, pesticides residues can contaminate agricultural and adjacent lands and become an ecotoxicological threat to non-target organisms, included humans. Nowadays, results almost impossible for many countries to decrease the use of pesticides without altering crop yields as agriculture gradually transformed into a high-tech system for satisfying the world's growing demands for food, feed, fiber and fuel [1,2]. It is known that pesticides can also be hazardous whether not appropriately employed since many of them may represent potential hazards to the environment due to the contamination of soil, water, air and food [3]. In addition, anthropogenic activities are continuously introducing large amounts of these compounds into the environment regardless of their persistence, bioaccumulation and toxicity and thus increasing their jeopardizing effects (www.epa.gov/pesticides).
Glyphosate is a non-selective herbicide widely used worldwide for post-emergent control of annual and perennial plants including weeds on a great variety of crops. It is a polar, highly water soluble substance that makes complexes easily. It binds tightly to the soil particles, reaching a usual half-life of 45-60 days in soil and persistence from 222 to 835 days [4]. By the other hand, Chlorpyrifos is a broad spectrum organophosphate insecticide that is widely used to control insect pest in agricultural fields. Likewise, Chlorpyrifos is a moderately persistent environmental contaminant, with a half-life ranging from several days to months [5]. High volumes of agrochemicals applied to great variety of crops, together with agricultural expansion and its persistence in the ecosystem generate great concerns due to the impact for the environment and large risk implicated for wildlife.
The ecotoxicological effects of pesticides can be assessed by monitoring the use of laboratory toxicity test. Earthworms, among soil organisms, are considered highly appropriate terrestrial model organisms for ecotoxicity test. They are powerful regulators of soil processes participating in the maintenance of its structure and regulation of organic matter dynamic [6-8]. Not only due to their natural contact with the soil but also for the ingestion of soil, earthworms can be easily influenced by pollutants. Thus, earthworms are sensitive indicators of anthropogenic stress factors and then are used as model organisms in the environmental risk assessments of chemicals [6,9]. In particular, the species Eisenia fetida has been widely used for soil toxicity assessments because standardized tests are available [10-12] Survival, growth, and reproduction are the usual endpoints for such tests, which provide eco-toxicologically relevant information. However, ecotoxicological approach by using biomarkers at low levels of biological organization is useful for damage detection before the community is affected [13]. The need to detect and assess the effects of contamination at sublethal levels has led to the development of molecular and cellular indicators of exposure to and effects of contaminants, referred as biomarkers [14,15].
The use cellular and molecular biomarkers can be complementary approach providing information about organism-stress response to toxicants before higher levels are affected [14]. In this sense, genotoxic evaluation is very important due to genotoxic influence can lead changes in one or more generations [16,17]. It can result in reduced fertility of soil populations, and thus causing biodiversity depletion [18].
It is well known that the single cell elelectrophoresis (SCGE) assay, also called comet assay, has been shown to be a sensitive and recommended method for the evaluation of DNA damage in individual cells induced by different xenobiotics [16,18-21]. In particular, the SCGE when applied on earthworms has resulted as a high sensitivity bioassay for evaluating the genotoxic damage induced for wide group of pollutants. Among them, metals [22-25] polycyclic aromatic hydrocarbons [23] and other organic compounds [26], ionic [27] and, overall, several pesticides [28-32] can be included.
Earthworm coelomocytes are the group of circulating cells present in the coelomic cavity [23,33,34,35]. Based on cytomorphometric, ultrastructural and cytochemical properties, coelomocytes can be classified into three major cell groups, namely eleocytes, hyaline amoebocytes and granular amoebocytes [36]. It has been reported that the proportion of the different cellular types of coelomocytes may be related to the health and the immune earthworm responses [23,28,35]. Thus, changes in cell proportions in the coelomic fluid of organisms exposed to different pollutants can be evaluated by means of this parameter as a reliable cytotoxic biomarker, as suggested elsewhere [15,37,38]. In agreement, it has been demonstrated that eleocyte proportions of another oligochaeta Dendrobaena veneta decreased after exposure to cadmium and copper [36].
The purpose of this study was to evaluate the toxicity of two commercial formulations of GLY and CPFon E. fetida exposed in vivo under laboratory conditions to treated soils and to its aqueous leachates as ex vivo exposure of extruded coelomocytes. DNA single-strand breaks, viability and coelomocyte counts of exposed organisms were employed as endpoints for genotoxicity and cytotoxicity, respectively. Pesticides were selected because they are commonly used as agricultural chemicals with intensive and overlapping applications in agricultural fields, not only in Argentinean soybean crops, but also around the world in many applications for the treatment of transgenic and non-transgenic agronomic crops [39].
Materials and Methods
Chemicals
Agrochemicals used included the 48% isopropylamine salt of glyphosate-based [N-(phosphonomethyl] glycine; CAS1071-83-6) commercial grade trade formulation Roundup® (Monsanto S.A.I.C., Buenos Aires, Argentina) and the 48% chlorpyriphos-based (O,O-diethyl O-3,5,6-trichloropyridin-2-ylphosphorothioate; CAS 2921-88-2) commercial grade trade formulation Terfos®(Chemotecnica S.A., Buenos Aires, Argentina). Hydrogen peroxide (H2O2, CAS 7722-84-1) was obtained from Sigma-Aldrich Co. (St. Louis, MO) whereas copper (II) chloride 2-hydrate (CuCl2.2H2O, CAS 10125-13-0) was purchased from Biopack Co. (Buenos Aires, Argentina). All other chemicals and solvents were of analytical grade. Nominal concentrations of GLY and CPF were controlled by HPLC-UV and GC-MS methods respectively according APHA [40].
Test organism
Specimens of E. fetida adults, average wet weight 300 mg, were purchased from local source (Luján, Buenos Aires, Argentina). Earthworms were maintained in moistened control soil (pH 6.6 ± 0.26, 25% sand, 48% slime, 27% clay, moisture 40-60% of water holding capacity, WHC 60 ± 5 mL/100 g), at room temperature (RT) under natural photoperiod and fed with 10% of alfalfa forage. The worms were allowed to acclimate to laboratory conditions for several weeks before testing. Specimens were maintained in plastic containers in a control soil corresponding to a natural soil of the experimental surrounding field of National University of Lujan, previously characterized elsewhere [24].
Pesticide exposure
The genotoxicity, cytotoxicity and trophic indexes in coelomocytes of E. fetida exposed in vivo and ex vivo were determinate. The control soil was artificially treated with both pesticides chosen. The applications rate corresponded to the manufacturers recommended application dose. Applied dose were CPF 1 and 2 L/ha (480 and 960 g CPF/ha) and GLY 2.5, 4 and 6 L/ha (1200, 1920 and 2880 g GLY/ha). The artificial treatment of soils was performed simulating the conditions of application followed by producers in the field. A soil surface of minimum depth and 0.04 m2 was used. Thus, soils sieved to 1000 µm were distributed in a homogeneous layer of 1 cm of depth in a glass vessel. Soil was treated with the corresponding amount of pesticide as application recommended rate of manufacturers. Commercial formulations were dispersed in Milli-Q water and applied into the soil by using a commercial sprayer. Once treated with pesticides, soils were mechanically homogenized and used immediately to avoid volatilization losses. To evaluate endpoints of ex vivo manner, coelomocytes were extruded of untreated organisms and incubated 1 h (RT) in the soil leachates. Soil leachates were prepared from pesticides-treated soils to evaluate the mobility of self inter environment compartments. Soil leachates were prepared according to US EPA [41] recommendations. For SCGE assay, solutions of PBS and H2O2 (100 µM in PBS) were used as negative and positive controls, respectively [19,23]. Solutions of PBS and CuCl2 were used as negative and positive controls, in cytotoxicity evaluation, respectively [15,38]. To evaluate endpoints of in vivo manner, coelomocytes were extruded of organism exposed during 7 and 14 days in pesticides-treated soils. For each test, 750 g fresh weight of the test medium (control soil and pesticides-treated soils) was placed into each plastic container and then adult earthworms with clitellum observable were added. The containers were covered with perforated plastic film to prevent the test medium from drying and kept under the test conditions for 7 and 14 days. Two replicates for each treatment were performed.
Coelomocyte extrusion
At the end of the exposure period either for in vivo and ex vivo treatments, a non-invasive extrusion method was used for collecting earthworm coelomocytes according to Di Marzio, et al. [23]. Earthworms were rinsed in tap water at RT and placed on a damp paper towel overnight to void gut contents during the extrusion procedure. Afterwards, organisms were immersed in an extrusion medium consisted in 5% v/v ethanol in saline solution (0.85% NaCl, 2.5 mg/mL EDTA, pH 7.5). A pooled castings of five organisms was obtained and then placed into centrifuge tubes containing 2 mL of extrusion medium/individual and incubated for 1 min at RT. Coelomic fluid containing the extruded cells was diluted in calcium and magnesium free phosphate-buffered saline (PBS), washed twice, and centrifuged at 2000 rpm (4 ℃, 10 min), and then coelomocyte pellets resuspended in 2 mL of PBS.
Coelomocyte counts and in vivo and ex vivo cytotoxicity
Extruded cells were counted using a counting chamber improved Neubauer hemocytometer. The extruded cells were characterized according to their morphology as eleocytes, amoebocytes or granulocytes according to Adamowicz and Wojtaszek and Adamowicz [34,36]. After cell counting, the following trophic indexes were calculated as follows:
Absolute trophic index earthworm (ATIE): En/Cn, where En is total eleocytes number average per individual/mL of celomic fluid and Cn is total coelomocytes number average per individual/mL of celomic fluid. Relative trophic index earthworm (RTIE): ATIE/wwf where wwf is wet weight without feces.
The cell viability was expressed as the percentage of viable cells measured with 0.4% of Trypan blue. One hundred cells were counted on each slide and three replicate slides were analyzed per specimen. Data were expressed as average of total viable coelomocytes per individual/mL of coelomic fluid. Copper, as CuCl2, was used as positive control according Irizar, et al. [38] and Svendsen and Week [15].
Single cell electrophoresis (SCGE) assay
The SCGE assay protocol proposed by Di Marzio, et al. [23] was used. Assays were performed under indirect incandescent light at 4 ℃. Gels were composed of three layers of agarose. The suspensions of earthworm's cells were diluted (1:2) with 1% low-melting-point agarose (LMPA) giving a final agarose solution of 0.66% and then 80 µL of the cell suspension were transferred to a slide having a thin layer of solidified 0.5% normal-melting agarose. The slides were covered with a coverslip and left on ice for 10 min to allow the second layer of agarose to solidify. The coverslip was gently removed, and 80 µL of 0.5% LMPA were spread over the second layer. A coverslip was placed on top of third layer and the agarose solidified. This last coverslip was removed and each slide was immersed in freshly prepared cold lysing solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris (pH 10), 1% N-laurylsarcosinate, 1% Triton X-100 and 10% dimethyl sulfoxide (DMSO) during 10 min. Slides were then placed in an electrophoresis tank and covered with electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH 13.5) for 25 min at RT to allow unwinding. Electrophoresis (300 mA, 30 min, 1 V/cm) was then performed in the some buffer. The slides were washed in the neutralization buffer (0.4 M Tris, pH 7.5, 10 min). Afterwards, slides were stained with 30 µL of 20 µ/mL ethidium bromide solution. The images of nucleoids were analyzed with Nikon Eclipse 600, microscope provided with epifluorescence (541-560 nm excitation filter and 590 nm emission filter) linked to an image analysis system (Image Pro Plus, V4.0, Media Cybernetics, Maryland, USA). The images obtained were analyzed with the CASP software [42]. Tail DNA% was used as final genotoxicity endpoint. The % Tail DNA comet assay parameter was chosen as it is not measured in arbitrary units, being more meaningful and advisable for regulatory purposes and for inter-laboratory comparisons [43].
Statistical analysis
Cytotoxicity and genotoxicity data were analyzed by non-parametric Kruskal-Wallis, and median and Dunn tests using Statistica software version 8.0 [44,45]. The Student t-test was used for pair comparisons in in vivo experiments between 7 and 14 days. The chosen level of significance was 0.05 unless indicated otherwise.
Results
Cell viability
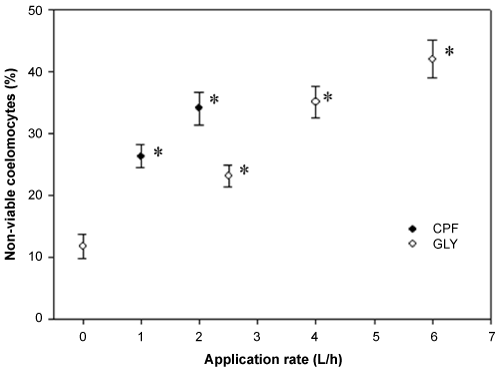
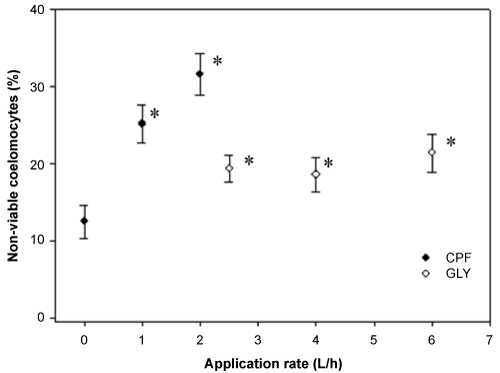
All assayed concentrations of CuCl2, ranged between 1 to 100 µg/mL, were statistically different with respect to negative controls (PBS). Coelomocyte cytotoxicity fell within the expected values showing confidence limits at 95% between 13.68 - 80.07 µg/mL, and LC50-1 hour mean of 33.10 µg/mL. The values for coelomocyte viability for ex vivo and in vivo exposure are indicated in Figure 1 and Figure 2, respectively. Coelomocytes exposed to aqueous leachate of pesticides-treated soils showed a significant increase (p < 0.05) in the percentage of non-viable cells (Figure 1). Both pesticides produced the increase of coelomocytes cytotoxicity in a positive concentration-response relationship. Earthworms exposed in vivo of pesticide-treated soils during 14 days showed a significant increase (p < 0.05) in the percentage of non-viable coelomocytes (Figure 2). CPF-based formulation showed an increase of non-viable cells concomitant with increase of application rates. On the contrary, GLY-based formulation showed a similar and significant increase (p < 0.05) of non-viable cells at all application rates evaluated. For the same application rate coelomocytes exposed of ex vivo manner showed higher toxicity than coelomocytes exposed of in vivo manner.
Trophic indexes (ATIE and RTIE)
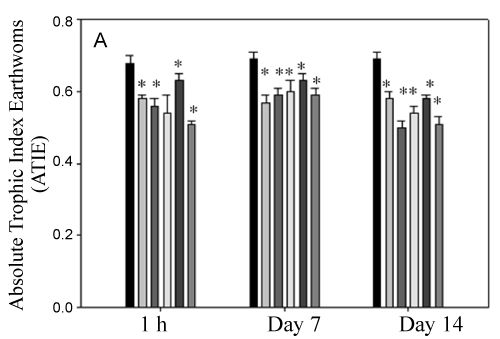
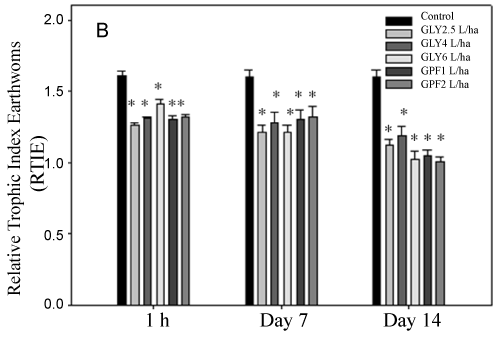
Values for calculated trophic indexes are showed in Figure 3A and Figure 3B. The number of eleocytes in control earthworms during in vivo and ex vivo exposure remained without significant differences among all of them during the experiment. After in vivo exposure to pesticides-treated soils, the total number of eleocytes decrease significantly (p < 0.05) after 7 and 14 days of treatment. ATIE and RTIE indexes, that have into account the total number of eleocytes, decreased for both pesticides and exposure times (p < 0.05).
SCGE assay
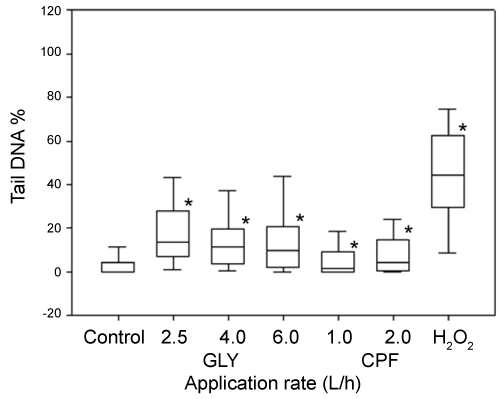
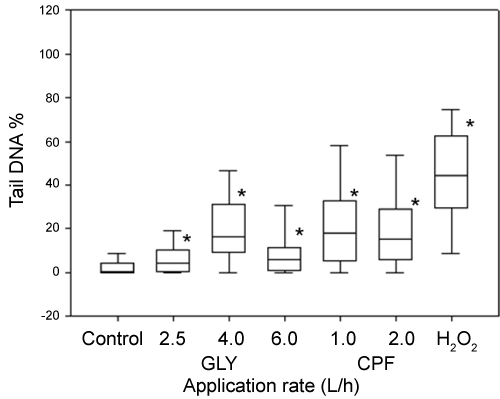
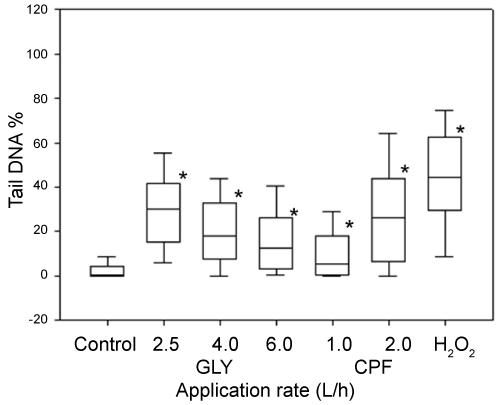
DNA damage, measured as % of tail DNA, in coelomocytes exposed to 100 µM H2O2 was statistically different with respect to PBS solutions (negative controls). All pesticides-treated soils leachates exerted genotoxic effects in coelomocytes after 1 h of exposure (Figure 4). In the case of DNA damage after 7 and 14 days of exposure, the entire evaluated application rates were statistically different with respect to the control soil for both pesticides (Figure 5 and Figure 6). With the respect to the exposure time, different results were observed between both pesticides. In the case of GLY-based formulation a significant increase (p < 0.05) was observed in DNA damage for the application rates of 2.5 and 6 L/ha between 7 and 14 days of exposure. By the other hand the application rate of 4 L/ha did not show difference between 7 and 14 days of exposure. In the case of CPF-based formulation, the application rate of 1 L/ha showed a significant decrease (p < 0.05) in the Tail DNA% between both exposure time. On the contrary, the application rate of 2 L/ha showed a significant increment in the Tail DNA% between 7 and 14 days of exposure.
Discussion
Pesticides usually enter the soil as sprays applied to crop plants from above ground, and in a lesser extend when applied directly on soils. In the environment, terrestrial organisms are most commonly exposed to application rate of pesticides recommended by manufacturers. The current study examined the toxic effects of two pesticides widely used at application rate recommended and over a non-target organism such as E. fetida. The results considering the viability response, DNA damage and trophic indexes showed that both pesticides exerted deleterious response in coelomocytes of E. fetida exposed in vivo and ex vivo.
Beside, results further demonstrated that it was possible to evaluate the DNA damage by using the SCGE assay, cytotoxicity and cellular proportions as non-invasive biomarkers starting from the coelomic cells or coelomocytes from exposed earthworms.
Ecological hazard assessment of chemicals has traditionally relied on the use of Standard Toxicity Tests (OECD, ISO), which in soils are based on short and long-term experiments using several terrestrial organisms [46]. It was recommended that an acute toxicity test with E. fetida was being employed as a valid test standard for soil evaluation [46]. Because of the low sensitivity of the acute endpoint mortality, alternative methods with more sensitive endpoints have to be checked as the chronic earthworm reproduction test [10]. However, the application of the latter is complicated because it requires 56 days of exposure against 14 days of acute exposure. Thus, it is necessary the development of sensitive biomarkers at short exposure times. In this aspect, the analysis of toxicity response taking into account cell types and trophic indexes was used as effective biomarker. Coelomocytes are free-circulating immune cells in the coelomic fluid, and have a central function in the earthworm's immunity against environmental pathogens and toxicants [28,35,36,38]. Coelomocytes are the first line of active defense. Due to this functional characteristic could led us to compare earthworm coelomocytes with human leukocytes as they share similar immunobiology [28]. Thus, the use of coelomocytes of E. fetida is an accurate technique for the toxicity assessment of pesticides in earthworms. The absolute and relative trophic index earthworm (ATIE and RTIE) were sensitive for to evaluate short and longer exposure times. These indexes take in account the density of eleocyte cells, which are mainly related with nutritive and immune functions [36]. The change in the relative proportions of coelomocytes seems indicate a physiological stress in earthworms provoked for the pesticides exposure [35].
Studies on the biological effects of currently used pesticides have increased in recent years. Evaluation of genotoxic effects of treated-soil pesticides and its leachate have acquired particular importance, especially in the case of a constant exposure as result of repeated applications. Thus, due to genotoxic influence can lead to changes in one or more generations [16,17], is very important to determinate in a sensitive manner any plausible interactions between pesticides and DNA. Pesticides tend to be very reactive compounds that can form covalent bonds with various nucleophilic centers of cellular biomolecules, including DNA [47]. Besides, it was also showed that several pesticides induce reactive oxygen species (ROS) formation which may be involved in the production of DNA-single strand breaks [30,32,48].
Two methods can be used to evaluate DNA damage: the micronucleus test and Comet assay, the latter being much more sensitive than the former [23,29]. In the present study, the SCGE or comet assay was used as a rapid and sensitive method for determine genotoxicity by measuring DNA damage such as single- and double-stranded DNA breaks as well as alkali-labile sites [16,20,21]. In the case of GLY, the results observed in the present study showed an increase of DNA migration in coelomocytes of E. fetida exposed both in vivo and ex vivo. These results are in accordance with previous reports of genotoxic effects provoked by GLY-based formulations by using several endpoints. Bolognesi, et al. [48] observed that GLY increase sister chromatid exchanges in human peripherical blood and adducts formation in kidney and liver cells in bone marrow cells. Grisolia [49] observed an increase in MN frequency in Tilapia rendalli fish. Vera-Candioti, et al. [50] observed the induction of primary DNA damage in peripheral blood cells of the ten spotted live-bearer fish Cnesterodon decemmaculatus. Also, different authors have obtained positive genotoxic results for GLY-based formulations through comet assay using a variety of cells and organisms. Clements, et al. [51] observed DNA damage in circulation blood cells of Rana catesbeiana tadpoles. Cavas and Konen [52] observed an increase of DNA damage on peripheral erythrocytes in Carassius auratus fish. Mañas, et al. [53] observed an increase in DNA migration in Hep-2 cells exposed in vitro manner. Schaumburg, et al. [54] observed an increase in DNA damage in Salvator merianae neonates after embryonic exposed to GLY-based formulations. In the case of earthworm, Casabé, et al. [29] observed no difference in DNA migration when earthworms were exposed to GLY-treated soils during 7 days. However, it has been reported that for GLY formulations, toxicity depends greatly on the surfactant employed in the formulation [55].
In our results, GLY-based formulations showed an increase in DNA damage in coelomocytes exposed in vivo manner between 7 and 14 days at application rates of 2.5 and 6 L/ha. Accumulation of DNA damage may occur either through and increase in the number of DNA-damaging events or due to a decrease in DNA repair capacity and/or antioxidant system [56]. Also this results shows that GLY-based formulations is persistent in the soil for 14 days in our assays conditions, according to its usual half-life of 45-60 days in soil and persistence from 222 to 835 days data [4].
In the case of CPF, we observed an increase of DNA migration due to the induction of DNA damage in coelomocytes of E. fetida exposed both in vivo and ex vivo. Few reports have determined the relationship between CPF and DNA damage. Exposure to CPF-based formulations, acutely or chronically, caused a dose-dependent increase in DNA damage in the liver and brains of rats [57] and also the production of reactive oxygen species that caused considerable cytotoxicity in PC12 cells [58]. By the other hand, Li, et al. [59] observed an increase in DNA migration on HELA and HEK293 cells exposed to CPF. In terrestrial organisms, Casabé, et al. [29] and Piola, et al. [31] observed an increase in DNA damage in coelomocytes of earthworm E. Andrei exposed to CPF.
With the respect to time of exposure, coelomocytes exposed to CPF-based formulations showed two responses. In organisms exposed at lowest application rate, DNA migration showed an decrease (p < 0.05) between 7 and 14 day of exposure. This result could be explained for the stimulation of DNA repair machinery. On the contrary, at highest application rate, DNA migration showed an increase (p < 0.05).
Both pesticides increased the extent of DNA migration in E. fetida coelomocytes exposed in vivo and ex vivo manner. This increase it is connected with the induction of cytotoxicity, since the Tripan Blue method showed that the tested concentration were not toxic to these cells.
When leachate effects are analyzed, we observed an increase of DNA damage in E. fetida coelomocytes exposed ex vivo to both pesticides. Previous reports have demonstrated that runoff is a one of the major source of non-point pesticide contamination of steams [60]. Thus, pesticides are capable to migrate into the liquid phase and representing, then, a high ecological risk for aquatic biota [39,61,62]. The behavior of pesticides on the environment will depend not only on their intrinsic properties but also on environmental conditions and agricultural practices. Thus, pesticides can exert deleterious effects in aquatic organisms as previously reported. Biomarkers are an important element in the ecological risk assessment of pesticide pollution. Also, pesticides genotoxicity is a matter of interest, and its environmental detection is an important topic. The different ways in which pesticides are being applied are continuously increasing, as well as the resultant risks. The use of pesticides is recommended, in order to obtain the beneficial effects in crops, but without to produce effects in the biota and in human health.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Acknowledgements
This study was supported by grants from the National University of Luján and the CONICET from Argentina.
References
- Heinemann JA, Massaro M, Coray DS, et al. (2014) Sustainability and innovation in staple crop production in the US midwest. International Journal of Agricultural Sustainability 12: 71-88.
- Larramendy ML, Soloneski S (2012) Integrated pest management and pest control - current and future tactics. InTech 668.
- WHO-FAO (2009) Pesticides residues in food. Rome: World health organization and food and agriculture organization of the united nations 1-426.
- Al-Rajab AJ, Amellal S, Schiavon M (2008) Sorption and leaching of 14C-glyphosate in agricultural soils. Agronomy for Sustainable Development 28: 419-428.
- Palma P, Palma V, Matosa C, et al. (2009) Assessment of the pesticides atrazine, endosulfan sulphate and chlorpyrifos for juvenoid-related endocrine activity using daphnia magna. Chemosphere 76: 335-340.
- Johnston AS, Hodson ME, Thorbek P, et al. (2014) An energy budget agent-based model of earthworm populations and its application to study the effects of pesticides. Ecological Modelling 280: 5-17.
- Lavelle P, Spain AV (2001) Functioning of the soil system. In: Lavelle P SA, Soil Ecology. Springer Netherlands, Dordrecht, 357-529.
- Sanchez-Hernandez JC (2006) Earthworm biomarkers in ecological risk assessment. Rev Environ Contam Toxicol 188: 85-126.
- Rombke J, Jansch S, Didden W (2005) The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol Environ Saf 62: 249-265.
- ISO (1998) Soil quality-effects of pollutants on earthworms (Eisenia fetida)-Part 2: Determination of effects on reproduction of eisenia (ISO 11268-2). International Standard Organization.
- OECD (1984) Guideline for Testing Chemicals N° 207. Earthworm acute toxicity test. Organization for Economic Cooperation and Development.
- OECD (2004) Guideline for Testing Chemicals N° 222. Earthworm reproduction test (Eisenia fetida/Eisenia andrei). Organization for Economic Cooperation and Development.
- Vasseur P, Cossu-Leguille C (2003) Biomarkers and community indices as complementary tools for environmental safety. Environ Int 28: 711-717.
- Calisi A, Lionetto MG, Schettino T (2009) Pollutant-induced alterations of granulocyte morphology in the earthworm Eisenia foetida. Ecotoxicol Environ Saf 72: 1369-1377.
- Svendsen C, Weeks JM (1997) Relevance and applicability of a simple earthworm biomarker of copper exposure. I. Links to ecological effects in a laboratory study with Eisenia andrei. Ecotoxicol Environ Saf 36: 72-79.
- Cotelle S, Ferard JF (1999) Comet assay in genetic ecotoxicology: a review. Environ Mol Mutagen 34: 246-255.
- Jha AN (2004) Genotoxicological studies in aquatic organisms: An overview. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 552: 1-17.
- Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23: 207-221.
- Collins AR, Oscoz AA, Brunborg G, et al. (2008) The comet assay: Topical issues. Mutagenesis 23: 143-151.
- Singh NP, McCoy MT, Tice RR, et al. (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175: 184-191.
- Tice RR, Agurell E, Anderson D, et al. (2000) Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35: 206-221.
- Bigorgne E, Cossu-Leguille C, Bonnard M, et al. (2010) Genotoxic effects of nickel, trivalent and hexavalent chromium on the Eisenia fetida earthworm. Chemosphere 80: 1109-1112.
- Di Marzio WD, Saenz ME, Lemiere S, et al. (2005) Improved single-cell gel electrophoresis assay for detecting dna damage in eisenia foetida. Environmental and Molecular Mutagenesis 46: 246-252.
- Di Marzio WD, Saenz ME, Montivero C, et al. (2007) Genotoxicity of aqueous elutions of industrial soils. Bulletin of Environmental Contamination and Toxicology 79: 483-487.
- Voua Otomo P, Reinecke SA, Reinecke AJ (2014) Using the comet assay to assess the combined and separate genotoxic effects of Cd and Zn in Eisenia andrei (Oligochaeta) at different temperatures. Bulletin of Environmental Contamination and Toxicology 92: 285-288.
- Liu Y, Zhou Q, Xie X, et al. (2010) Oxidative stress and DNA damage in the earthworm Eisenia fetida induced by toluene, ethylbenzene and xylene. Ecotoxicology 19: 1551-1559.
- Guo Y, Liu T, Zhang J, et al. (2016) Biochemical and genetic toxicity of the ionic liquid 1-octyl-3-methylimidazolium chloride on earthworms (eisenia fetida). Environmental Toxicology and Chemistry 35: 411-418.
- Casabe N, Piola L, Fuchs J, et al. (2007) Ecotoxicological assessment of the effects of glyphosate and chlorpyrifos in an Argentine soya field. Journal of Soils and Sediments 7: 232-239.
- Han Y, Zhu L, Wang J, et al. (2014) Integrated assessment of oxidative stress and Dna damage in earthworms (eisenia fetida) exposed to azoxystrobin. Ecotoxicol Environ Saf 107: 214-219.
- Piola L, Fuchs J, Oneto ML, et al. (2013) Comparative toxicity of two glyphosate-based formulations to Eisenia andrei under laboratory conditions. Chemosphere 91: 545-551.
- Wang J, Wang J, Wang G, et al. (2016) DNA damage and oxidative stress induced by imidacloprid exposure in the earthworm Eisenia fetida. Chemosphere 144: 510-517.
- Zang Y, Zhong Y, Luo Y, et al. (2000) Genotoxicity of two novel pesticides for the earthworm, Eisenia fetida. Environ Pollut 108: 271-278.
- Hayashi Y, Engelmann P, Foldbjerg R, et al. (2012) Earthworms and humans in vitro: Characterizing evolutionarily conserved stress and immune responses to silver nanoparticles. Environ Sci Technol 46: 4166-4173.
- Adamowicz A, Wojtaszek A (2001) Morphology and phagocytotic activity of coelomocytes in Dendrobaena veneta (Lumbricidae). Zoologica Polonidae 46: 91-104.
- Homa J, Niklinskab M, Plytycz B (2003) Effect of heavy metals on coelomocytes of the earthworm Allolobophora chlorotica: The 7th international symposium on earthworm ecology, cardiff, wales, 2002. Pedobiologia 47: 640-645.
- Adamowicz A (2005) Morphology and ultrastructure of the earthworm Dendrobaena veneta (Lumbricidae) coelomocytes. Tissue and Cell 37: 125-133.
- Curieses SP, Saenz ME, Larramendy ML, et al. (2016) Ecotoxicological evaluation of foundry sands and cosmetic sludges using new earthworm biomarkers. Ecotoxicology 25: 914-923.
- Irizar A, Duarte D, Guilhermino L, et al. (2014) Optimization of NRU assay in primary cultures of Eisenia fetida for metal toxicity assessment. Ecotoxicology 23: 1326-1335.
- Di Marzio WD, Saenz ME, Alberdi JL, et al. (2010) Environmental impact of insecticides applied on biotech soybean crops in relation to the distance from aquatic ecosystems. Environmental Toxicology and Chemistry 29: 1907-1917.
- APHA-AWWA-WPCF (1998) Standard methods for the examination of water and wastewater. In: Franson M, (20th edn), American Public Association, American Water Works Association, Water Environment Federation, Washington.
- US EPA (1991) Ecological Assessment of hazardous waste sites: a field and laboratory reference, Washington.
- Konca K, Lankoff A, Banasik A, et al. (2003) A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 534: 15-20.
- Kumaravel TS, Jha AN (2006) Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res 605: 7-16.
- Zar JH (2010) Biostatistical analysis. Pearson, Boston.
- Sparks T (2000) Statistics in ecotoxicology. Wiley, New York.
- Moser H, Rombke J (2009) Ecotoxicological characterization of waste: Results and experiences of an international ring test. Springer 308.
- Bagchi D, Bagchi M, Hassoun EA, et al. (1995) In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104: 129-140.
- Bolognesi C, Bonatti S, Degan P, et al. (1997) Genotoxic activity of glyphosate and its technical formulation roundup. Journal of Agricultural and Food Chemistry 45: 1957-1962.
- Grisolia CK (2002) A comparison between mouse and fish micronucleus test using cyclophosphamide, mitomycin C and various pesticides. Mutat Res 518: 145-150.
- Vera-Candioti J, Soloneski S, Larramendy ML, et al. (2013) Evaluation of the genotoxic and cytotoxic effects of glyphosate-based herbicides in the tenspotted live-bearer fish cnesterodon decemmaculatus (jenyns, 1842). Ecotoxicol Environ Saf 89: 166-173.
- Clements C, Ralph S, Petras M, et al. (1997) Genotoxicity of select herbicides in Rana catesbeiana tadpoles using the alkaline single-cell gel DNA electrophoresis (comet) assay. Environmental and Molecular Mutagenesis 29: 277-288.
- Cavas T, Konen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22: 263-268.
- Manas F, Peralta L, Raviolo J, et al. (2009) Environmental Toxicology and Pharmacology 28: 37-41.
- Schaumburg LG, Siroski PA, Poletta GL, et al. (2016) Genotoxicity induced by Roundup® (Glyphosate) in tegu lizard (Salvator merianae) embryos. Pesticide Biochemistry and Physiology 130: 71-78.
- Wagner N, Reichenbecher W, Teichmann H, et al. (2013) Questions concerning the potential impact of glyphosate‐based herbicides on amphibians. Environmental Toxicology and Chemistry 32: 1688-1700.
- Marques A, Guilherme S, Gaivao I, et al. (2014) Progression of DNA damage induced by a glyphosate-based herbicide in fish (Anguilla Anguilla) upon exposure and post-exposure pediods - insights into the mechanisms of genotoxicity and DNA repair. Comp Biochem Physiol C Toxicol Pharmacol 166: 126-133.
- Mehta A, Verma RS, Srivastava N (2008) Chlorpyrifos-induced DNA damage in rat liver and brain. Environ Mol Mutagen 49: 426-433.
- Crumpton TL, Seidler FJ, Slotkin TA (2000) Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Brain Res Dev Brain Res 121: 189-195.
- Li D, Qingchun H, Miaoqing L, et al. (2015) The organophosphate insecticide chlorpyrifos confers its genotoxic effects by inducing DNA damage and cell apoptosis. Chemosphere 135: 387-393.
- Jergentz S, Pessacq P, Mugni H, et al. (2004) Linking in situ bioassays and population dynamics of macroinvertebrates to assess agricultural contamination in streams of the Argentine pampa. Ecotoxicology and Environmental Safety 59: 133-141.
- Gonzalez M, Miglioranza KS, Aizpun JE, et al. (2010) Assessing pesticide leaching and desorption in soils with different agricultural activities from Argentina (Pampa and Patagonia). Chemosphere 81: 351-358.
- Bunzel K, Schafer RB, Thran D, et al. (2015) Pesticide runoff from energy crops: A threat to aquatic invertebrates?. Science of The Total Environment 537: 187-196.
Corresponding Author
Walter Di Marzio, Comisión Nacional de Investigaciones Científicas y Técnicas (CONICET); Programa de Investigación en Ecotoxicología, Departamento de Ciencias Básicas, Universidad Nacional de Luján - Luján, Argentina, Tel: +542323-423979.
Copyright
© 2018 Curieses SP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.