Cyto and Genotoxicity of Positive and Negative Coated Silica Nanoparticles on Celomocytes of Earthworms Eisenia fetida (Oligochaeta, Annelida)
Abstract
To understand and assess the effects of nanoparticles (NPs) on the environment, should be well established quantitatively the concentration-response relationships. Also relate the potential effects on selected variables of response with the exposure to sub-lethal levels of NPs. In this work, we describe the ecotoxicological evaluation of negative and positive coated silica NPs (Si-Nps) on coelomic cells from Eisenia fetida. The cytotoxicity of earthworm coelomocytes, expressed as LC50-1 hour, was equal to 73.94 and 116.93 µg/mL for positive and negative Si-NPs, respectively. Genotoxicity were determined on the basis that the Si-NPs promote the generation of reactive oxygen species (ROS) once added on cell membranes, or entered in cells. In this case both types of NPs were genotoxics even at the lowest tested concentration equal to 1 µg/mL.
Keywords
Eisenia fetida, Silica nanoparticles, Genotoxicity, Cytotoxicity
Introduction
The nanotechnology has been broadly developed in the fields of medicine, engineering, and manufactured products containing materials at the nanoscale. These typically exhibit unique physical properties and highly chemical reactivity. Engineered nanomaterials (ENs) present tremendous opportunities for industrial growth and development and hold great promise for the enrichment of the lives of citizens, in medicine, electronics and numerous other areas [1]. However, there are considerable gaps in our knowledge concerning the potential hazardous effects of ENs on human health and on the environment. The nanoscale, which allows for new beneficial properties also open the doors to new or different potential hazards. Because nanotechnology is so novel and complex, there may be real difficulties to single out, quantify and manage the potential risks that might be involved, especially the long-term ones. The main key concerns are centered around the potential health and environmental hazards of nanoparticles (NPs) and nanomaterials, and the corresponding ethical, legal and social advisory (ELSA) issues. The effects of ENs are being thoroughly investigated with a better understanding of NPs toxicology, of their fate and behavior, and of the life cycle of nanomaterial-embedded products thus the potential hazards can be minimized for consumers, workers and the environment. This research will be useful for public and occupational health as well as for the successfulness and sustainability of the nanotechnology industry [1]. To assess potential hazards originating from accidental exposure of nanomaterials, their toxicological effects have been reviewed from the environmental, health, toxicological, and scientific perspectives [2-4]. Although the toxicology of carbon nanomaterials and quantum dots has become a subject under intensive investigation, other engineered nanomaterials have not received as much attention [5,6]. Silica NPs (Si-NPs) are one of the commonly used particles in nanotechnology-based products. They are used in a wide spread application such as chemical industry, cosmetics, medicine and agriculture [7]. Si-NPs surface is the main factor of their importance for the biomedical applications. They are used for biological sample preparation as support of biomolecules. Porous silica particles are also of importance as a carrier for genes and drugs delivery. Si-NPs are good material to design complex systems for biomedical applications, due to their stability, low toxicity and ability to be functionalized by coating with a range of molecules and polymers. Si-NPs applications include as additive for rubber and plastics, as strengthening filler for concrete, as non-toxic platform for biomedical applications such as drug delivery and theranostics. Mono or multilayer Si-NPs are used as drug delivery, the advanced features as probes give them a substantial advantage for use in the detection of single molecules, bioimaging and extraction of cells and cellular components, as well as disease targeting Wang, et al. [7]. Jin, et al. [8] indicates that the luminescent silica nanoparticle is a promising labeling reagent for various biomedical applications.
However, limited numbers of studies are currently available in the literature on the impact on earthworm coelomocytes [9]. The cyto and genotoxicity of functionalized Si-NPs with different charge on Eisenia fetida coelomocytes is not reported. Choi, et al. [10] studied the cyto and genotoxicity of Si-NPs in mammalian cell lines, using the dye exclusion, comet, and mouse lymphoma assays. Authors reported an inhibition concentration for 20% of the assayed L5178Y and BEAS-2B cell populations (IC20) of Si-NPs, ranged between 2.441-2.363 mg/mL, 2.324-0.537 mg/mL, respectively. Comet assay demonstrated that treating L5178Y and BEAS-2B cells with Si-NPs induced approximately 2-fold increases in tail moment (% tail DNA* nucleoid tail length). The results of this study indicate that Si-NPs can cause primary DNA damage and cytotoxicity in cultured mammalian cells. Park, et al. [11] observed that smaller size (20 nm) were more toxic than the larger size (> 100 nm) on viability of mammalian keratinocytes. Also, they reported that negative charge of Si-NPs were more cytotoxic and generated more reactive oxygen species (ROS) than those with positive charge using skin fibroblast cells. According these authors, ROS production is the major contributing factor of chemical toxicity. Van der Ploeg, et al. [12,13] studied the sensitivity of immune cells (coelomocytes) of Lumbricus rubellus after in vivo C60 exposure. They reported tissue damage appeared to occur without accompanying increased immune responses during in vivo exposure. Coelomocytes exposed in vitro to C60 showed no decrease of their cellular viability, but demonstrated a decrease in gene expression of the cytokine-like protein CCF-1, indicating immunosuppression. Murugadoss, et al. [14] concluded that the design of safe(r) SiNPs for food, medical, and other applications will only be possible when physico-chemical characteristics can be unambiguously linked to toxicity. In this study, we wanted to evaluate the cytotoxicity of Si-NPs on the viability of celomocytes extracted using a non-invasive method from E. fetida. Considering Si-NPs with negative and positive charges with similar absolute values. Also, once the concentration-response relationship was established determining the genotoxicity of sub-lethal concentrations on coelomocytes.
Materials and Methods
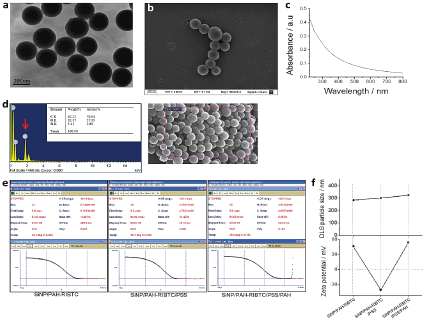
To couple Rhodamine to polymer, 1 mL of Polyallylamine hydrochloride (PAH) solution was mixed in a round bottom flask with 20 ml of MilliQ water, 0.1 g of NaOH and 94.6 mg of Rhodamine B Isothiocyanate (RBITC, Sigma-Aldrich, mixed isomers I), and left for 24 hours in the dark under stirring. The polymer was washed from unreacted RBITC by dyalizing during three days against 0.5M NaCl using a cellulose dyalisis membrane with 18kDa pore size (Sigma D9652 Dialysis Tubing Cellulose Membrane). The final product was a solution (called here after "PAH-RBITC") of 24 monomeric units of polyelectrolyte per dye molecule, with a concentration of 9.44 × 10-3 M expressed in monomeric units of polyelectrolyte. Silica nanoparticles were prepared according to the well stablished Stöber, et al. [15]. Glass material was washed with alcoholic potassium hydroxide and rinsed with water. To prepare 250 nm particles, NH3 (30%, 2 mL), absolute ethanol (48.75 g) and water (13.13 mL) were mixed and stirred vigorously. Then tetraethoxysilane (TEOS, 3.04 mL) was added at once, with a syringe, at the vortex of the solution. Half an hour later the solution started to turn white; stirring was continued for 24 hours, after which the particles were washed to remove ammonia with 10 centrifugation cycles of 20 min at 7000 g and resuspended in water. The synthesis was carried out at room temperature. The resulting particles were characterized by TEM, UV-Vis, IR spectroscopy, DLS and Zeta-potential (ZP) as described in Scodeller, et al. [16]. Particle concentration is expressed as mg of SiO2/mL and was controlled by weighing and dispersing the air-dried material. The weight of a single 250 nm SiNP, using the density value of bulk Silica, is 2.2 × 10-14 g. For the experiments the started solution was 0.5 mg/mL particle concentrations, which corresponds to 23 × 109 NP/mL. No particle loss was detected during the centrifugation cycles. A suspension of Si-NPs (10 mL of 0.5 mg/mL in water) was added dropwise, while stirring, to 10 mL of PAH-RBITC described previously, at room temperature. After 30 min of incubation, the particles were washed to remove the excess of PAH-RBITC by 3 centrifugation cycles of 1000 g and 30 min and dispersed in 10 mL of water (this solution is hereafter called Si-NPs/PAH-RIBTC). To synthesize the negative particles, 10 mL of Si-NPs/PAH-RBITC were added dropwise, while stirring, to a 1% solution of Polystyrene sulfonate in water (PSS, Sigma Aldrich 75 Kda). After 30min, the particles were washed to remove unbound PSS by 3 centrifugation cycles of 1000 × g and 30 min and dispersed in 10 mL of water. This sample is hereafter called Si-NPs/PAH-RBITC/PSS and 5 mL of these were used as the negatively charged particle sample. To synthesize the positively charged particles, 5 mL of Si-NPs/PAH-RBITC/PSS solution were added dropwise, while stirring, to a 1% solution of PAH in water. After 30 min, the particles were washed to remove unbound PSS by 3 centrifugation cycles of 1000 × g and 30 min and dispersed in 5 mL of water. This sample is hereafter called Si-NPs/PAH-RBITC/PSS/PAH and 5 mL of these were used as the positively charged particle sample. See Supplementary Figure S1.
Eisenia fetida adults, average wet weight 300 mg, were purchased from local source (Luján, Buenos Aires). Earthworms were maintained in moistened control soil (pH 6.6 ± 0.26, 25% sand, 48% slime, 27% clay, moisture 40-60% of water holding capacity, WHC 60 ± 5 mL/100 g), at room temperature (RT), under natural photoperiod; fed with 10% of alfalfa forage. The worms were allowed to acclimate to laboratory conditions for several weeks before testing. A non-invasive extrusion method was used for collecting earthworm coelomocytes according to Di Marzio, et al. [17]. The pooled of five organisms was placed into centrifuge tubes containing 2 mL of EM/individual and incubated for 1 min at RT. Coelomic fluid containing the extruded cells was diluted with calcium and magnesium free phosphate buffered saline (PBS), washed twice, and centrifuged at 2000 rpm at 4℃ during 10 min. The final pellets were resuspended in 2 mL of PBS. Extruded cells were counted using a counting chamber improved Neubauer hemocytometer. Coelomocytes were characterized according to Adamowicz and Wojtaszek [18] and Adamowicz [19] (see Supplementary Figure S2-A). To evaluate in vitro cytotoxicity, coelomocytes were incubated 1 h at RT, in the following Si-NPs concentrations 276, 138, 69, 34 and 17 µg/mL, PBS was used as negative control. The cell viability was expressed as the percentage of viable cells by 0.4% of Trypan blue. One hundred cells were counted on each slide and three replicate slides were analyzed per specimen. After one hour of exposure cells were observed under epifluorescence microscopy as described below. The assayed concentrations for genotoxicity were chosen based on cytotoxicity results. Cell exposures were conducted by immersing slides containing the triple-layer agarose gels used for the comet assay in PBS (negative control), hydrogen peroxide (positive control) and sub-lethal concentrations calculated using equation (2) of SNP 1 and 10 µg/mL. One hundred cells were analyzed, per duplicate. SCGE assay protocol proposed for Di Marzio, et al. [17] was used. The images of coelomocyte nucleoids were analyzed with epifluorescence microscope Nikon, Eclipse 600 (541-560 nm excitation filter and 590 nm emission filter) linked to an image analysis system (Image Pro Plus, V4.0, Media Cybernetics, Maryland USA). The images obtained in the experiments were analyzed with the software program CASP [20]. DNA migration was measured as % of Tail DNA as final genotoxicity endpoint. % Tail DNA parameter was chosen as it is not measured in arbitrary units, being more meaningful and advisable for regulatory purposes and for inter-laboratory comparisons [21].
Concentration-response curves (CRCs) of SNP were fitted using the Weibull model: E = 1-exp(-exp(α + β log10(C)))(1) where E is the effect or response expressed as fraction of a maximum possible effect (0 < E < 1), C the concentration of SNP, α, β, model parameters. Data were fitted using Newton algorithm to finds the minimum of the sum of squares. And the inverse of equation (1) used to choose sub-lethal concentration as: C = 10(ln(-ln(1-E))- α)/β. For genotoxicity data a square root transformation was applied to Tail DNA (%) to stabilize the variances and approximate a normal distribution. These data were analyzed by a one-way ANOVA and Dunnet test to detect differences between treatments and control, and Tukey test to detect differences in genotoxicity between Si-NPs with different charges. All statistical analysis was made using Statistica V8 [22,23].
Results and Discussion
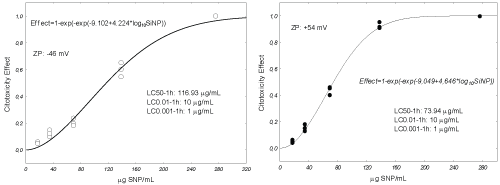
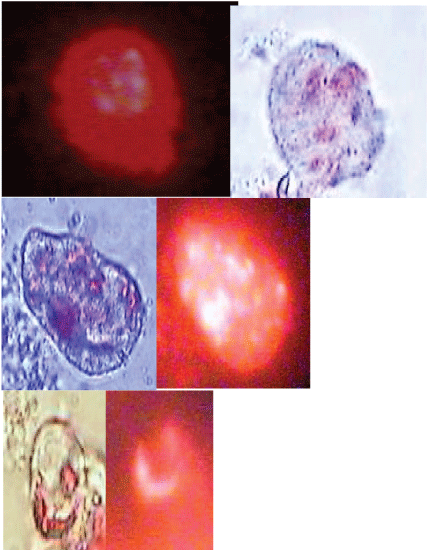
In Figure 1 and Figure 2 the relationship between the concentrations of Si-NPs and viability of celomocytes is indicated. Considering the LC50-1 h positively charged Si-NPs were approximately twice more toxic than negatives. The concentrations causing "no-effects" 0.01 and 0.001%, estimated using equation 2, were 10 and 1 µg/mL, respectively. These concentrations were used to determine the genotoxic effects of the Si-NPs evaluated. Celomocytes exposed for one hour were observed by epifluorescence microscopy. This observation allowed proving that both negative and positive NPs, formed aggregates on the cell membranes of eleocytes causing the lysis of the cells (Supplementary Figures S2-B). Moreover, in our observations we did not observe that the Si-NPs were swallowed by the amebocytes. However, Hayashia and Engelmann [9] remarked that the phagocytic population of the coelomocytes, amoebocytes, seems a susceptible target of nanomaterials, and as result of which indirect responses by chloragocytes (elecocytes) are conceivable.
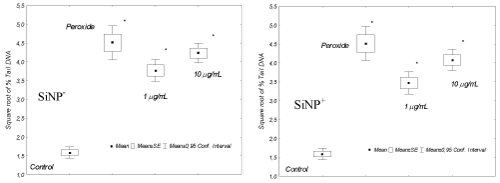
Regarding the genotoxicity on coelomocytes exposed to the Si-NPs, both types, positives and negatives were genotoxic respect to control cells. Because the % of tail DNA of these, incubated during 1 h at 1 and 10 µg Si-NPs/mL, was significantly different with respect cells exposed to PBS or negative controls. However, not significant differences (p > 0.05) were observed when comparing % tail DNA between positive and negative Si-NPs for both assayed concentrations. Earthworm immune system consists of coelomocytes and humoral components (antimicrobial, cytolytic and pattern recognition molecules) directed towards non-self materials in a natural non-specific manner [24]. Cytochemical, immunological and functional approaches characterized three major subpopulations among which amoebocytes and granulocytes participate in the cellular mechanisms as phagocytosis and encapsulation; while eleocytes contribute more to homeostasis and humoral immunity [25-27]. This selective cellular uptake could have different toxicological implications for celomocytes exposed to nanomaterials.
Metal-based nanomaterials that readily dissolve and liberate bioactive metal ions, Ag-NPs represent the most well-studied type of NPs [28]. Free Ag+ ions, the product of oxidative dissolution, itself is highly biologically active and reacts with proteins and DNA of the cellular components in a similar manner as reactive oxygen species (ROS) [9]. Hayashi, et al. [29] and Curieses, et al. [30] observed, at the molecular level, a cascade of stress responses initiating from oxidative stress genes to immune genes downstream following short-term exposure to Ag-NPs in coelomocytes. The proposal of these authors about the mode of action of the NP on the amebocytes, is that once incorporated into the cytoplasm triggers the liberated metal causing oxidative stress. Similarly, the Si-NPs aggregate on the cell membrane of eleocytes could facilitate entry of silica and this indirectly damage DNA through the generation of ROS [9]. In this study we observed Si-NPs aggregates on the membranes of these cells and found that they cause DNA damage. ENs are incorporated into a growing variety of products ranging from common household items to novel medical technologies. Life cycle release studies have identified wastewater treatment facilities (WWTPs) as a significant exposure pathway to aquatic and terrestrial ecosystem [31-34]. The Si-NPs predicted concentrations in biosolids, based on life cycle release models and measurements was 5-123 mg/kg and 0.03-6.74 µg/L concentrations in effluent using market study production estimates [35]. We could propose that the discharge of liquid effluents could exert genotoxic effects on organisms in the receiving environments. Moreover, we should evaluate in vivo exposure to solid wastes and their aqueous eluates, for a fully environmental risk characterization of their final disposal.
Conclusions
In our study we determined the quantitative relationships between coated silica NPs concentrations and coelomocytes cytotoxicity. In this regard, positively charged Si-NPs were approximately twice more toxic than negatives. Also we evaluated genotoxicity of sub-lethal concentrations for these NPs. Positive and negative Si-Nps were similarly genotoxicity even at the lower exposure concentration of 1 µg/mL. We observed that silica nanoparticles were aggregated on the cellular membrane of eleocytes.
Declaration of Interest
The authors declare no conflicts of interest.
References
- http://ec.europa.eu/research/industrial_technologies/safety-of-health-and-environment_en.html.
- Colvin VL (2003) The potential environmental impact of engineered nanomaterials. Nat Biotechnol 21: 1166-1170.
- Oberdorster G, Oberdorster E, Oberdorster J (2005) Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113: 823-839.
- Hardman RA (2006) Toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ Health Perspect 114: 165-172.
- Donaldson K, Aitken R, Tran L, et al. (2006) Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 92: 5-22.
- Nel A, Xia T, Madler L, et al. (2006) Toxic potential of materials at the nanolevel. Science 311: 622-627.
- Wang L, Zhao W, Tan W (2008) Bioconjugated silica nanoparticles: Development and applications. Nano Research 1: 99-115.
- Jin Y, Kannan S, Wu M, et al. (2015) Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol 20: 1126-1133.
- Hayashi Y, Engelmann P (2013) Earthworm's immunity in the nanomaterial world: New room, future challenges. Invertebrate Survival J 10: 69-76.
- Choi H, Kim Y, Song M, et al. (2011) Genotoxicity of nano-silica in mammalian cell lines. Toxicol Environ Health Sci 3: 7-13.
- Park YH, Bae HC, Jang Y, et al. (2013) Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Molec Cell Toxicol 9: 67-74.
- Van der Ploeg MJC, Handy RD, Heckmann LH, et al. (2013) C60 exposure induced tissue damage and gene expression alterations in the earthworm Lumbricus rubellus. Nanotoxicology 7: 432-440.
- Van der Ploegab MJC, Van den Bergb JHJ, Bhattacharjeebc S, et al. (2014) In vitro nanoparticle toxicity to rat alveolar cells and coelomocytes from the earthworm Lumbricus rubellus. Nanotoxicology 8: 28-37.
- Murugadoss S, Lison D, Godderis L, et al. (2017) Toxicology of silica nanoparticles: An update. Arch Toxicol 91: 2967-3010.
- Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26: 62-69.
- Scodeller P, Catalano PN, Salguero N, et al. (2013) Hyaluronan degrading silica nanoparticles for skin cancer therapy. Nanoscale 5: 9690-9698.
- Di Marzio WD, Saenz ME, Lemiere S, et al. (2005) Improved single cell gel electrophoresis assay for the earthworm Eisenia foetida. Environmental and Molecular Mutagenesis 46: 246-252.
- Adamowicz A, J Wojtaszek (2001) Morphology and phagocytotic activity of coelomocytes in Dendrobaena veneta (Lumbricidae). Zoologica Poloniae 46: 91-104.
- Adamowicz A (2005) Morphology and ultrastructure of the earthworm Dendrobaena veneta (Lumbricidae) coelomocytes. Tissue Cell 37: 125-133.
- K Konca, A Lankoff, A Banasik, et al. (2003) A cross platform public domain PC image analysis program for the comet assay. Mutation Research 534: 15-20.
- Kumaravel TS, Jha AN (2006) Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res 605: 7-16.
- Zar JH (2010) Biostatistical Analysis. Pearson Publisher and Distributor, Boston, USA.
- Sparks T (2000) Statistics in Ecotoxicology. John Wiley & Sons Publisher and Distributor, New York, USA.
- Cooper EL, Kauschke E, Cossarizza A (2002) Digging for innate immunity since Darwin and Metchnikoff. BioEssays 24: 319-333.
- Engelmann P, Molnar L, Palinkas L, et al. (2004) Earthworm leukocyte populations specifically harbor lysosomal enzymes that may respond to bacterial challenge. Cell Tissue Res 316: 391-401.
- Engelmann P, Palinkas L, Cooper EL, et al. (2005) Monoclonal antibodies identify four distinct annelid leukocyte markers. Dev Comp Immunol 29: 599-614.
- Opper B, Nemeth P, Engelmann P (2010) Calcium is required for coelomocyte activation in earthworms. Mol Immunol 47: 2047-2056.
- Liu J, Sonshine DA, Shervani S, et al. (2010) Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 4: 6903-6913.
- Hayashi Y, Engelmann P, Foldbjerg R, et al. (2012) Earthworms and humans in vitro: Characterizing evolutionarily conserved stress and immune responses to silver nanoparticles. Environ Sci Technol 46: 4166-4173.
- Curieses SP, Garcia-Velasco N, Urionabarrenetxea E, et al. (2017) Responses to silver nanoparticles and silver nitrate in a battery of biomarkers measured in coelomocytes and in target tissues of Eisenia fetida earthworms. Ecotoxicol Environ Saf 141: 57-63.
- Hansen SF, Michelson ES, Kamper A, et al. (2008) Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology 17: 438-447.
- Gottschalk F, Nowack B (2011) The release of engineered nanomaterials to the environment. J Environ Monit 13: 1145-1155.
- Gottschalk F, Sonderer T, Scholz R, et al. (2010) Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environ Toxicol Chem 29: 1036-1048.
- Gottschalk F, Sun T, Nowack B (2013) Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ Pollut 181: 287-300.
- Lazareva A, Keller A (2014) Estimating potential life cycle releases of engineered nanomaterials from wastewater treatment plants. ACS Sustainable Chem Eng 2: 1656-1665.
Corresponding Author
Walter Di Marzio, Comisión Nacional de Investigaciones Científicas y Técnicas (CONICET); Programa de Investigación en Ecotoxicología, Departamento de Ciencias Básicas, Universidad Nacional de Luján - Luján, Argentina, Tel: +542323-423979.
Copyright
© 2018 Di Marzio WD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.