Ozonation Efficacy in the Treatment of Soil-Borne Phytophthora sojae in Cultivating Soybeans
Abstract
Ozonation was studied for inactivating Phytophthora sojae, a predominant soybean pathogen that causes root and stem rot, and pre-and post-emergence soybean damping-off. Typically, fungicides are used to treat soils to control the damage from P. sojae to soybean production. An environmentally friendly method of ozonation was studied for inactivating P. sojae, a model Phytophthora pathogen that affects a wide range of high-value crops. Assays of artificially inoculated soil samples with P. sojae were treated with different doses of gaseous ozone. This study showed that a dosage of 0.47 g.kg-1 O3 in the soil totally prevented root and stem-rot disease incidence by P. sojae. The findings of this research clearly indicate that ozonation is an efficient alternative to chemical fungicides in the inhibition of Phytophthora diseases in the soil, hence a balancing feedback loop reinforcing the soil system as natural capital.
Keywords
Ozone, Phytophthora sojae, Soil disinfection, Soil ozonation, Soil borne pathogens, Sustainability
Introduction
Phytophthora is an important phytopathogen that means literally 'plant destroyer'. With more than 80 known species, Phytophthora is an oomycete from the once proposed kingdom Chromalveolata now residing in the superphylum heterokonta. Phytophthora spp. attack a wide range of agriculturally-important plants, resulting in billions of dollars in losses worldwide each year [1]. Phytophthora infestans was behind the infamous Irish famine in the 1840s, which destroyed all potato production due to potato late blight. Phytophthora produces several kinds of spores to survive under different soil conditions, the most predominant of which are (i) sporangia, asexual sac-like multinucleate spores, (ii) oospores, which are non-motile sexual spores specialized for survival in the absence of a host-plant and adverse conditions, and (iii) zoospores, which are dispersal spores adapted to move with water, locate the host-plant, and disseminate the pathogen [2]. Asexual spores (sporangia and zoospores) are often targeted by treatments to manage Phytophthora, because they represent a vulnerable phase in the pathogen life cycle. Also, they are exposed to the environment and have limited nutrient reserves, which prevent them from persisting for long outside a host [2].
Phytophthora sojae is one of the important species of Phytophthora. It can infect soybeans at all growth stages and causes seed rot, pre- and post-emergence 'damping off' and root and stem rot of older plants, with an annual cost worldwide of U.S. $1-2 billion [3]. Seedlings infected with P. sojae show lesions anywhere between the root, hypocotyls and cotyledon, turn brown, wilt, and die [4]. Like the other Phytophthora species, P. sojae persists in soils as oospores that can survive for many years without a host, either in the crop residue or in the soil after the residue decomposes [3].
Cultural practices, development of resistant varieties, organic amendments, fungicides and fumigants are all adopted in the control of Phytophthora diseases. However, each control measure has some drawback. Based on the biological knowledge of Phytophthora, and understanding the ecological processes that could suppress the disease, the most important cultural practice in the control of Phytophthora diseases is the management of soil moisture since the pathogen's spores disperse with free moisture and through water. However, controlling soil moisture is not always manageable, as in the case of P. sojae, one of the predominant soybean pathogens, in production regions with poorly drained soils and heavy rain occurrence [5]. The estimated reduction in soybean yield due to P. sojae in 1994 was 560,300 metric tons, and mild symptoms, referred to as hidden damage, may reduce yield by as much as 40% [6,7]. Organic treatments, such as composts and soil amendments, did not reduce soil populations of P. capsici causing pepper root and crown rot, although they provided some control of the disease incidence [8]. The use of resistant varieties is not a durable solution, because the pathogen in many instances has adapted quickly and become resistant [9]. In addition, some of the developed resistant varieties to Phytophthora do not possess desirable horticultural characteristics that are accepted by growers [10], or in some cultivars, they possess excellent horticultural characteristics combined with resistance to one phase of the pathogen, but not to its other phases [11,12]. Chemical fungicides that are mostly used in the control of Phytophthora spp in high-value crops are metalaxyl (trade name Ridomil), mefenoxam (trade name Ridomil Gold), phosphite (salt of phosphorous acid), fosetyl-Al (trade name Aliette), and soil fumigants i.e. methyl bromide (MeBr), metam sodium and chloropicrin. The development of resistance to metalaxyl [13-15], and to mefenoxam [15-18], the limited efficiency in disease control of fosetyl-Al [19] and phosphite [20], and the environmental repercussions of these fungicides and of soil fumigants, especially MeBr [21-24], metam sodium [25,26] and chloropicrin [27] necessitate the search for more efficient, eco-friendly, and durable alternatives to control the 'plant destroyer' especially for high-value crops.
Soil is natural capital, which is, by definition 'a stock of natural ecosystems that yields a flow of valuable ecosystem goods or services into the future [28]. This means that soils (i.e. arable lands) are stocks that can provide an indefinitely sustainable flow of plant production. Soil also provides many services as a system including plant anchorage and support, as well as plant debris (wastes) decomposition and mineralization by physical weathering and biotic activities, thus maintaining endless nutrient availability to plants. This flow of services requires that the soil functions as a system, whose sustainability hinges on soil biodiversity.
The use of synthetic pesticides in the treatment of Phytophthora and other soil pests, especially broad-spectrum and soil sterilizing fumigants (e.g. MeBr) affects the soil fauna and flora and disrupts the cyclic processes that make the soil a functional system and natural capital. Such processes are vital to the survival of plants and, by consequence, to higher living organisms depending on them in the food chain. Examples of vital cyclic processes are: nutrient mineralization and/or transformation (Nitrobacter, Nitrosomonas), nutrient fixation into the soil (nitrogen fixing bacteria), mobilization and soil aeration (soil worms), and symbiosis with plant roots helping in nutrient absorption (rhizobacteria, mycorrhizae). The harm of pesticides extends beyond the location where first applied. Pesticides' residues and byproducts deposited in nature could be transported by water, air, soil and animals to undesired places where further harm to the environment could occur [29]. Accordingly, pesticides are considered amongst the most important non-point source pollutants [30], which are best controlled through the adoption of suitable alternatives.
Resilience helps the soil recover from the damage of pesticides to keep providing ecological services to a certain degree. Hence, the sustainability of soil services hinges greatly on the degree of resilience it holds. However, resilience is limited to the extent of biodiversity and other parameters like soil texture, structure and organic matter content. Therefore, the stock of soil fertility and services become endangered when resilience drops to a certain soil biodiversity threshold. Upon disease incidence, the use of synthetic pesticides affects both phytopathogens and beneficial microorganisms, depleting the stock of soil biota. With the decline in beneficial microorganisms, phytopathogens overgrow at their expense, and the plants suffer disease outbreak [31]. The persistent dependence on pesticides results in the disruption of natural biological control systems, and has been reported having adverse effects on non-target organisms with pest outbreaks, widespread resistance development, and detrimental repercussions on the environment and human health [32]. Once more, the need for pesticides grows to put phytopathogens under control, while soil fertility and productivity drop. Accordingly, the use of synthetic pesticides is considered a reinforcing feedback loop (that reinforces the depletion of the stock of soil productivity) because it leads to soil biodiversity-depletion [33]. To offset this negative impact, a restoring intervention with balancing feedback should be exerted to reinforce the stock and stabilize the soil biodiversity hence the stock of soil productivity at a sustainable level. An ideal balancing intervention could be the control of phytopathogens below the damage threshold, without affecting the soil biodiversity [34].
Ozone is a potent oxidant and it has been used successfully against numerous pathogens including viruses, bacteria, protozoa, fungi and metazoa [35-42]. Ozone is often used to disinfect drinking water and wastewater [43,44], and disinfest ships' ballast water [45,46] due to its oxidizing properties. Ozone has also been used in mold prevention on stored corn [47]. Scanning electron microscopy showed that ozone causes damage in the form of blebs on the surface of the protein coat at the basement of the honeycomb-like structures of Toxocara canis eggs, a nematode parasite of dogs and other canines [48]. Ozone is also capable of diffusing across bacterial membranes and reacting with cytoplasmic biomolecules, such as DNA, which results in cell death [49]. Furthermore, ozone reacts with biomolecules such as proteins, carbohydrates and polyunsaturated fatty acids bound to albumin, dyes, and is involved in lipid peroxidation [50,51].
In contrast with other disinfection methods and conventional fungicides used in the treatment of soil pathogens (e.g. metalaxyl, mefenoxam, MeBr, metam sodium, and chloropicrin), the use of ozone as disinfection method has the advantage because it is environmentally friendly and not a source of pollution. This qualifies ozone to be considered as a balancing feedback loop to the soil system as natural capital. To our knowledge, no previous published research has been conducted on soil ozonation against an oomycete. The high oxidative power of ozone, its efficiency in inhibiting pathogens without leaving toxic residues in the environment, the limited research conducted on the use of ozone as a soil fumigant, and the absence of research on soil ozonation as an oomycete treatment, had encouraged this research. Furthermore, the economic importance of Phytophthora, and the need for balancing feedback loops to offset the reinforcing loops and maintain the system sustainability, as in seeking efficient and environmentally safe alternatives to the use of fungicides, has justified the need for this research.
Sopher, et al. reported the successful use of gaseous ozone for soil fumigation in increasing plant yield and minimizing the damaging effects of soil pathogens for a range of crops and soils under different climatic conditions [52]. They reported that positive effects of pre plant ozone application were due to the decrease in soil pathogen populations and increased nutrient availability. However, they recommended further studies to accurately predict specific responses achieved from ozonation under different soils, plants, and environmental factors (crops, soils, pathogens and climatic conditions). Nevertheless, to our knowledge no further studies were conducted on this topic.
Therefore, the main objective of this study was to investigate soil treatment with gaseous ozone in controlling P. sojae, as a model Phytophthora pathogen that affects a wide range of high-value crops.
Methods and Materials
Experimental investigations for this study were conducted in the environmentally controlled greenhouse of the Department of Horticulture at Iowa State University (ISU). Soil for this experiment was collected from Hinds Farm (an ISU research farm, near Ames, Story County, Iowa). This soil belongs to the Clarion-Nicolett-Webster 'principal association area', and Zenor soil series per the Iowa Soil Properties and Interpretations Database-ISPAID. The soil was analyzed for texture and organic matter content. The soil texture was sandy with low organic matter and organic carbon content (Table 1).
Inoculum preparation
To evaluate the effect of ozonation on P. sojae, soil was deliberately infested with P. sojae rice inoculum, treated with ozone at various dosages, then seeded with susceptible soybean cultivar (Sloan), and incubated for two weeks. To prepare the soil samples, soil was first sterilized through autoclaving (dry heat at 170 °C for 60 min) to eliminate any undesired pathogens, and then the soil was artificially inoculated with rice infested with P. sojae [11]. The isolate of P. sojae R7-2a (pathotype 1d, 2, 3a, 5, 6, 7) (acquired from Dr. Anne Dorrance, Department of Plant Pathology at Ohio State University) was used in this study. For long-term storage, the isolate was first plated on DV8++ (diluted V8 juice agar plus antibiotics neomycine sulfate and chloramphenicol) and after 7 days, plugs ~2 mm2 of P. sojae mycelia were transfered to sterilized tube including sterile water, at room temperature without the presence of any light (complete darkness). To prepare P. sojae rice inoculum, two-week old agar plugs of R7-2a were transferred to rice that had been autoclaved twice for 45 min on two consecutive days, and incubated for two weeks at room temperature, with daily break of clumps that were built in the plastic bag. The rice was dried for two consecutive days at room temperature, before it was mixed with the autoclaved soil.
Experiments were conducted in a greenhouse using 16 oz PVC pots. Each pot was first filled with 150 g of sterilized soil, then 15 cc of P. sojae-infested rice was placed in a layer, and finally the inoculum layer was covered by adding 300 g of sterilized soil. The pots were flooded with deionized water for 24 hours (h), then drained for another 24 h until the moisture content approached ~300 mb matrix potential [48,53]. The pots were then placed in polyethylene bags and incubated in a greenhouse for a total of two weeks (greenhouse temperature was maintained at 25 °C for 16 h to simulate day hours, and at 21 °C for 8 h to simulate night hours). Oospores will germinate and form sporangia during this period. Plastic bags from pots were removed after the two-week period, and then pots were flooded again for 24 h period and then drained for 48 h. The last flooding procedure is required to disperse zoospores, emerging from sporangia in the rice inoculum layer, throughout the soil in the pot.
Experimental design
After considering the available resources (facilities, equipment, time, etc.) for conducting the experiments and the possible sizes of treatment effects, we decided to prepare a total of five runs of the experiments, or five 'batches.' Each batch consisted of 23 to 25 pots of P. sojae-infested soil: 8 pots were non-treated control samples, and 15 to 17 pots were treated samples at different ozone doses. There were six treatments consisting of ascending doses of ozone generated by increasing the time of ozonation (10, 13, 15, 17, 20 and 25 min per 450 g soil corresponding to ~0.47, 0.73, 0.79, 1.1, 1.2, and 1.41 g.kg-1 O3, respectively). Treatment samples (number of pots per treatment dose) and non-treated control samples (number of pots) per batch are listed in (Table 2). Each batch was treated separately on a different day, including four of the six treatments for batches 1 to 4 and three of the treatments for batch 5, and one set of eight control pots per batch. Every pot in each batch was sown 10 Sloan seeds on the same day, one day per batch, from November 10 to 14, 2012. Control samples, consisting of eight pots per batch of P. sojae-infested soil, sown with Sloan seeds and incubated without treating with ozone, served to confirm inoculation success by revealing disease symptoms on seeds and seedlings, and these were compared with soil samples treated with ozone.
The experiments were conducted by following the same procedures for each batch; batches were used solely to make the experiments feasible and manageable. Thus, batch was not considered as a blocking factor and was not expected to account for much variation in the data. Since we were most interested in identifying the differences between the control and the treatments, the sample size for the control was the most important as it affects all the standard errors of these differences. Thus, we used 40 pots for the control to achieve better precisions for all these comparisons. In addition, the control pots took less effort and cost, increasing the total sample size of the experiments for given amount of resources. Furthermore, if the treatments turned out to be like each other but different from the control (as it happened to be the case), more pots for the control better balanced the amount of information available between the control and the treatments. The number of pots for the treatments also varied, mainly due to our desire for more information around the middle range of the dosage levels (the low and high dosage levels were added just in case we did not get the right range of the effective dosage levels). After the experiments were done, we realized that simpler and more balanced experiments could have been used; for example, we could use six batches of 20 pots, with each batch containing 4 pots for the control and four of the six treatments, so that we would have 24 pots for the control and 16 pots for each treatment.
Experimental treatments and incubation
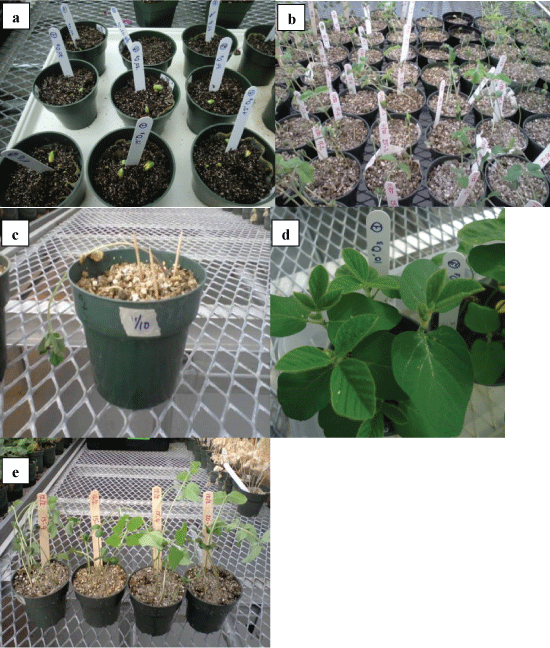
Soil in each pot (weighing 450 g) was ozonated at a flow rate of 0.5 L/min. Doses of ozonation in this experiment varied from 0.47 to 1.41 gram of ozone per kilogram of soil, by incrementally increasing the ozonation time (Figure 1a).
Following ozonation, pots were placed again in the greenhouse (where temperature of 25 °C was maintained for 16 h during the day, and temperature of 21 °C was maintained for 8 h during the night). Ten soybean seeds of cultivar Sloan, which is susceptible to P. sojae, were placed on the surface of the soil in each pot and covered with 2.5 cm of wet coarse vermiculite (Figure 1b), flooded for 24 h and drained for another 24 h. Each pot was flooded separately, to avoid cross contamination between treatments if any. The germination rate of Sloan seeds used in this experiment was 96.5%. The pots were then placed into plastic bags for three days to prevent drying out during seed germination. Three days later, bags were removed and the pots were flooded again for 24 h and then placed on benches to drain. Over the next 15 days, pots were monitored for symptoms.
Monitoring and assessment of treatment procedures
Evaluation of treatment efficiency was done by monitoring the treated (ozonated) and non-treated (control) samples and assessing the symptoms of infection with P. sojae. Typically, symptoms of infection with P. sojae include: seed rot, root rot, collapsed hypocotyls of emerging seedlings, and stem lesions. Any recorded symptom among these latter thus reveals the disease incidence (Figure 1c).
Ozonation
The ozone generator used was a 1000BT-12 Triogen Model TOG C2B, generating 1 g O3/h from pure oxygen by corona discharge, where the conversion of oxygen to ozone occurs in a reaction cell excited by a high-voltage potential. The reactor was made of glass (Figure 1a), and all tubing was made of silicone material. The operating volume of the reactor was 1.5 L (Figure 1a). In each test, the ozone flow rate per min was maintained at 1 L min-1L-1 gas-flow/L volume of soil sample. The feed and excess unreacted ozone were measured by the iodometric wet-chemistry method [54]. The amount of ozone absorbed by the soil sample was determined by difference [55].
Data analysis
For each of the 120 pots, we recorded its batch number, treatment level (control or one of the six treatments), number of seeds germinated, number of seedlings with symptoms, and number of germinated seeds not emerged and with symptoms. Thus, our data consisted of 120 rows, one row per pot. Because these germination data (e.g. the number of seeds germinated among the 10 seeds planted for each pot) follow a binomial distribution, we analyzed the data using the binomial regression (with the logistic link function) and R software (version 3.1.0, The R Foundation for Statistical Computing). The response variable for all these models is the number of seeds germinated per pot. We started with batch (five levels) and treatment (seven levels) as predictors. The likelihood ratio test shows that batch is not significant (P-value = 0.956) and may be dropped from the model. This confirmed our pre-experiment belief that batch would not affect the responses. (For all the models we considered, batch is insignificant, with very large P-values.) The fitted binomial model with treatment as the predictor has the Cox and Snell R-square of 0.48, with the following estimated coefficients and P-values (note that, for example, 1.04e-4 = 0.000104 below).
| Intercept (Control) | 10 min | 13 min | 15 min | 17 min | 20 min | 25 min | |
|---|---|---|---|---|---|---|---|
| Estimate | -0.08 | 1.546 | 1.565 | 1.018 | 0.738 | 0.55 | 0.327 |
| P-value | 0.424 | 3.46E-07 | 2.42E-10 | 4.82E-07 | 1.04E-04 | 0.0076 | 0.107 |
Based on the fitted model, we can estimate the percentage of germinated seeds (% emergence) for each treatment level. For example, for the control and 10 min treatment, the estimated % emergence are exp (-0.080)/(1 + exp (-0.080)) = 0.48 (48%) and exp (-0.080 + 1.546)/(1 + exp (-0.080 + 1.546)) = 0.81 (81%). More detailed results are given in Table 3.
Results
Seedling emergence (Figure 2a) started at day three after sowing soybean seeds in all pots except pots that received higher ozonation doses (1.1, 1.2 and 1.4 g.kg-1 O3) when it occurred on day four in these pots. All emerged seedlings in all treatments were free of any disease symptoms like root and stem rot, stem lesions, collapse of hypocotyls and damping-off (Table 3, Figure 2b, Figure 2d and Figure 2e) whereas seedlings in non-treated (control samples) showed different levels of disease incidence (Table 3 and Figure 2c). In addition, it was observed that the emergence rate of seedlings was negatively correlated with the dose of ozone treatment (Table 3). The analysis results are presented in '% emergence' and '% disease incidence' related to infection with P. sojae of seed, root rot, stem lesions, collapse of hypocotyls, and seedling damping-off that were observed in non-ozone treated pots (Table 3), with the margin of error for the 95% confidence interval (CI) in parentheses, except for the % disease incidence for the six treatments, for which the upper confidence limits are in parentheses (the lower confidence limits are 0). Note that the CIs for the % emergence were obtained from the above fitted binomial model; the CI for the % disease incidence for the control was from the standard (approximate) formula for a binomial proportion with 400 trials (400 seeds); the upper limits for the % disease incidence for the six treatments were from exact calculations based on their respective binomial distributions (e.g., for the 10 min treatment, we have 8 pots, or 80 seeds).
We also considered treating the treatment or dosage factor as a quantitative variable. The lowest (control) and highest dosage level (1.41) had the lowest percentage of emergence, making the binomial model fit poor; we thus focused on the 80 observations from the six treatments, with three possible predictors: time, dose, and rate of ozonation (dose/time), as shown in Table 3 above. We used the Akaike information criterion (AIC) to select which predictors to include in the model. The best model includes dose as the only predictor, with the Cox and Snell R-square of 0.29 and the following estimated coefficients and P-values:
| Intercept | Dose | |
|---|---|---|
| Estimate | 2.224 | -1.424 |
| P-value | 3.60E-14 | 2.45E-07 |
Discussion
The appearance of P. sojae disease symptoms on control pots seedlings and the absence of these symptoms in treated pots confirmed our belief that pots not treated with ozone resulted in infected seedlings from pathogens present in the soil after artificial infestation with P. sojae rice inoculum. The exemption of treated pots from any P. sojae-related disease symptoms concluded that ozonation of soil resulted in healthy seedlings free from pathogen damage.
Since the variation in the rate of ozonation was small among the six treatments and time was highly correlated with dose (with a correlation coefficient of 0.975), only dose was in the best binomial model selected using the AIC criterion (with the P-value = 2.45e-7), when we considered the 80 observations from the six treatments and treating these as quantitative factors. Because seeds were sown after the treatment, they were not directly subjected to the effect of rate of ozonation, and the germplasm would not be harmed. Germination is defined as 'the emergence of the radicle through the seed coat' [56], while emergence is the superficial outgrowth of the seedling shoot from the soil. Most non-emerged seeds in the treated pots had germinated. This observation confirms the explanation about the rate of germination.
Symptoms seen in non-ozone treated pots were attributed to infection from P. sojae for three reasons: (i) disease-like symptoms from pathogens were observed only in non-treated pots, (ii) the soil in the pots was autoclaved at the beginning of the experiment eliminating the possibility from other diseases except from P. sojae, and (iii) disease symptoms matched those usually seen in P. sojae-infected soybean, namely seed and root rot, stem lesions, collapse of hypocotyls, and seedling damping-off that were observed (Figure 2c). These results also show that the ozonation of P. sojae-infected soil was seen highly efficient because even the pots treated with the lowest dose 0.47 g.kg-1 O3 (10 min) resulted in an average of 81% seedling emergence rate of healthy plants (Table 3 and Figure 2d). The non-emergence of seedlings in the treated samples could not be attributed to the direct harm to the germplasm by ozone, since 95% of the non-emerged seeds were germinated, and the ozonation process was done in the absence of seeds. A possible explanation for the observation of lowered seed emergence in response to the increased ozonation dosages, could be that higher dosages result in lowering the viability of beneficial microorganisms responsible for many vital processes in promoting plant growth, like rhizobacteria, which, by consequence, decrease emergence [57]. Examples of mechanisms that these microorganisms promote are nutrient mineralization, solubilisation and immobilization, induced plant resistance and pathogens suppression, growth promotion and increased yield [57,58]. In addition, ozonation might form oxidized products with potential deleterious properties, like the oxidized bromide ion that upon reaction with water or soil constituents might form mildly toxic hypobromous and bromate ion or tribromomethane [24]. Ozone concentrations of 0.2-0.3 mg.L-1 caused root injury when immersing cucumber plant root in ozonated water [59]. Kottapalli, et al. found that an exposure of barley seeds to ozone at the dose 11 mg.g-1.min-1 O3-barley for 30 min resulted in significant reduction in barley germination energy [60].
However, in the current research, the seeds were not directly exposed to ozone, but were sown after ozonation. Since the lowest ozone dose (0.47 g.kg-1 O3) was as good as inhibiting 100% of the disease without affecting or harming seed germination, residual ozone toxicity would not be a practical limitation. Future experiments may be conducted with lower dosage levels, which may lead to even less toxicity. The occurrence of soil borne disease and its severity depend on the populations of both the pathogen and disease-suppressing organisms in the soil [31]. Pesticides reduce the diversity of soil microorganisms, and break the balance between beneficial and phytopathogenic organisms. In healthy soils, beneficial organisms suppress disease-causing organisms; however, breaking the balance between these fosters resistant pathogens [58,61]. Accordingly, it is important to study the effect of soil ozonation on non-targeted organisms at the effective dose to treat the pathogen.
This study focused on the feasibility of the technique and not on the viability of ozonation for mass use in agriculture. Treating a field of soybean with ozone would be impractical because row crops occupy large areas, needing huge amounts of oxygen and large ozone generators to generate enough ozone to treat soil to a minimum depth of 15 cm. In addition, ozone gas application to the soil would be done using irrigation system pipes or shanks (as fumigation gas), and the soil covered by tarp or impermeable nylon mulch to reduce the fumigant emission and increase ozone residence time in the soil to maximize its pesticidal activity, which is undoable for field crops.
Hence, a practical application of this treatment would be in high-value cash crops like greenhouse crops. Finally, we recommend investigating the efficiency of ozone in the control of seed borne pathogens (e.g. ozonation of seedlings, potato seed tubers) based on the promising results of this work.
Conclusions
The overall results of this study clearly indicate that ozonating soil contaminated with P. sojae at a dose of 0.47 g.kg-1 O3 is sufficient to minimize any harmful impact on seed germination and plant health. These are promising results in regards to the system of soil biodiversity, where ozonation soil treatment proved to be a viable controller of soil pests [36]. Hence, ozonation could be an intervention with balancing feedback effect on the stock of soil biodiversity, alleviating the externalities of hidden environmental costs of synthetic pesticides use, hence maintaining the sustainability of soil productivity. In addition, given that ozone does not leave toxic residues in nature, we conclude that ozonation, when integrated with other eco-friendly practices, becomes a sustainable alternative to the conventional treatments against soil pathogens such as Phytophthora. Accordingly, it entitles it for application in organic agriculture. However, further research must concentrate on the economics of ozonation to control disease effects on soil pathogens.
Acknowledgements
The authors of this research want to acknowledge and express sincere thanks to the US Department of State for providing an International Fulbright S&T Award to the first author for carrying out this research at Iowa State University. In addition, we express appreciation and thanks to the US DOE Ames Laboratory at Iowa State University for providing the lab facility needed to conduct this research, and to the reviewers for their valuable suggestions that significantly improved the quality of this paper.
References
- NSF Current (2013) Genome info from "plant destroyers" could save trees, beans and chocolate.
- Judelson HS, Blanco FA (2005) The spores of Phytophthora: weapons of the plant destroyer. Nat Rev Microbiol 3: 47-58.
- Tyler BM (2007) Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol 8: 1-8.
- Dorrance AE, Berry SA, Anderson TR, et al. (2008) Isolation, Storage, Pathotype Characterization, and Evaluation of Resistance for Phytophthora sojae in Soybean. Plant Health Progress.
- Grau CR, Dorrance AE, Bond J, et al. (2004) Fungal diseases. In: Shibles RM, Harper JE, Wilson RF, Shoemaker RC, Soybeans: Improvement, Production, and Uses. (3rd edn), American Society of Agronomy, Inc, USA, 679-763.
- Schmitthenner AF, Bhat RG (1994) Useful methods for studying Phytophthora in the laboratory. OARDC Special Circulation.
- Schmitthenner AF (2000) Phytophthora rot of soybean. In: Plant Health Progress. The American Phytopathological Society. (4th edn), USA.
- Kim KD, Nemec S, Musson G (1997) Control of Phytophthora root and crown rot of bell pepper with composts and soil amendments in the greenhouse. Applied Soil Ecology 5: 169-179.
- Duncan J (1999) Phytophthora-an abiding threat to our crops. Microbiology Today 26: 114-117.
- Barksdale TH, Papavizas GC, Johnston SA (1984) Resistance to foliar blight and crown rot of pepper caused by Phytophthora capsici. Plant Disease 68: 506-509.
- Cafe-Filho AC, Duniway JM (1995) Effects of furrow irrigation schedules and host genotype on Phytophthora root rot of pepper. Plant Disease 79: 39-43.
- Ristaino JB, Johnston SA (1999) Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Disease 12: 1080-1089.
- Deahl KL, SP DeMuth, Sinden SL, et al. (1995) Identification of mating types and metalaxyl resistance in North American populations of Phytophthora infestans. American Potato Journal 72: 35.
- Dowley LJ, O'Sullivan E (1985) Monitoring metalaxyl resistance in populations of Phytophthora infestans. Potato Research 4: 531-533.
- Pennisi AM, Agosteo GE, Cacciola SO, et al. (1998) Insensitivity to metalaxyl among isolates of Phytophthora capsici causing root and crown rot of pepper in southern Italy. Plant Disease 82:1283.
- Lamour KH, Hausbeck MK (2001) Investigating the spatiotemporal genetic structure of Phytophthora capsici in Michigan. Phytopathology 91: 973-980.
- Mathis WL, Williams-Woodward J, Csinos AS (1999) In: sensitivity of Phytophthora capsici to mefenoxam in Georgia. Phytopathology 89: 201-208.
- Parra G, Ristaino J (1998) Insensitivity to Ridomil Gold (mefenoxam) found among field isolates of Phytophthora capsici causing Phytophthora blight on bell pepper in North Carolina and New Jersey. Plant Disease 82: 711.
- Browne GT, Becherer HE, Vazquez MR, et al. (1999) Phytophthora control on strawberry, almond and walnut without methyl bromide. Proceedings of Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, San Diego, USA.
- Forster HG, Adaskaveg JE, Kim DH, et al. (1998) Effect of phosphite on tomato and pepper plants and on susceptibility of pepper to Phytophthora root and crown rot in hydroponic culture. Plant Disease 82: 1165-1170.
- Gan J, Yates SR, Anderson MA, et al. (1994) Effect of soil properties on degradation and sorption of methyl bromide in soil. Chemosphere 29: 2685-2700.
- Ristaino JB, ThomasW (1997) Agriculture, methyl bromide, and the ozone hole: can we fill the gaps? Plant Disease 81: 964-977.
- Yagi K, Williams J, Wang NY, et al. (1993) Agricultural soil fumigation as a source of atmospheric methyl bromide. In: Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences 90: 8420-8423.
- Yates SR, Gan J, Ernst FF, et al. (1996) Methyl bromide emissions from a covered field. Experimental conditions and degradation in soil. Journal of Environmental Quality 25: 184-192.
- Cone JE, Wugofski L, Balmes JR, et al. (1994) Persistent respiratory health effects after a metam sodium pesticide spill. Chest J 106: 500-508.
- Macalady JL, Fuller ME, Scow KM (1998) Effects of metam sodium fumigation on soil microbial activity and community structure. J of Environmental Quality 27: 54-63.
- Gan J, Yates SR, Ernst FF, et al. (2000) Degradation and volatilization of the fumigant chloropicrin after soil treatment. J of Environmental Quality 29: 1391-1397.
- Costanza R (2008) Stewardship for a 'full' world. Current History 107: 30-35.
- Yadav IC, Devi NL, Syed JH, et al. (2015) Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries : A comprehensive review of India. Sci Total Environ 511: 123-137.
- Gregoire C, Elsaesser D, Huguenot D, et al. (2009) Mitigation of agricultural nonpoint-source pesticide pollution in artificial wetland ecosystems. Environ Chem Lett 7: 205-231.
- Abawi GS, Widmer TL (2000) Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Applied Soil Ecology 15: 37-47.
- Cloyd RA (2012) Indirect effects of pesticides on natural enemies. In: Soundararajan RP, Pesticides-Advances in chemical and botanical pesticides. InTech, India, 127-150.
- Wright D, Meadows DH (2008) Thinking in systems. Chelsea Green Publishing Company, USA.
- Nutter FW, Teng PS, Royer MH (1993) Terms and concepts for yield, crop loss, and disease thresholds. Plant Disease 77: 211-216.
- Kobayashi F, Ikeura H, Ohsato S, et al. (2011) Disinfection using ozone microbubbles to inactivate Fusarium oxysporum f. sp. melonis and Pectobacteriumcarotovorum subsp. carotovorum. Crop Protection 30: 1510-1518.
- Msayleb Nahed (2014) Soil ozonation as a sustainable alternative to fumigation with methyl bromide and treatments with synthetic pesticides, Iowa State University, USA.
- Mun S, Cho SH, Kim TS, et al. (2009) Inactivation of Ascaris eggs in soil by microwave treatment compared to UV and ozone treatment. Chemosphere 77: 285-290.
- Ortade Velasquez MT, Rojas-Valencia MN, Vaca-MieM (2002) Destruction of helminth eggs (Ascarissuum) by ozone: second stage. Water Resources 2: 227-233.
- Orta de Velasquez MT, Martinez JL, Monje-Ramirez I, et al. (2004) Destruction of helminth (Ascarissuum) eggs by ozone. Ozone Science & Engineering 26: 359-366.
- RamIrez-Cortina CR, Alonso Gutierrez MS, Bautista JD, et al. (2005) Ozonation: An effective process for Toxocara canis eggs inactivation. In 17th World Ozone Congress, Strasbourg.
- Sankaran S, Khanal SK,van Leeuwen J(H), et al. (2008) Ozone as a selective disinfectant for nonaseptic fungal cultivation on corn-processing wastewater. Bioresource Technology 99: 8265-8272.
- Thomas V, Loret JF, Jousset M, et al. (2008) Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ Microbiol 10: 2728-2745.
- Van Leeuwen J(H) (1996) Reclaimed water-an untapped resource. Desalination 106: 233-240.
- Van Leeuwen J(H), Pipe-Martin C, Lehmann RM (2003) Water reclamation at South Caboolture, Queensland, Australia. Ozone Science & Engineering 25: 107-120.
- Oemcke DJ, van Leeuwen J(H) (2004) Seawater ozonation of Bacillus subtilis spores: implications for the use of ozone in ballast water treatment. Ozone: Science & Engineering 26: 389-401.
- Oemcke DJ, Van Leeuwen J(H) (2005) Ozonation of the marine dinoflagellate algae Amphidinium sp.-implications for ballast water disinfection. Water Research 39: 5119-5125.
- White SD, Murphy PT, van Leeuwen J(H), et al. (2010) Controlling deterioration of high-moisture maize with ozone treatment. J of Stored Products Research 46: 7-12.
- Ooi HK, Lin CL, Wang JS (1998) Effect of ozone treatment on Toxocara caniseggs. J of Veterinary Medicine Science 60: 169-173.
- Ishizaki K, Kazuyuki S, Kazunobu M, et al. (1987) Effect of ozone on plasmid DNA in Escherichia coliin situ. Water Resources 21: 823-827.
- Bocci Velio (2005) Ozone a new medical drug. Springer, The Netherlands.
- Zhu W, Koziel JA, Van Leeuwen J(H), et al. (2013) Ozonation-based decolourization of food dyes for recovery of fruit leather wastes. J of Agric and Food Chem 61: 8198-8206.
- Sopher CD, Graham DM, Rice RG, et al. (2002) Studies on the use of ozone in production agriculture and food processing. In: Proceedings of the International Ozone Association, Pan American Group Annual Conference, Raleigh-Durham, 1-15.
- Tsao PH (1983) Factors affecting isolation and quantitation of Phytophthora from soil. In: Phytophthora: its biology, taxonomy, ecology and pathology. American Phytopathological Society, USA 219-236.
- International Ozone Association, Standardization Committee-Europe (1987) Iodometric method for the determination of ozone in process gas, Paris, France: International Ozone Association, European-African Group.
- Van Leeuwen J(H) (2015) Measuring ozone dosage. Letter to editor, Ozone Sci and Eng 37: 191-192.
- Mac Donald MB, Kwong F (2005) Flower seeds: biology and technology. CABI, USA, 372.
- Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J of Microbiology and Biotechnology 28: 1327-1350.
- Kloepper JW, Hume DJ, Scher FM, et al. (1988) Plant growth-promoting rhizobacteria on canola (rapeseed). Plant Disease 72: 42-46.
- Matsuo M (1993) Ozone sterilizing for the plant pathogenic fungi in the solution of soilless culture: The case of microconidia of Fusarium oxysporum cucumerinum. J of the Japanese Society of Agricultural Machinery 55: 105-111.
- Kottapalli B, Wolf-Hall CE, Schwarz P (2005) Evaluation of gaseous ozone and hydrogen peroxide treatments for reducing Fusarium survival in malting barley. J Food Prot 68: 1236-1240.
- Sullivan P (2004) Sustainable management of soil-borne plant diseases.
Corresponding Author
J (Hans) van Leeuwen, Department of Agricultural & Biosystems Engineering, Department of Civil, Construction and Environmental Engineering, Department of Food Science and Human Nutrition, Iowa State University, 476 Town Engineering, Ames, IA 50011-3232, USA, Tel: +1-515-294-5251.
Copyright
© 2017 Msayleb N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.