Low HDL and Elevated Triglyceride-Glucose Index and HOMA-IR Associated with Multisystem Inflammatory Syndrome in Children (MIS-C)
Abstract
Background: Multisystem inflammatory syndrome in children (MIS-C) is a post infectious hyper inflammatory syndrome that develops 2-4 weeks after COVID-19. Impaired glucose-insulin metabolism and hypertriglyceridemia have been shown as risk factors for MIS-C. Triglyceride-glucose index (TyG) is a simple, cheap, and easily accessible marker calculated using fasting serum glucose and triglyceride values. Therefore, we aimed to evaluate the association between metabolic disorders and MIS-C development and to compare it with COVID-19.
Methods: A retrospective case-control study, which included 49 COVID-19 patients, and 68 MIS-C patients, was conducted at a tertiary-level university hospital. All demographic characteristics, laboratory findings, and hospital courses were retrospectively recruited from electronic medical records.
Results: Gender, standard deviation scores (SDS) of weights, and height did not significantly differ among the groups (p > 0.05). Higher triglyceride (TG) levels and lower high-density lipoprotein (HDL-C) levels were associated significantly with MIS-C; p levels were < 0.001 and < 0.001, respectively. The mean level of the homeostatic model assessment of insulin resistance (HOMA-IR) was significantly higher (5.5 vs.2.5; p < 0.001), insulin resistance was more common (60.3% vs. 22.4%; p < 0.001), and the mean level of TyG was significantly higher (4.8 ± 0.28 vs. 4.4 ± 0.25; p < 0.001) in MIS-C patients than COVID-19 patients. Increased age, lymphopenia, thrombocytopenia, monocytopenia, hypoalbuminemia, and higher levels of NT-pro BNP were significantly associated with pediatric intensive care unit (PICU) admission, p levels were 0.02, < 0.001, 0.013, 0.007, 0.002, < 0.001, 0.006 respectively. The ROC curve analysis showed HDL-C (< 30 mg/dL) with the highest AUC value of 0.902 and a sensitivity of 83.3 and a specificity of 100%. The AUC values of TyG, TG, and HOMA-IR were 0.871, 0.866, and 0.686, respectively.
Conclusions: Metabolic abnormalities, including lower HDL-C and higher levels of HOMA-IR and TyG index associated with MIS-C.
Keywords
COVID-19, HOMA-IR, Multisystem Inflammatory Syndrome in Children (MIS-C), Prognosis, TyG index
Abbreviations
ANC: Absolute Neutrophil Count; ALC: Absolute Lymphocyte Count; BMI: Body Mass Index; CBC: Complete Blood Count; CDC: Centers for Diseases and Prevention; COVID-19: Coronavirus Disease; CRP: C-Reactive Protein; EF: Ejection Fraction; Hb: Hemoglobin; HDL-C: High-Density Lipoprotein; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; IGG: Immunoglobulin G; IVIG: Intravenous Immunoglobulin; LDL-C: Low-Density Lipoprotein; LV: Left Ventricular; MIS-C: Multisystem Inflammatory Syndrome in Children; NT-Probnp N-Terminal Pro-Brain Natriuretic Peptide; PCR: Polymerase Chain Reaction; PICU: Pediatric Intensive Care Unit; PLT: Platelet Count; SARS-Cov-2: Severe Acute Respiratory Syndrome Coronavirus 2; SDS: Standard Deviation Scores; TG: Triglyceride; Tyg: Triglyceride-Glucose Index; WBC: White Blood Cell; WHO: World Health Organization
Introduction
Coronavirus Disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported from Wuhan City, China, and subsequently spread worldwide and leading to more than 659 million cases and 6.6 million deaths as of January 9, 2023 [1]. Initially, children appear to be less affected than adults, showing milder symptoms until the emergence of the novel hyper inflammatory condition MIS-C: Multi-inflammatory syndrome associated with Severe acute respiratory syndrome coronavirus 2 [2-4]. Until January 3, 2023, the Centers for Diseases and Prevention (CDC) reported over 9333 MIS-C cases in the United States, and mortality occurred in nearly 0.81% of the cases [5]. SARS-CoV-2 polymerase chain reaction (PCR) is usually negative and antibody testing positive in MIS-C patients; therefore, MIS-C has been suggested as a post infectious hyper inflammatory syndrome caused by delayed interferon response and slow viral clearance [6-9]. It is still unclear why only 1% of SARS-CoV-2 infected children subsequently developed MIS-C. In recent studies, overweight, asthma, ethnicity (black or Asian), and genetic abnormalities in the SOCS1, XIAP, or CYBB genes have been reported as risk factors for MIS-C [9,10]. Although the severity of COVID-19 is likely multifactorial, studies have shown that people with COVID-19 who have comorbidities such as prematurity, diabetes, complex genetic disorders, chronic lung disease or asthma, heart disease, neurologic disorders, obesity have a high risk of poor prognosis [11,12]. Recently, obesity has been recognized as a significant risk factor for coronavirus disease-related prognosis [13]. During the COVID-19 pandemic, quarantine precautions, including lockdown and distance education, have mostly affected children, contributing to increased obesity among all age groups. Although impaired metabolic conditions (characterized by hypertension, dyslipidemia, and hyperglycemia) are associated significantly with obesity, impaired metabolic conditions might also be present in those with normal weight or overweight [14].
Insulin resistance has been reported to be associated with the severity of the disease and poor clinical outcomes in COVID-19 patients [14]. Several studies demonstrated the triglyceride-glucose (TyG) index, a reliable, simple marker of insulin resistance which is calculated using fasting triglyceride and fasting glucose measurements [15,16]. A high TyG index is a significant predictor of poor prognosis in both the general population and patients with various diseases [17,18]. A recent study showed insulin resistance, evaluated through the TyG index, positively correlated with total and central body adiposity and shorter time spent in lively activities [19]. From the beginning of the pandemic, we observed that most MIS-C patients had high triglyceride levels, even in patients with normal weight.
The first aim of this study was to evaluate the association of TyG index and metabolic abnormalities with MIS-C and COVID-19. To the best of our knowledge, this is the third and the largest comprehensive study which evaluated higher TyG index and the homeostatic model assessment of insulin resistance (HOMA-IR) association with MIS-C.
Study Design and Study Population
A single-center retrospective study was conducted between March 11, 2020, and September 30, 2022, at Ege University Children's Hospital, a tertiary-level university hospital in Turkey. According to the Turkish Ministry of Health's COVID-19 Guideline, diagnosed and confirmed as COVID-19 were defined as cases in which SARS-CoV-2 was detected by molecular methods from nasal and throat swab samples [20]. The MIS-C group consisted of 68 children, and the COVID-19 group consisted of 49 children. The patients in the COVID-19 group were suitable if they did not develop MIS-C at least three months after primer infection and did not have a history of severe COVID-19 because any patients in the MIS-C group have a history of severe or critical COVID-19. None of the patients has been vaccinated against COVID-19. COVID-19 vaccines have been available since September 2021 in Turkey and have been recommended by the Turkish Ministry of Health. The diagnosis of MIS-C was established according to the criteria defined by the Centers for Disease Control and Prevention in May 2020 [5].
The patients with an underlying disease, including metabolic disorders and chronic medical conditions such as cardiopulmonary diseases, liver diseases, diabetes mellitus, steroid therapy, endocrinological and neuromuscular disorders, and the patients under any treatment that can alter the TyG index were excluded.
Data Collection
A standardized form was used to collect epidemiological data, clinical symptoms, and laboratory findings. Body mass index (BMI) is a person's weight in kilograms divided by the square of height in meters. Obesity was defined as more than 2 SDS of the World Health Organization (WHO) Growth Reference median for children 5-19 years of age and as height-weight greater than 3 SDS of the WHO Child Growth Standards median for children under five years of age [21].
Laboratory analysis on admissions, such as whole blood count [white blood cell (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), hemoglobin (Hb), platelet count (PLT), eosinophil, monocytes], biochemical parameters including triglyceride (TG), total cholesterol, high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), insulin and Glucose, albumin, C-reactive protein (CRP), D-dimer, fibrinogen and N-terminal pro-brain natriuretic peptide (NT-proBNP) values were recorded. A complete blood count (CBC) was performed on Sysmex XN-3100™ Automated Hematology System (Sysmex). CRP was measured immunoturbidimetrically (CRPL4, Tina-quant CRP IV) in human serum on Cobas c systems (Roche Diagnostics GmbH) (Roche, Cobas®).
The guideline has recommended that TG and LDL-C levels ≥ 130 mg/dL, total cholesterol ≥ 200 mg/dL, and HDL-C levels < 40 mg/dL be considered abnormal for children and adolescents [22]. Enzymatic, colorimetric assays measured cholesterol and triglyceride. HDL-C was measured by a homogeneous enzymatic colorimetric assay. The Fried wald equation calculated LDL-C for 'patients' TG levels < 400 mg/dL and directly measured by homogeneous enzymatic colorimetric assay for 'patients' TG levels ≥ 400 mg/dL. Glucose, insulin, and lipid values were measured in the first three days and under fasting conditions of all patients. Glucose was measured by the enzymatic reference method with hexokinase. Insulin was measured by the electrochemiluminescence immunoassay method using the sandwich principle with two monoclonal antibodies specific for human insulin.
All chemistry parameters were measured on Roche Cobas® 8000 modular analyzers (Roche Diagnostics).
The homeostatic model assessment of insulin resistance, our primary outcome measure, was calculated as a product of fasting insulin and Glucose using a standard equation (fasting insulin [µIU/mL] × fasting blood glucose [mg/dL]/405) [23,24]. Based on this score and previously published studies, clinically significant insulin resistance was defined using the HOMA-IR cut-point ≥ 3 in children [25,26]. The TyG was calculated as the Ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. A logarithmic scale expresses the TyG index [27].
Combined nasopharyngeal and or opharyngeal swab specimens were collected in a viral transport medium, including VNat (Bioeksen, Turkey). Our Molecular Virology laboratories tested all samples using the Bio-speedy® SARS CoV-2 Double Gene RT-qPCR (Bioeksen, Turkey). This assay amplifies and detects two targets (ORF1ab and N) of the virus with a limit of 200 genomes per mL. The human gene target RNAse P (RP) was measured in each sample for use in internal control. Reverse transcription-polymerase chain reaction (RT-PCR) was performed using the Rotor-Gene (Qiagen, Luxemburg). Results were considered positive if the signal was detected (Ct < 35) for RP, ORF1ab, and N genes.
Anti-Spike immunoglobulin G(IgG) and IgM antibodies were detected in serum samples using rapid lateral flow immunoassay (LFIA) (Colloidal Gold-Hotgen, Germany).
Echocardio graphic data were recorded from clinical notes and electronic record systems. Depressed left ventricular (LV) function was defined as an LV ejection fraction (EF) of < 55%.
Ethics and Consent
All parents/legal guardians gave signed informed consent to this study. The Research Ethics Committee of Ege University and the Turkish Ministry of Health obtained approval for the study (Ethical decision No. 21-8T/61).
Statistical Analysis
Statistical analysis was performed using SPSS statistical package (version 25 for Windows). Data were expressed as means ± SD or medians (interquartile range) for continuous variables or percentages for categorical variables, depending on the normality distribution. The Shapiro-Wilk test was used to evaluate our data's normal distribution of the parameters. The Student t-test and the Chi-square test were appropriate for statistical comparisons between the groups. The Mann-Whitney U test was used to compare differences in nonparametric data. The Kruskal Wallis One-Way ANOVA test was used to compare the three groups. Correlation analysis was used to determine the correlation between HOMA-IR and TyG levels.To identify the TyG index cutoff point for prediction in MIS-C children, the ROC curve was analyzed using software (MedCalc® software). Binary logistic regression was performed to identify TyG index levels associated with the risk of glucose metabolic condition. Statistical significance of differences and correlations were defined p-value of < 0.05.
Results
The study groups consisted of 49 patients with confirmed COVID-19 and 68 MIS-C cases. The mean age was 146.2 ± 50 and 104.1 ± 58.7 months in the COVID-19 and MIS-C groups, respectively. The mean age was significantly higher in the COVID-19 group than in other groups (p < 0.001). 22 (44.9%) of COVID-19 and 36 (52.9%) of MIS-C patients were male. There was no significant difference between COVID-19, and MIS-C patients regarding gender, weight-SDS, height-SDS, and BMI-SDS (p = 0.391, p = 0.087, p = 0.527, p = 0.104 respectively) (Table 1). The clinical characteristics and laboratory data are shown in Table 1. SARS-CoV-2 IgG antibodies were positive in 68 (100%) of the MIS-C group. Mortality was not observed in COVID-19 and MIS-C cases.
MIS-C patients had significantly higher mean values of TG and lower mean values of cholesterol, HDL-C, and LDL-C than COVID-19 patients (p < 0.001, p < 0.001, p = 0.043, p < 0.001, respectively).
The mean value of insulin and glucose was significantly higher in MIS-C patients than in the COVID-19 group (22.3 ± 19 vs. 11.7 ± 8.7 mU/L, 93.6 ± 24.8 vs. 86.3 ± 6.7 mg/dL) (p < 0.001, p = 0.024). MIS-C patients significantly had higher mean HOMA-IR levels than the COVID-19 group (p < 0.001). The results are shown in Table 1. The rate of insulin resistance was significantly higher in the MIS-C group than in the COVID-19 group (p < 0.001).
The mean level of the TyG index was significantly higher in the MIS-C group than in the COVID-19 group (p < 0.001). However, it was not significantly different among pediatric intensive care unit (PICU) and non-PICU groups (Table 2).
The mean platelet and albumin in the PICU group were significantly lower than in the non-PICU group (p = 0.013, p < 0.001). The PICU group had significantly higher mean values of NT-pro BNP than non-PICU (p = 0.006), (Table 2).
Thirty-six (52.9%) MIS-C patients presented with features of circulatory shock and required PICU care for circulatory support. Thirty-eight of the 68MIS-C patients did not response to intravenous immunoglobuline (IVIG) therapy. Eight of the 68 MIS-C patients had low ejection fraction. When children with MIS-C in the PICU are classified according to coronary artery involvement, IVIG response, inotropic support, and levels of HOMA-IR, we evaluated for TyG index; we did not find a significant difference in these data. However, the TyG index was higher in MIS-C patients with unresponsive to IVIG, coronary artery involvement, and higher HOMA-IR levels (p > 0.05).
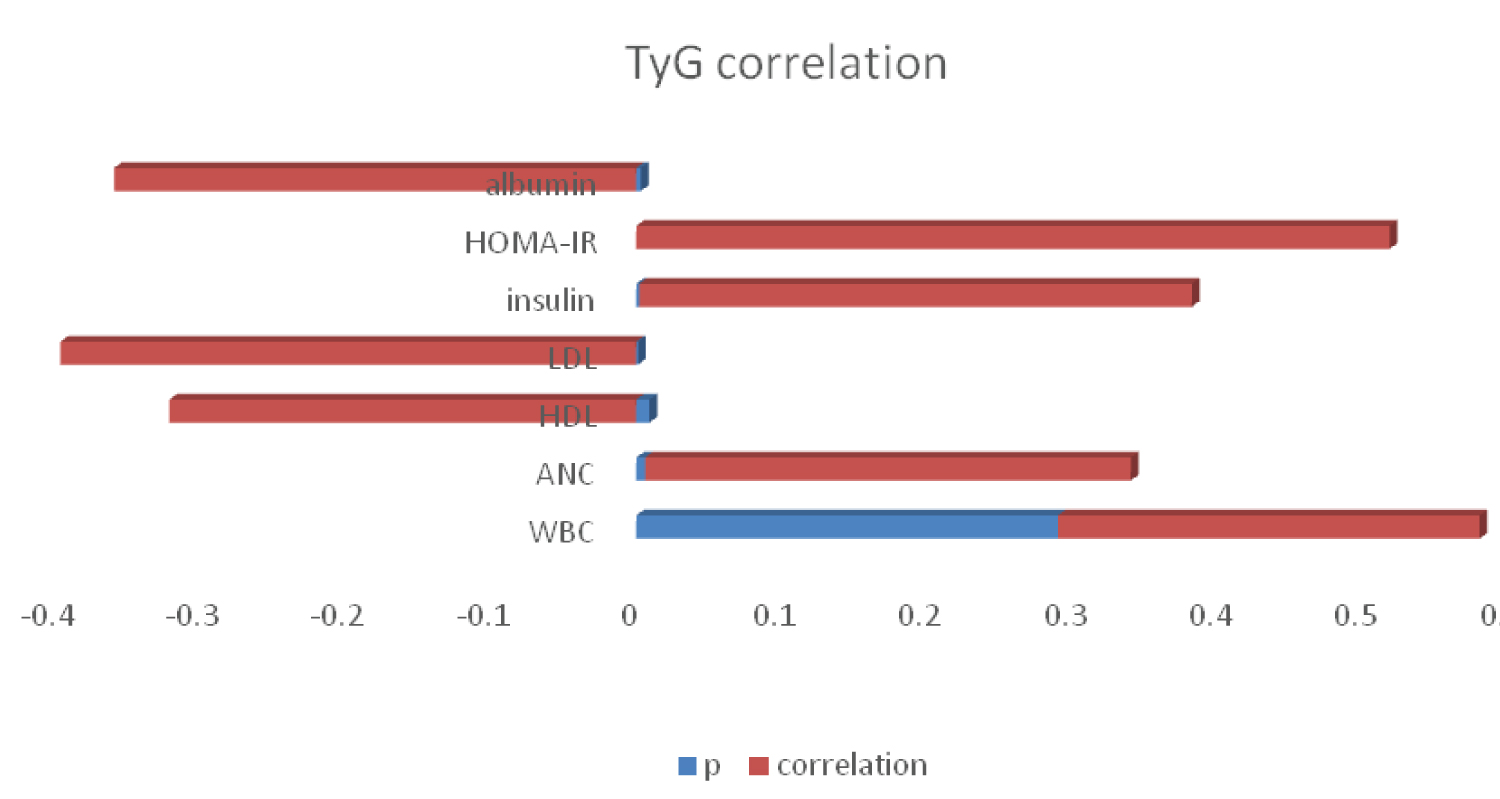
TyG index was negatively correlated with HDL-C (r = -0.321, p = 0.009), LDL-C (r = -0.396, p = 0.001), and albumin (r = -0.359, p = 0.003) and positively correlated with WBC (r = 0.390, p = 0.018), ANC (r = 0.334, p = 0.006), insulin (r = 0.380, p = 0.002) and HOMA-IR (r = 0.518, p < 0.001) in the MIS-C group (Figure 1).
HOMA-IR was negatively correlated with LDL-C (r = -0.316, p = 0.015), response to the IVIG (r = -0.316, p = 0.012), and positively correlated with age (r = 0.306, p = 0.015), TG (r = 0.399, p = 0.001), coronary artery involvement (r = 0.279, p = 0.027) in the MIS-C group.
Response to the IVIG treatment, coronary artery involvement, admission to PICU, and needing inotropes weren't correlating with the TyG index.
Multivariate logistic regression analysis revealed that predictors of MIS-C were the TyG index, HDL-C, and TG (Table 3).
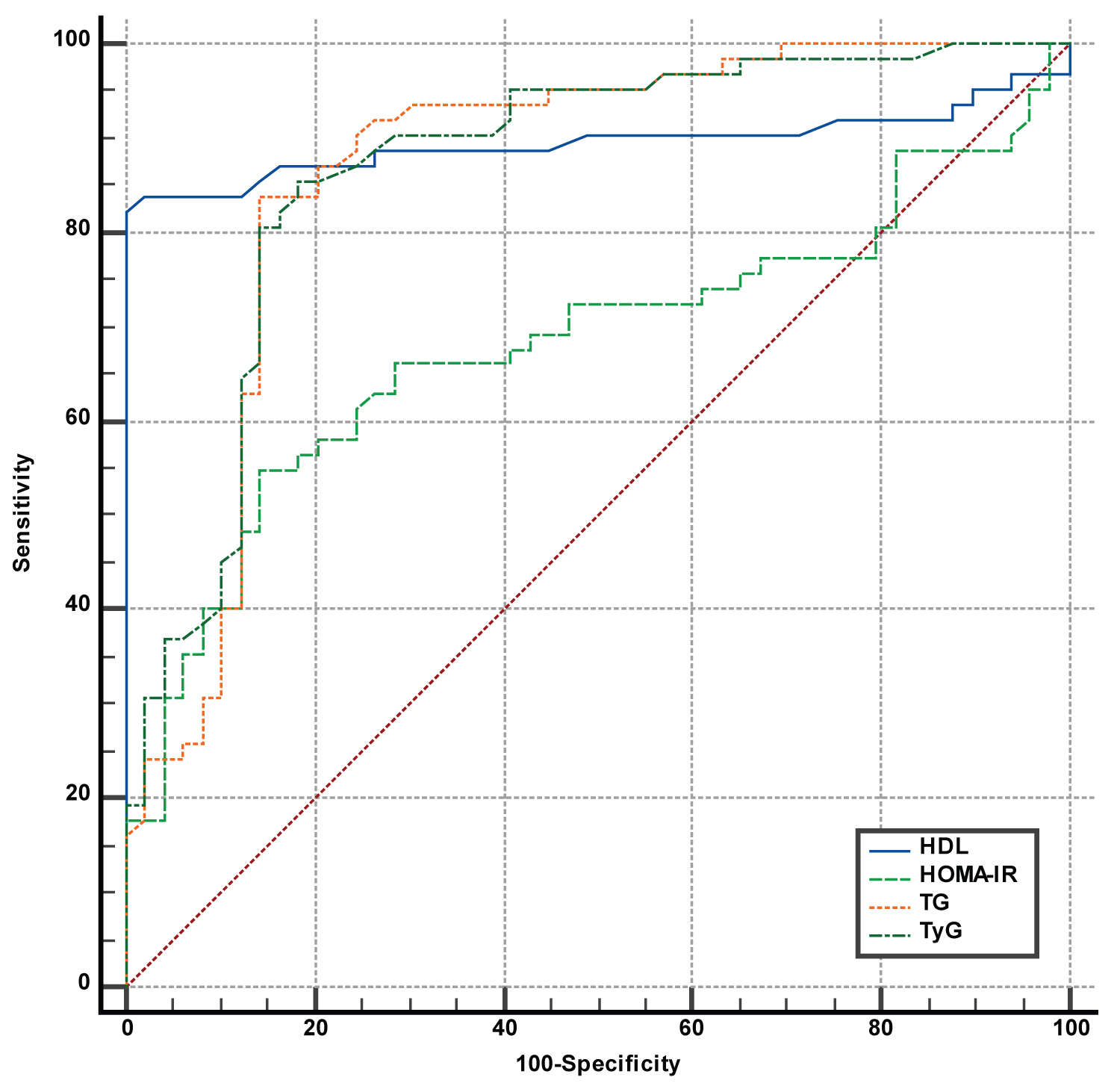
ROC analysis showed the best area under curve (AUC) level of 0.902 (95% Confidence interval (CI ): 0.832-0.949) with a sensitivity of 83.3% and specificity of 100% for HDL-C with a cutoff < 30 mg/dL. The sensitivity, specificity, and AUC values for the TyG index, TG, and HOMA-IR were 0.87, 0.866, and 0.686, respectively (Table 4). The ROC curves are shown in Figure 2.
Discussion
The COVID-19 pandemic affected over 660 million people and caused over 6 million deaths. Children seemed luckier and developed less severe diseases until the appearance of MIS-C. However, the mechanism was still unclear, and it is still an open question "Why only less than 1% of children develop MIS-C after COVID-19?". We investigated this possible interaction and showed a significant association between elevated TyG index and HOMA-IR levels and low levels of HDL-C with MIS-C, even in non-obese patients. Furthermore, higher levels of HOMA-IR were associated with PICU admission of MIS-C patients. To the best of our knowledge, this is the third and the largest study demonstrating this significant association. The first study had a control group consisting of confirmed COVID-19 cases who did not develop MIS-C. This study adds the significant associations between low HDL-C, higher levels of TyG, and HOMA-IR and MISC; meanwhile, insulin resistance significantly increases the risk of PICU admission in MIS-C patients.
Hyperglycemia can also be seen in severe diseases, not only in diabetes. Due to increased hormone and cytokine levels, unexplained hyperglycemia may be a sign of infection or inflammation [28]. In the short term, hyperglycemia can impair fluid balance and the immune system, as well as cause inflammation [29,30]. The studies showed that white blood cell function abnormalities are associated with hyperglycemia. However, glucose control improves these abnormalities [31]. Observational studies showed that hyperglycemia was a risk factor for severe complications during acute illness in patients without diabetes [32,33]. Insulin secretion increases to prevent hyperglycemia. Our study showed that MIS-C patients with the more noticeable cytokine release and inflammation also had higher insulin and glucose values. Insulin resistance was significantly more frequent in MIS-C than in COVID-19.The TyG index is a simple surrogate marker of insulin resistance. Previous studies, including COVID-19 adults, showed the potential role of the TyG index and markers of insulin resistance as an indicator for the severe complications of COVID-19 [17,18,34,35].
Calcaterra, et al. [36] evaluated 30 MIS-C patients and found all patients had pathological levels of TyG index, and 17 of 18 patients, of whom HOMA-IR levels were available, had pathological levels. In our study, 87.7% of the MIS-C cases had elevated TyG indexes nearly three-fold more common than COVID-19 cases, and 60.3% had insulin resistance. Moreover, the low level of HDL-C was 5-fold more frequently detected in MIS-C cases. The current study adds to the literature by showing the significant association between MIS-C and metabolic abnormalities, including elevated TyG index, higher basal insulin, HOMA-IR, and low HDL-C levels, contributing to MIS-C pathogenesis.
Ren, et al. [35] demonstrated a higher TyG index was significantly more common in severe and deceased COVID-19 patients. We did not determine an association between MIS-C severity and elevated TyG index. However, we showed a significant association between insulin resistance and PICU admission. None of the MIS-C patients died during the study period. A large population-based study from Korea by Chang, et al. [34] evaluated 3887 patients and suggested the TyG index was a good predictor of disease severity in COVID-19 patients. They defined the study's primary outcomes as the development of severe complications of COVID-19, such as mechanical ventilation, intensive care unit care, high-flow oxygen therapy, and mortality within two months after the diagnosis of COVID-19. They determined that the TyG index was positively associated with severe complications of COVID-19 (adjusted odds ratio: 1.42, 95%CI [1.12-1.79]) by the multivariate logistic regression analysis.
Zheng, et al. [37] showed TyG index decreased at the positive and re-positive SARS-CoV-2 RNA stages and increased at the negative stage. They suggested the TyG index may be a reliable marker for identifying the re-positive of COVID-19 patients and determining the stage of the patient's disease. MIS-C has dined as a post-infection hyper inflammatory syndrome that can be accepted as the negative stage. As they suggested, we found a high proportion of patients with elevated TyG index, and all patients' PCR results were negative for SARS-CoV-2 on admission. However, we did not re-check their TG levels in the follow-up period. Biter, et al. [38] investigated the predictive value of the TyG index for in-hospital mortality in non diabetic COVID-19 patients with myocardial injury. They showed TyG index cutoff value greater than 4.97 showed 82% sensitivity and 66% specificity in the prediction of in-hospital death in non diabetic COVID-19 patients with myocardial damage. MIS-C can be presented with myocardial injury and diminished EF. However, we did not show a significant correlation between an elevated TyG index and a lower EF.
Chen, et al. [39] demonstrated that an increased TyG index is associated with impaired b-cell function regardless of the Glucose metabolic conditions. The TyG index is an alternative indicator for predicting b-cell dysfunction. A large study by Rohani-Rasaf, et al. [40] investigating 1288 confirmed COVID-19 cases showed that an elevated TyG index was significantly associated with the severity and mortality of the disease. In this study, we also showed that MIS-C cases had significantly higher HOMA-IR levels, and higher HOMA-IR levels were associated with PICU admission.
Hypertriglyceridemia and low levels of HDL-C were associated considerably with MIS-C. Chen, et al. [41] showed that changes in total cholesterol and HDL-C levels were significantly associated with the TyG index. Similarly, we showed a significant correlation between lower HDL-C and TYG index and both significantly associated MIS-C. The ROC curve analysis showed the highest AUC level for HDL-C lower than 30 mg/dL. Furthermore, it showed 100% specificity in the prediction of MIS-C. It is an easy and cheap marker that can rule out MIS-C. Despite the low patient numbers in our study, it showed great specificity, which may be confirmed with larger and prospective studies. Rohani-Rasaf, et al. [40] revealed that the TyG and TG/HDL-C indexes are biochemical markers of the severe prognosis of COVID-19. Zinello, et al. [42] highlighted that increased proinflammatory cytokines may causethese changes in lipid profile in COVID-19 infection via upregulation of scavenger receptor class B type 1. We showed that TG levels and TYG index were higher, and HDL-C was lower in MIS-C.
Several studies have found a positive correlation between the TyG index, white blood cell count, and CRP levels [43,44]. Inflammation can result in damage of the vascular endothelium. This can lead blood contents to leak into the perivascular spaces, causing further vascular damage [45]. Monocytopenia is related to the severity of COVID-19 and ICU admission in a study (OR, 3.28 [95% CI, 1.4-7.68]) [46]. Abrams, et al. [6] also demonstrated a significant association with decreased levels of thrombocyte and lymphocyte with PICU admission, whereas they did not evaluate monocyte levels in MIS-C cases. We evaluated the hematological parameters; we showed that patients with decreased thrombocyte, lymphocyte, and monocyte levels were associated with PICU admission.
The treatment protocol for MIS-C includes glucocorticoids and intravenous immunoglobulin, which may cause glycemic fluctuation. However, the initial HOMA-IR and TyG indexes before treatment protocol initiation were considered in this study. Therefore, the effects of glucocorticoids have been excluded.
Limitations
This is a retrospective, single-center, and small-size study. Some data may be missed due to retrospective design. Another limitation is the lack of longitudinal follow of the patients.
On the other hand, this study has several advantages, to be the third and the largest study evaluating the association between elevated TyG index and higher HOMA-IR and lower HDL-C with MIS-C. The second advantage of this study is having a control group including confirmed COVID-19 cases who did not develop MIS-C.
Conclusions
This study first demonstrated a significant association between metabolic abnormalities such as hypertriglyceridemia, higher HOMA-IR levels, TyG index, low HDL-C levels, and MIS-C. Elevated HOMA-IR levels were significantly associated with PICU admission in MIS-C patients. Furthermore, fasting triglyceride and Glucose are cheap and widely available. Therefore, the TyG index may be helpful in limited settings. MIS-C cases should be followed for long-term outcomes such as diabetes and metabolic abnormalities and consulted with a Pediatric Endocrinologist. Further prospective and more extensive studies are needed. By the end of the pandemic, new variants of SARS-CoV-2 may result in increased cases of MIS-C, and pediatricians should still be conscious of MIS-C.
What is Already Known on this Topic?
Potential risk factors for MIS-C are impaired glucose-insulin metabolism and hypertriglyceridemia.
What does this Study add?
Metabolic abnormalities, including lower HDL-C and higher levels of HOMA-IR and TyG index associated with MIS-C patients.
Funding Sources
None.
References
- World Health Organization (2023) WHO Multisystem inflammatory syndrome in children and adolescents with COVID-19.
- Jones VG, Mills M, Suarez D, et al. (2020) COVID-19 and kawasaki disease: Novel virus and novel case. Hosp Pediatr 10: 537-540.
- Verdoni L, Mazza A, Gervasoni A, et al. (2020) An outbreak of severe Kawasaki-like disease at the Italian epicenter of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 395: 1771-1778.
- Riphagen S, Gomez X, Gonzalez Martinez C, et al. (2020) Hyper inflammatory shock in children during COVID-19 pandemic. Lancet 395: 1607-1608.
- (2023) Centers of diseases and prevention (CDC).
- Abrams JY, Godfred Cato SE, Oster ME, et al. (2020) Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: A Systematic Review. J Pediatr 226: 45-54.e1.
- Rowley AH (2020) Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 20: 453-454.
- Radia T, Williams N, Agrawal P, et al. (2021) Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr Respir Rev 38: 51-57.
- Guimarães D, Pissarra R, Reis Melo A, et al. (2021) Multisystem inflammatory syndrome in children (MISC): A systematic review. Int J Clin Pract 75: e14450.
- Chou J, Platt CD, Habiballah S, et al. (2021) Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J Allergy Clin Immunol 148: 732-738.e1.
- Choi JH, Choi SH, Yun KW (2022) Risk factors for severe COVID-19 in children: A systematic review and meta-analysis. J Korean Med Sci 37: e35.
- Swann OV, Holden KA, Turtle L, et al. (2020) Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ 370: m3249.
- Lockhart SM, O'Rahilly S (2020) When two pandemics meet: why is obesity associated with increased COVID-19 mortality? Med (NY) 1: 33-42.
- Turkey ministry of health, general directorate of public health. COVID-19 Guide.
- (2021) World Health Organization. Childhood overweight and obesity: WHO.
- Montori VM, Bistrian BR, McMahon MM (2002) Hyperglycemia in acutely ill patients. JAMA 288: 2167-2169.
- McCowen KC, Malhotra A, Bistrian BR (2001) Stress-induced hyperglycemia. Crit Care Clin 17: 107-124.
- Stefan N, Schick F, Häring HU (2017) Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab 26: 292-300.
- Dikaiakou E, Vlachopapadopoulou EA, Paschou SA, et al. (2020) Τriglycerides-glucose (TyG) index is a sensitive marker of insulin resistance in greek children and adolescents. Endocrine 70: 58-64.
- Brito ADM, Hermsdorff HHM, Filgueiras MS, et al. (2021) Predictive capacity of triglyceride-glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: A systematic review. Crit Rev Food Sci Nutr 61: 2783-2792.
- Liu XC, He GD, Lo K, et al. (2021) The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med 7: 628109.
- Park K, Ahn CW, Lee SB, et al. (2019) Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care 42: 1569-1573.
- Vieira Ribeiro SA, Fonseca PCA, Andreoli CS, et al. (2019) The TyG index cutoff point and its association with body adiposity and lifestyle in children. J Pediatr (Rio J) 95: 217-223.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 129: S1-S45.
- Matthews DR, Hosker JP, Rudenski AS, et al. (1985) Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419.
- Bonora E, Targher G, Alberiche M, et al. (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23: 57-63.
- Tao LC, Xu JN, Wang TT, et al. (2022) Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc Diabetol 21: 68.
- Yin J, Li M, Xu L, et al. (2013) Insulin resistance determined by homeostasis model assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr 5: 71.
- Simental Mendía LE, Rodríguez Morán M, Guerrero Romero F (2008) The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6: 299-304.
- Bistrian BR (2001) Hyperglycemia and infection: Which is the chicken and which is the egg? JPEN J Parenter Enteral Nutr 25: 180-181.
- McMahon MM, Bistrian BR (1995) Host defenses and susceptibility to infection in patients with diabetes mellitus. Infect Dis Clin North Am 9: 1-9.
- Capes SE, Hunt D, Malmberg K, et al. (2000) Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet 355: 773-778.
- Capes SE, Hunt D, Malmberg K, et al. (2001) Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke 32: 2426-2432.
- Chang Y, Jeon J, Song TJ, et al. (2022) Association of triglyceride-glucose index with prognosis of COVID-19: A population-based study. J Infect Public Health 15: 837-844.
- Ren H, Yang Y, Wang F, et al. (2020) Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol 19: 58.
- Calcaterra V, Bosoni P, Dilillo D, et al. (2021) Impaired glucose-insulin metabolism in multisystem inflammatory syndrome related to SARS-CoV-2 in children. Children (Basel) 8: 384.
- Zheng Y, Wang J, Ding X, et al. (2022) The correlation between triglyceride-glucose index and SARS-CoV-2 RNA Re-positive in discharged COVID-19 patients. Infect Drug Resist 15: 3815-3828.
- Biter HI, Kalyoncuoglu M, Tosu AR, et al. (2022) Prognostic value of the TyG index for in-hospital mortality in nondiabetic COVID-19 patients with myocardial injury. Rev Assoc Med Bras (1992) 68: 1297-1302.
- Chen M, Zhu B, Chen D, et al. (2021) COVID-19 may increase the risk of insulin resistance in adult patients without diabetes: A 6-month prospective study. Endocr Pract 27: 834-841.
- Rohani Rasaf M, Mirjalili K, Vatannejad A, et al. (2022) Are lipid ratios and triglyceride-glucose index associated with critical care outcomes in COVID-19 patients? PLoS One 17: e0272000.
- Chen Z, Wen J (2022) Elevated triglyceride-glucose (TyG) index predicts impaired islet β-cell function: A hospital-based cross-sectional study. Front Endocrinol (Lausanne) 13: 973655.
- Zinellu A, Paliogiannis P, Fois AG, et al. (2021) Cholesterol and triglyceride concentrations, COVID-19 severity, and mortality: A systematic review and meta-analysis with meta-regression. Front Public Health 9: 705916.
- Nam KW, Kwon HM, Jeong HY, et al. (2020) High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: A cross-sectional study. Cardiovasc Diabetol 19: 53.
- Zhao Q, Zhang TY, Cheng YJ, et al. (2020) Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: Results from an observational cohort study in China. Cardiovasc Diabetol 19: 108.
- Pantoni L (2010) Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9: 689-701.
- Vanhems P, Gustin MP, Elias C, et al. (2021) Factors associated with admission to intensive care units in COVID-19 patients in Lyon-France. PLoS One 16: e0243709.
Corresponding Author
Sahbudak Bal Zumrut, MD, Division of Infectious Disease, Department of Pediatrics, Medical School of Ege University, Bornova/Izmir, 35100, Turkey, Tel: 905054423192, Fax: 902323889900.
Copyright
© 2023 Zumrut SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.