The Correlation between MicroRNA-199a and White Adipose Tissue in C57/BL6J Mice with High-Fat Diet
Abstract
Understanding is emerging about microRNAs as mediators in the regulation of white adipose tissue (WAT) and obesity. The expression level of miR-199a in mice was investigated to test our hypothesis: miR-199a might be related to fat accumulation. And we try to find its target mRNA, which perhaps could propose strategies with a therapeutic potential affecting the fat storage. C57/BL6J mice were randomly assigned to either a control group or an obesity model group (n = 10 in both groups). Control mice were fed a normal diet (fat: 10 kcal %) ad libitum for 12 weeks, and model mice were fed a high-fat diet (fat: 30 kcal %) ad libitum for 12 weeks to induce obesity. At the end of the experiment, we used DEXA to test body fat mass and used enzyme-linked immunoassays method to measure the free fatty acids (FFAs) and triglycerides (TGs) in WAT. Fat cell size was measured by hematoxylin-eosin (H&E) staining method and counted by MetaMorph-based method. QRT-PCR method was used to test the expression level of microRNA-199a and key adipogenic transcription factors, including peroxisome proliferator activated receptor gamma2 (PPARγ2), CCAAT/enhancer binding proteins alpha (C/EBPα), adipocyte fatty acid-binding protein (aP2). The fat mass of the model group was higher than that of the control group (P < 0.05). In addition, the concentrations of the FFAs and TGs were higher (P < 0.05) and the adipocyte size was bigger (P < 0.05) in the model group. Up-regulated expression of miR-199a was observed in control group. Decreased levels of miR-199a were accompanied by high expression levels of SREBP-1c. We found that the 3'-UTR of SREBP-1c mRNA has a predicted binding site for miR-199a. Based on the current discoveries, we suggest that miR-199a may exert its action by binding to its target mRNA and mediated SREBP-1c inactive or down-regulated to inhibit WAT hypertrophy. Therefore, if the predicted binding site is confirmed by further research, miR-199a may have therapeutic potential for obesity.
Introduction
Obesity is closely linked to metabolic syndrome, and it is a risk factor for various metabolic diseases, including type 2 diabetes, hypertension, hyperlipidemia and atherosclerosis [1-3]. Excessive storage of white adipose tissue (WAT) is the main manifestation of obesity. WAT has been characterized as an endocrine organ that participates in energy metabolism [4]. Although WAT is important in the regulation of metabolism, excessive fat accumulation in WAT is associated with metabolic syndrome [5]. Several key adipocytokines (e.g., adipocyte fatty acid-binding protein [aP2], CCAAT/enhancer binding proteins alpha [C/EBPα], peroxisome proliferator activated receptor gamma2 [PPARγ2], and sterol regulatory element binding protein-1c [SREBP-1c]) play a significant role in the regulation of fat storage [6,7], which in turn will affects the normal function of WAT.
MicroRNAs (miRNAs) are highly conserved, single-stranded non-coding RNAs [8]. At present, numerous studies have confirmed that miRNAs are involved in the regulation of various human tumors, such as ovarian cancer, colorectal cancer, and others [9,10]. Accumulating evidence has recently shown that miRNAs participate in the regulation of adipose tissue function, affecting adipose tissue metabolism and related diseases [11,12]. Many miRNAs are dysregulated in the WAT of obese animals and human subjects, potentially contributing to the pathogenesis of obesity-associated complications [4,13,14]. Despite wide studies above [4,11-14], we also want to know the relationship between miRNAs and key adipocytokines, and whether their interaction leads to adipose tissue dysfunction. Further exploration of more miRNAs is required. Identification and exploration of more obesity-related miRNAs will be helpful for better understanding of fat accumulation and WAT dysfunction.
Among the many miRNAs that have been identified in humans, we focus on miR-199a because of its potential effect on obesity. Previous studies showed miR-199a was highly expressed in 3T3-L1 preadipocytes, and was a suppressor of adipogenic differentiation [15,16]. Several studies have reported that miR-199a participates in the regulation of adipogenesis [17,18], which indicates that miR-199a may play an important regulatory role in obesity. However, there is currently no report on the relationship between miR-199a and obesity.
In the present study, an obesity model was established in C57/BL6J mice. Free fatty acids (FFAs), triglycerides (TGs), adipocyte counts, and fat mass were observed among the model and control groups. The expression level of miR-199a in mice was investigated to test our hypothesis that miR-199a may be associated with fat accumulation. Furthermore, the study aimed to establish its target mRNA, which may propose strategies with a therapeutic potential that affect fat storage.
Materials and Methods
Animals
All experimental protocols were approved by the Shandong University Institutional Animal Ethics Committee, and all procedures were performed in accordance with ethical standards. Male C57/BL6J mice (n = 20) were obtained from PLA Nanjing Military Medical College (Nanjing, China). The weight of mice were 18~22 g (4~6 weeks), and housing conditions was as follows: environment temperature 22~25 ℃, relative humidity 55%~65%, 12 h light and 12 darkness cycle. The C57/BL6J male mice were randomly assigned to one of two groups (n = 10 per group for this pilot study). In the control group, mice were fed a normal diet (10% kcal from fat) ad libitum for 12 weeks. In the model group, mice were fed a high-fat diet (30% kcal from fat) ad libitum for 12 weeks to induce obesity. The diet was ordered from Shandong University. Weight measurements were performed weekly. At the end of the experiment, adipose tissue mass was scanned in vivo using digital dual-energy X-ray (DEXA) scanners (Norland at Swissray. Fort Atkinson, WI, USA). After all of the mice were euthanized, WAT samples were dissected to detect associated indicators of obesity.
Body fat mass
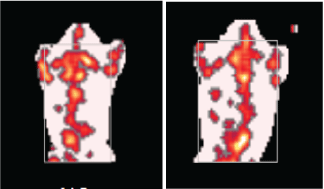
Fat mass was measured using digital DEXA scanners. DEXA data were processed for study of morphometry. Mice were anesthetized for ~1 h by injection of pentobarbital sodium (30 mg/kg bodyweight). The anesthetized mice were individually placed on a foam board and the scanning arm of the DEXA scanner assessed the fat mass (in grams) from neck to tail. After several minutes, the fat mass was recorded (Figure 1).
FFAs and TGs levels in WAT
Subcutaneous adipose tissue and visceral adipose tissue were dissected, weighed, and stored at -80 ℃. Lipids from subcutaneous adipose tissue were extracted by the Folch method as previously described [19], and the levels of FFAs and TGs were measured with the use of enzyme-linked immunoassays (Shanghai Tongwei reagent biological technology Co., Ltd., China) and the kit catalogue numbers were TWp002083 and TWp003393. The concentrations of FFAs and TGs were calculated based on a standard curve, which is made standard concentration (μmol/L) as abscissas and optical density (OD) value (l g (1/trans)) as ordinate.
Adipocyte size measurement
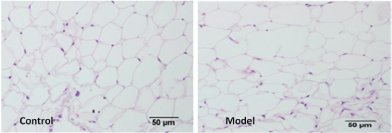
Adipose tissue was harvested, fixed, sectioned and stained with hematoxylin-eosin (H&E). And ~5000 adipocytes per mouse were counted using the MetaMorph-based method [20] under the light microscope (× 200 X, scale bar = 50 μm, OLYMPUS C o., Ltd., Japan) to show the distribution of adipocytes sizes (Figure 2).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from adipose tissue, which was isolated using an RNeasy Lipid Tissue Mini kit (Qiagen GmbH, Hilden, Germany) and stored at -80 ℃. The purity, concentration, and integrity of the RNA were analyzed by micro-ultraviolet spectrophotometry (Thermo Scientific NanoDrop 2000/2000c, USA) and agarose gel electrophoresis. Total RNA was reverse-transcribed with the ReverTra Ace qP CR RT kit (TOYOBO life science Co., Ltd., Japan) to obtain total cDNA. qRT-PCR was performed with SYBR® Green qPCR Master Mix (Shanghai TOYOBO biological technology Co., Ltd., China) in a LightCycler® 480 system (Roche Group, Switzerland). The expression level of each mRNA was normalized to β-actin. SYBR Green fluorescence quantification method was used and thermocycling was 40 Ct. DNAase (Shanghai TOYOBO biological technology Co., Ltd., China) was used to eliminate DNA interference. The primer sequences and cycling conditions used are listed in Table 1. TaqMan™ MicroRNA reverse transcription kit (Applied Biosystems, USA) was optimized to convert total RNA into cDNA when used with specific microRNA primers supplied with the TaqMan™ MicroRNA Assay (has-miR-199a-000498, Thermo Fisher Scientific, USA). The expression level of miRNA was normalized to endogenous snRNA U6 (ThermoFisher Company, USA).
Functional analyses
We check the structure of these molecules by nucleotide database (http://www.microrna.org, https://www.ncbi.nlm.nih.gov/nuccore), and the functional link between these molecules was analyzed using the Miranda program [21] to find theoretical binding site for miR-199a.
Statistical analyses
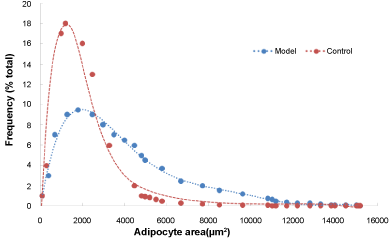
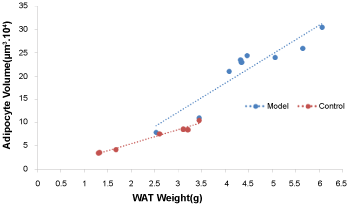
We used SPSS 22.0 and Excel software to analyze the data. The distribution of adipocyte sizes and the linear relationship between average adipocyte volume and subcutaneous WAT weight was calculated. The distribution of adipocytes sizes was showed using the frequency function in Excel to calculate the mean radius of adipocytes. Results are expressed as medians (P25, P75) or means ± SD according to normality test, which correspondingly used two independent-sample nonparametric tests or t-test to compare the medians or means between the two groups. A correlational analysis was calculated and is shown with correlation coefficient. A P-value < 0.05 was considered statistically significant.
Results
Changes in lipid metabolism induced by high-fat diet
The body fat mass (from neck to tail) was determined using DEXA scanners (Figure 1). The fat mass of the model group was higher than that of the control group (Table 2; P = 0.009). Under a magnified visual field (x 200) of the light microscope, an average number of 236 adipocytes were counted in the control group and 200 in the model group (Figure 2). The adipocyte count was lower in the model group than in the control group (Table 2; P = 0.012). In addition, the distribution of adipocyte size was showed in Figure 3 and the mean radius of adipocyte in subcutaneous WAT of mice was 27.1 μm and 34.3 μm in control group and model group (Figure 3). A linear relationship to weight of subcutaneous WAT observed between model group and control group (Figure 4) and the model group was higher in adipocyte volume. The FFA and TG levels in the WAT are presented in Table 2. The concentrations of FFA (Table 2; P < 0.05) and TG (Table 2; P < 0.05) were higher in the model group than in the control group.
Changes in the expression levels of miR-199a, aP2, C/EBPα, PPARγ2, and SREBP-1c
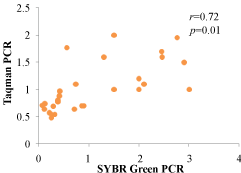
The miR-199a expression level in WAT was measured using RT-qPCR and TaqMan Probe method (Table 3). Correlation of the expression between the SYBR Green PCR method and TaqMan probe PCR method in microRNA-199a was showed in Figure 5 and it showed a very good consistency. The miR-199a expression level was upregulated in the control group and was higher when compared with the model group (Table 3, P < 0.01).
To further confirm the role of miR-199a in obesity, the expression levels of four key adipogenic transcription factors, including PPARγ2, C/EBPα, aP2, and SREBP-1c were evaluated. The expression levels of aP2, C/EBPα, PPARγ2 and SREBP-1c were higher in the model group than in the control group. Aside from the expression level of C/EBPα (Table 3; P = 0.246), the increased expression levels of the three other indicators (SREBP-1c, PPARγ2, aP2) were statistically significant (Table 3; P = 0.0035, 0.013 and 0.021).
Correlation between miR-199a and aP2, C/EBPα, PPARγ2, and SREBP-1c
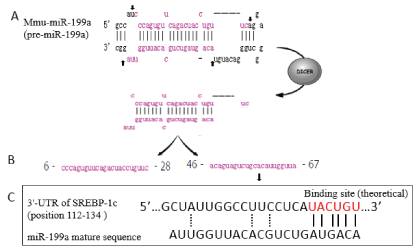
The correlation (r) between miR-199a and the four key adipogenic transcription factors is presented in Table 4. SREBP-1c expression levels were negatively correlated with the miR-199a expression level (r = -0.7222, P = 0.002). No statistically significant correlation was observed between miR-199a and aP2, C/EBPα, or PPARγ2 expression levels (P > 0.05). The functional link between these molecules was analyzed using the Miranda program [21]. It was found that the 3'-UTR of SREBP-1c mRNA has a theoretical binding site for miR-199a. The miR-199a processing and recognition of the mRNA target sites are presented in Figure 6.
Discussion
The results of our study showed decreased expression level of miR-199a in the WAT of the model group. The expression levels of key transcriptional regulation factors related to adipocyte differentiation and fat accumulation, including aP2, PPARγ2, and SREBP-1c, were up-regulated in the WAT of the model group. Among them, SREBP-1c was negatively correlated with the miR-199a expression level.
Obesity is an energy balance disorder that is characterized by increased lipid storage in adipocytes (hypertrophy) as well as an increased number of adipocytes (hyperplasia) [22]. Adipocyte hypertrophy is the main reason of adult obesity. Generally speaking, it is not only caused by increased fatty acid synthesis from carbohydrates and fat intake by organs and low energy expenditure, but also by a malfunction of fat tissue. The typical diameter of an adipocytes is 0.3-0.9 μm, but it can be about 20-fold larger in hypertrophic adipose tissue [20,23]. In our study, a high-fat diet was used to create an obesity model. The fat mass was higher in the model group, and adipocyte sizes in the model group were bigger than in the control grouped.
The function of WAT is to store excess energy in the form of TGs and to convert them into FFAs and glycerol to provide energy upon demand. When the excess calories overwhelm the storage capacity of adipocytes, excess fat is stored in the liver, leading to fat deposition and insulin resistance [24]. As an endocrine organ, WAT plays a crucial role in controlling whole body metabolism by secreting adipokines and storing FFAs [25], and increased WAT mass via hyperplasia and hypertrophy results in adipocyte dysfunction [26]. In our study, the FFA and TG levels of the model group were higher than those of the control group. This finding implies a disorder in lipid metabolism in the hypertrophic adipose tissue.
MiRNAs, as transcription factors in the adipocyte differentiation process, were previously demonstrated to regulate adipogenesis and fat storage [11]. A better understanding of the regulation of adipogenesis is crucial to the development of novel therapeutic strategies for obesity and its associated metabolic syndromes. Existing data demonstrated that miR-33 reduces the oxidation of fatty acids and inhibits the production of high-density lipoprotein (HDL), and it appears to be up-regulated in the individuals with obesity [27]. In addition, the miR-222 expression level was found to be up-regulated in the serum of obese subjects, whereas miR-221 was down-regulated [28]. The expression levels of both types of miRNAs are related to body mass index (BMI), waist circumference measurement, fat distribution, and HDL concentration [28]. Many studies have also shown that miRNAs can be used as targets in the treatment of obesity and obesity-related chronic diseases [4,13,29].
As shown in Figure 6, pre-miR-199a has a hairpin structure. This hairpin structure is processed into a miR-199a duplex by the RNase Dicer through cleavage at several sites in the hairpin. The miRNA is then unwound into two strands: miR-199a-3p (right) and miR-199a-5p (left). A previous study showed that miR-199a was highly expressed in 3T3-L1 preadipocytes [30], and was a suppressor of adipogenic differentiation [15]. Other several researches have shown that miR-199a participates in the regulation of adipogenesis [17,18], suggesting that miR-199a may play an important regulatory role in obesity. However, the potential role of miR-199a in adipogenesis and hypertrophy of WAT has not yet been demonstrated in animal models or in human subjects. In our study, the result showed a decreased expression level of miR-199a in the WAT of the model group. Based on these results, we hypothesized that the decreased miR-199a expression was associated with lipid accumulation. We then conducted the experiments to test this hypothesis.
We also measured the expression levels of key transcriptional regulation factors that are related to adipocyte differentiation and fat storage. PPARγ, which is specifically expressed in WAT, is involved in lipid formation in mature adipocytes [31]. C/EBPα also stimulates adipogenesis and works together with PPARγ in the process of adipocyte differentiation [32]. In addition, other transcription factors, including aP2 and SREBP-1c are related with to fatty acid metabolism and glucose metabolism [33-35]. Our results showed that the expression levels of aP2, PPARγ2, and SREBP-1c were up-regulated in the WAT of the model group. The results were consistent with previous reports [31]. Moreover, SREBP-1c expression was negatively consistent with the miR-199a expression level (Table 4), implying a potential mechanism between obesity and the miR-199a. In the control group, SREBP-1c expression level was higher. When induced by a high-fat diet, the expression decreased significantly in line with miR-199a expression. It showed that miR-199a may be relevant to obesity induced by a high-fat diet and the potential mechanism might be associated with SREBP-1c.
We analyzed the functional link between SREBP-1c and miR-199a and found that the 3'-UTR of SREBP-1c mRNA has a theoretical binding site for miR-199a (Figure 6). Based on our findings, we speculate that miR-199a may exert its action by binding to its target mRNA and mediated SREBP-1c inactive or down-regulated to inhibit WAT hypertrophy. As reported in the literature, miRNAs function is by partially pairing to sequences located in the 3'UTR of target mRNA [36]. If the predicted binding site for miR-199a in the 3'UTR of SREBP-1c is confirmed in further research, therapeutic strategies affecting the function of miR-199a may be possible (e.g., chemically modified complementary inhibitors). However, future research would first need to verify the direct role of miR-199a by using gene silencing or knockout technology specifically in WAT.
In conclusion, the present study showed that miR-199a expression is decreased by a high-fat diet, and a higher level of miR-199a is correlated with increased expression of SREBP-1c. We analyzed the functional link of these molecules and found that the 3'-UTR of SREBP-1c mRNA has a theoretical binding site for miR-199a. Further research is needed to confirm our speculation. If the predicted binding site is confirmed in future research, potential therapeutic strategies affecting the function of miR-199a may be proposed for obesity and related-diseases.
Acknowledgments
The authors declare no conflicts of interest that would prejudice the impartiality of this scientific work. This work was sponsored by National Natural Science Foundation (NSFC 81370966) of China.
References
- Kadakia MB, Fox CS, Scirica BM, et al. (2011) Central obesity and cardiovascular outcomes in patients with acute coronary syndrome: observations from the MERLIN-TIMI 36 trial. Heart 97: 1782-1787.
- Malik VS, Willett WC, Hu FB (2013) Global obesity: Trends, risk factors and policy implications. Nat Rev Endocrinol 9: 13-27.
- Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Invest 106: 473-481.
- Alexander R, Lodish H, Sun L (2011) MicroRNAs in adipogenesis and as therapeutic targets for obesity. Expert Opin Ther Targets 15: 623-636.
- Grundy SM (2015) Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest 45: 1209-1217.
- Zhao X, Xiaoli, Zong H, et al. (2014) Inhibition of SREBP transcriptional activity by a boron-containing compound improves lipid homeostasis in diet-induced obesity. Diabetes 63: 2464-2473.
- Chung S, Kim YJ, Yang SJ, et al. (2016) Nutrigenomic Functions of PPARs in Obesogenic Environments. PPAR Res.
- Wang J, Chen J, Sen S (2016) MicroRNA as biomarkers and diagnostics. J Cell Physiol 231: 25-30.
- Du Z, Sha X (2017) Demethoxycurcumin inhibited human epithelia ovarian cancer cells' growth via up-regulating miR-551a. Tumour Biol 39.
- Han Y, Zhao Q, Zhou J, et al. (2017) miR-429 mediates tumor growth and metastasis in colorectal cancer. Am J Cancer Res 7: 218-233.
- Seton-Rogers S (2012) MicroRNAs: Editing changes the meaning. Nature Reviews Cancer 12: 797.
- Van Rooij E, Olson EN (2012) MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 11: 860-872.
- Peng Y, Yu S, Li H, et al. (2014) MicroRNAs: emerging roles in adipogenesis and obesity. Cell Signal 26: 1888-1896.
- Hilton C, Neville MJ, Karpe F (2013) MicroRNAs in adipose tissue: their role in adipogenesis and obesity. Int J Obes (Lond) 37: 325-332.
- Shi XE, Li YF, Jia L, et al. (2014) MicroRNA-199a-5p affects porcine preadipocyte proliferation and differentiation. Int J Mol Sci 15: 8526-8538.
- Ouyang D, Ye Y, Guo D, et al. (2015) MicroRNA-125b-5p inhibits proliferation and promotes adipogenic differentiation in 3T3-L1 preadipocytes. Acta Biochim Biophys Sin (Shanghai) 47: 355-361.
- Gu N, You L, Shi C, et al. (2016) Expression of miR-199a-3p in human adipocytes is regulated by free fatty acids and adipokines. Mol Med Rep 14: 1180-1186.
- Song J, Gao L, Yang G, et al. (2014) MiR-199a regulates cell proliferation and survival by targeting FZD7. PLoS One 9: e110074.
- Breil C, Abert Vian M, Zemb T, et al. (2017) "Bligh and Dyer" and folch methods for solid-liquid-liquid extraction of lipids from microorganisms. Comprehension of solvatation mechanisms and towards substitution with alternative solvents. Int J Mol Sci 18: 708.
- Parlee SD, Lentz SI, Mori H, et al. (2014) Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol 537: 93-122.
- Wang S, Kim J, Jiang X, et al. (2014) GAMUT: GPU accelerated microRNA analysis to uncover target genes through CUDA-miRanda. BMC Med Genomics 7: S9.
- Wang QA, Tao C, Gupta RK, et al. (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine 19: 1338-1344.
- Kim JI, Huh JY, Sohn JH, et al. (2015) Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol 35: 1686-1699.
- Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548-2556.
- Choe SS, Huh JY, Hwang IJ, et al. (2016) Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 7: 30.
- Roberts R, Hodson L, Dennis AL, et al. (2009) Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia 52: 882-890.
- Su D, Zhang R, Hou F, et al. (2017) Lychee pulp phenolics ameliorate hepatic lipid accumulation by reducing miR-33 and miR-122 expression in mice fed a high-fat diet. Food Funct 8: 808-815.
- Ortega FJ, Mercader JM, Catalan V, et al. (2013) Targeting the circulating microRNA signature of obesity. Clin Chem 59: 781-792.
- Kolfschoten IG, Roggli E, Nesca V, et al. (2009) Role and therapeutic potential of microRNAs in diabetes. Diabetes Obes Metab 11: 118-129.
- Kajimoto K, Naraba H, Iwai N (2006) MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA 12: 1626-1632.
- White UA, Stephens JM (2010) Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol 318: 10-14.
- Zuo Y, Qiang L, Farmer SR (2006) Activation of CCAAT/enhancer-binding protein (C/EBP) alpha expression by C/EBP beta during adipogenesis requires a peroxisome proliferator-activated receptor-gamma-associated repression of HDAC1 at the C/ebp alpha gene promoter. J Biol Chem 281: 7960-7967.
- Kralisch S, Fasshauer M (2013) Adipocyte fatty acid binding protein: a novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 56: 10-21.
- Kolehmainen M, Vidal H, Alhava E, et al. (2001) Sterol regulatory element binding protein 1c (SREBP-1c) expression in human obesity. Obes Res 9: 706-712.
- Wang H, Kouri G, Wollheim CB (2005) ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci 118: 3905-3915.
- Abelson JF, Kwan KY, O'roak BJ, et al. (2005) Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science 310: 317-320.
Corresponding Author
Xia Wang, Associate Professor, Department of Maternal and Child Health Care, School of Public Health, Shandong University, 44 Wenhuaxi Road, Jinan, Shandong 250012, PR China.
Copyright
© 2018 Liu D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.