Feeding Practices of Open Abdomen Patients: An Assessment of Energy Intake and Clinical Outcomes
Abstract
Purpose
The open abdomen (OA) procedure in the damage control setting has become more common. Optimizing nutritional support for OA patients continues to pose a challenge for surgeons. The objective of this study was to review current practices using the Penn State equation to determine if these critically ill patients were adequately fed.
Methods
A retrospective study was conducted on 33 patients admitted to the ICU with OA for ≥ 7 days at a Level 1 Trauma Centre between January 2010 and September 2013. Daily caloric and protein intakes were measured by tabulating the total enteral (EN) and/or parenteral nutrition (TPN) received the total relevant fluid and medication infusions. Patient demographics, standard outcomes and Penn State target were recorded. The optimal energy needs were defined as ± 10% of the target.
Results
The median age was 47 with 85% males. At 7 days, 6% of patients met 90% of mean target calories and protein requirements. EN was successfully introduced in 21.2% of the patients while 42.4% of the patients received TPN, 27.2% received combined nutrition and 9% did not received any form of nutrition support. At 8-14 days, 24% reached the caloric target with 55% achieving protein target. Twenty percent of them received EN, while 43.3% received TPN and 40% received both TPN and EN. By the total 14 days of admission, 9% achieved the mean protein and caloric target. Unadjusted survival was higher in the group that met their target protein needs at 8-14 days 100% vs. 64% (P = 0.011). TPN use was higher in the group who achieved the optimal protein intake target 68% vs. 13% (P = 0.002).
Conclusions
The vast majority of OA patients were insufficiently fed during their ICU stay. Patients who achieved their protein target at 14 days had a higher survival rate. TPN use was also higher in the group who achieved the optimal protein target. Further studies are needed to identify the impacts of underfeeding on OA patients.
Introduction
Implementation of damage control surgery and the open abdomen technique has showed improved survival in trauma and acute general surgery emergencies [1]. However, this technique has also created new challenges in the management of patients with a significant abdominal wall defect. One of the main challenges lies in optimizing resting energy expenditure (REE). Thus, under-feeding or over-feeding remains problematic due to uncertainties regarding the prediction of energy needs at different disease states as well as individual variations [2]. Early studies were conducted to explain the responses of injury and its influence on caloric and protein requirements. These studies showed that nutrition support after trauma should be dynamically adjusted according to metabolic responses. This is because the trauma itself can induce a series of dynamic metabolic responses with different characteristics in three stages: the ebb phase, flow phase, and recovery phase [3,4]. The ebb phase typically lasts 12 to 48 hours, followed by the flow phase, which generally lasts 7 to 10 days, and finally the anabolic or recovery phase, which may extend to months [5,6]. In the course of the flow phase, hyper-metabolism occurs as the body attempts to rehabilitate itself while maintaining organ functionality. This phase is characterized by insulin resistance and hyperglycemia, which increase pro-inflammatory cytokine production [7]. Cerra, et al. study reported that cytokines increased daily energy needs by 10 to 20% [8]. Thus, even well-nourished patients may develop protein-energy malnutrition within 7 to 10 days of intensive care unit (ICU) admission [9].
Prediction equations are promptly available and universally used to estimate resting energy expenditures. In particular, the Penn State equation is widely used for critically ill, mechanically ventilated, and trauma patients to estimate the resting energy expenditure if the metabolic cart is not accessible [10,11]. Both the 1998 and 2003 Penn State equations were found to be unbiased and valid by Franken field and colleagues, who found the 1998 Penn State equation to be 68% accurate, and the 2003 Penn State equation to be 72% accurate [12].
Challenges Associated with the Open Abdomen Technique
Open abdomen patients often present with multiple injuries that require multiple surgeries, and they are also the most sick, critically ill, and subsequently the most hyper-metabolic of all surgical and trauma patients [13]. This hyper-metabolic state renders achieving caloric and protein targets extremely difficult. In addition, an open abdomen technique induces a significant source of protein and nitrogen loss in these critically ill patients, as confirmed by Cheatham, et al. [14]. Moreover, large amounts of protein loss across these wounds can result in changes in oncotic pressure at the capillary bed level. Protein loss can also induce the further loss of circulating volume into the interstitial space [15]. Furthermore, abdominal wall closure may not be possible either after major trauma or in septic patients for many reasons [16,17]. Massive intestinal edema, risk of acute compartment syndrome, multiple re-explorations of the abdomen, as well as a triad of hypothermia, coagulopathy, and acidosis together may lead to prolongation of the hyper-metabolic state [6,18,19]. The purpose of this study was to compare our current practice with the Penn State equation target to determine if open abdomen patients were adequately fed.
Materials and Methods
Study design
A retrospective review of all trauma and general surgery admissions from 1 January 2010 to 1 September 2013 was performed to identify patients who underwent exploratory laparotomy and subsequently required an open abdomen for seven days or more as a part of the damage control technique or after the development of acute compartment syndrome. These patients were subsequently transferred to the intensive care unit. Data for the review were obtained from hospital charts and from a prospectively collected ICU database.
Patient selection and data collection
The study includes a 14-day tracking period of all trauma and surgical patients who were admitted to the ICU and who had an open abdomen for seven days or more. For the purpose of the study, patients who had definitive fascial closure were no more considered an open abdomen patients. Demographic data included: age, sex, mechanism of injury, admission weight, body mass index (BMI), Injury Severity Score (ISS), Acute Physiology and Chronic Health Evaluation II (APACHE II), as well as initial albumin and pre-albumin, and hospital and ICU length of stay.
Nutritional assessment data and calculations
On patient admission, clinical nutritionist calculated energy expenditure based on the 2003 Penn State equation by using Mifflin St. Jeor equation as follow [10,20].
| Penn State equation | 0.96 × (Mifflin St. Jeor) + 167 × (maximum temperature) + 31 × (minute ventilation) - 6212 |
| Mifflin St. Jeor equation | Men: 10 (weight kg) + 6.25 (height cm) - 5 (age) + 5 |
| Women: 10 (weight kg) + 6.25 (height cm) - 5 (age) - 161 |
Total daily energy and protein needs derived from both enteral and parenteral nutrition formulae were calculated from each patient's ICU flow sheets and the clinical dietitian's orders. Total enteral nutrition (EN), such as Peptamen AF 1.2, Peptamen 1.5, Vivonex Plus, Isosource 1.5, and Promote, as well as Total Parenteral Nutrition (TPN), any relative fluids such as dextrose and any medical infusion such as Propofol were calculated to determine the total kcal/day and protein in each cubic centimeter. Average nutritional intake was calculated and divided by prescribed nutritional target to get the mean percent target for three different timelines: first week, second week, and two weeks of ICU admission. Optimal energy needs were defined as ± 10 % of the mean target. The mean percent goal per ICU day was calculated as follows: [21]
Independent variables such as route of feeding, technique of closure, duration of open abdomen, ventilation days, and any clinical outcomes such as sepsis, pneumonia, fistula, tracheostomy, wound infection, and intra-abdominal sepsis were also reviewed.
Statistical analyses
All data was entered into a Microsoft Excel Work sheet. Statistical analyses were performed using the SAS System, version 9.3 (SAS Institute, Cary, NC). Patient characteristics at baseline were summarized using proportions, means with standard deviation and medians with ranges as appropriate. Student t, Kruskal-Wallis and chi square tests were used to compare clinical variables between patients who had achieved optimal caloric and those who had not. Similar analyses were carried out for optimal protein intake. P values were reported.
Results
Patient characteristics
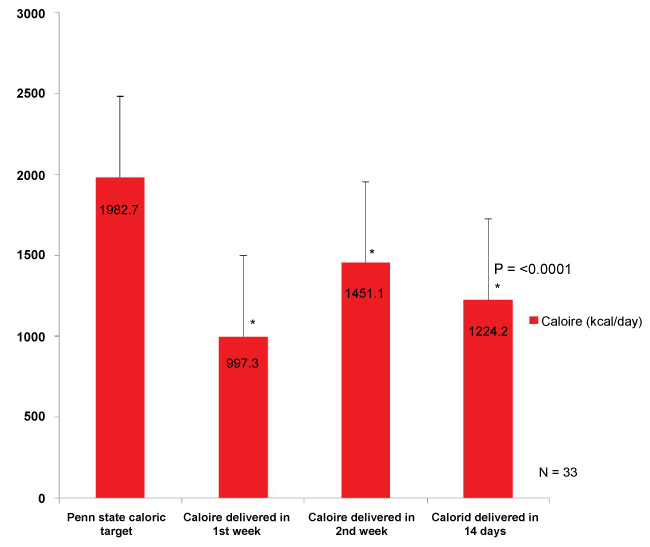
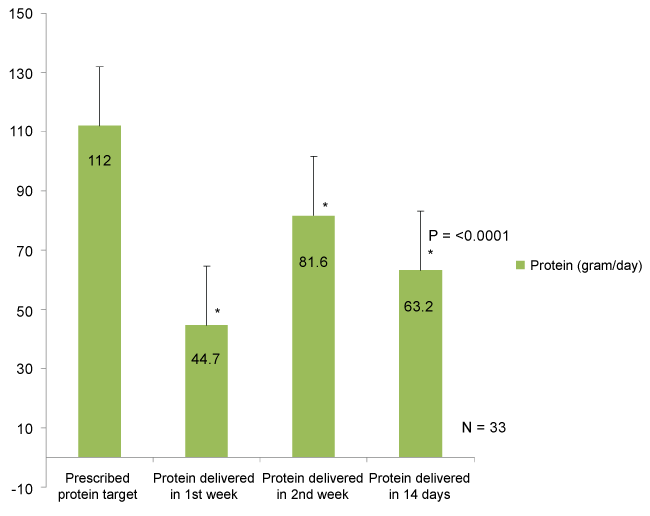
During the study period, the total open abdomen patients were 110. Thirty-five patients who had an open abdomen for seven days or more were enrolled in the study. Two of those patients were excluded due to incomplete data, leaving 33 patients for analysis (Table 1). The median patient age was 47 years. Eighty-five percent of the patients were males. Seventy percent of the patients were trauma patients, while 30% were general surgery patients. Blunt mechanisms of injury were more common than penetrating trauma (60% vs. 40%). Thirty percent of the patients were obese, with a BMI ≥ 30. Among the 33 patients with open abdomens, the mean prescribed caloric and protein target was 1982.7 ± 422 Kcal/day and 112 ± 27 gram/day, respectively.
Route of feeding and nutritional targets
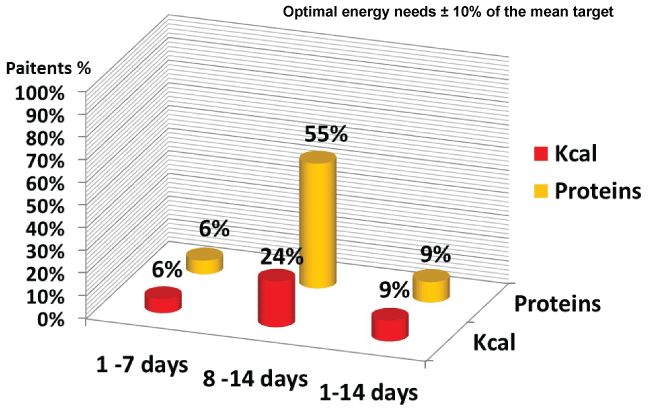
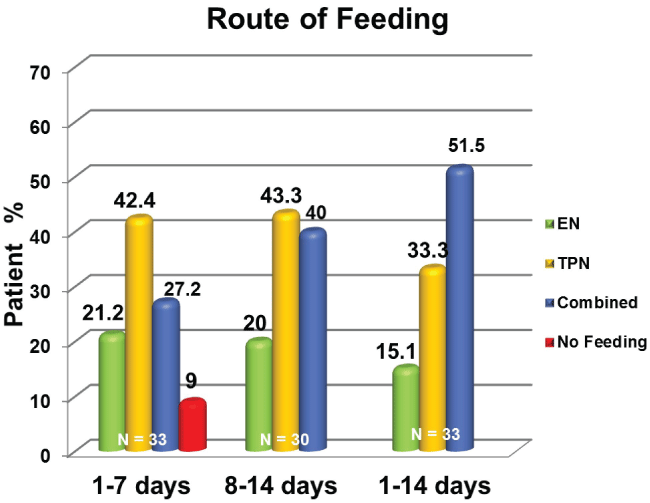
During three different points in time, first week, second week, and two weeks of ICU admission , average energy and protein intakes were significantly lower compared to prescribed nutritional targets with a statistically significant difference (p = < 0.0001) (Figure 1 and Figure 2). The patients' mean percent goals were calculated and compared at all three different timelines (Figure 3). Route of feeding was also evaluated at the same timeline (Figure 4).
In the first week, the average daily calorie and protein intakes delivered were 997.3 ± 380 Kcal/day and 44.6 ± 27.5 gram/day, respectively. Enteral nutrition was successfully introduced in 21.2% of the patients, while 42.4% received TPN, 27.2% received combined nutrition (EN + TPN), and 9% did not receive any form of nutrition support. Six percent of patients met more than 90% of their target calories and protein. During the second week, the average daily calorie and protein intakes delivered were 1451.1 ± 680 Kcal/day and 81.6 ± 43.9 gram/day, respectively. Twenty percent of patients received EN, while 43.3% received TPN, and 40% received both TPN and EN. Fifty-five percent of the patients achieved ≥ 90% of their protein intake target, while 24% achieved the optimal caloric target.
Over two weeks of ICU admission, an average delivered calorie was found to be significantly lower as opposed to prescribed caloric target (1224.2 ± 439 vs. 1982.7 ± 422.8 Kcal/day, p < 0.0001). Similarly, an average delivered protein was significantly lower compared with prescribed protein target (63.2 ± 30.5 vs. 112 ± 27 gram/day, p < 0.0001).
Thirty-three percent of patients received TPN. Only 15.1% received EN and 51.5% received combined TPN and EN nutritional support. Nine percent of the patients achieved the optimal caloric and protein targets.
Clinical outcomes
In the second week, patients were divided into two groups on the basis of the optimal and suboptimal protein targets (Table 2A). Univariate analysis showed no significant difference in patients' baseline characteristics, such as age, gender, BMI, Injury Severity Score (ISS), APACHE II score, as well as prescribed caloric and protein targets, was identified. As expected, the group who met ≥ 90% of the protein target, had a significant higher average delivered protein than the suboptimal group (115.8 ± 17.5 vs. 49.4 ± 35.9 gram/day, p = < 0.0001). There was no statistically significant difference between both groups in clinical outcomes such as sepsis, pneumonia episodes, duration of open abdomen, and length of ICU stay. However, the unadjusted survival rate was significantly higher in the group who met ≥ 90 % of the protein target (100% vs. 64%, p = 0.011). Also, the proportion of TPN use in the group who achieved the optimal protein target was significant compared to the suboptimal group (68% vs. 13%, p = 0.002) (Table 2B). The average initial day of TPN use was earlier in those who met ≥ 90% of the protein target compared to the suboptimal group (3 ± 1 vs. 6 ± 3.5 days, p = 0.017).
During the same period, a univariate analysis was performed between the optimal and suboptimal caloric target (Table 3A). Between day 8-14, the patients who met ≥ 90% of the caloric target received, on average, 1994.7 ± 436 Kcal/day, while those who met < 90% of the prescribed target received, on average, 1304.8 ± 665 Kcal/day, (P = 0.021). Furthermore, there was no statistically significant difference between both groups in baseline characteristics data, such as age, gender, ISS, APACHE II score, prescribed caloric and protein targets, as well as the route of feeding or any standard clinical outcomes such as survival, pneumonia episodes, sepsis, the length of ICU stay, or the duration of open abdomen (Table 3B).
Discussion
The fundamental goal of nutritional support is to meet energy and protein needs and to minimize protein catabolism. This study investigated the adequacy of nutritional support over 14 ICU days in 33 patients who had an open abdomen for seven days or more after trauma or general surgery emergencies. We observed that critically ill patients were insufficiently fed during their two-week stay at the ICU according to traditional nutritional targets. In previous studies, malnutrition seemed to be a considerable problem in the surgical ICU [22,23]. Similarly, in Canadian ICUs, Heyland, et al. [24] reported that 16% of patients who stayed more than three days in the ICU did not receive any nutritional support. Furthermore, during their first 12 days in the ICU, the patients who received nutritional support achieved only 56% to 62% of their estimated energy needs.
Nutritional Intake and Outcomes
To the best of our knowledge, this is the first study performed that explore the sufficiency of the nutrition offered to open abdomen patients with delayed fascial closure. The primary aim of the study was to determine if open abdomen patients were adequately fed. The data elaborated that mean delivered calories and protein were significantly lower compared to prescribed target during different timelines. Given that the delivered energy and protein were collected from various sources such as TPN, dextrose infusion as well as medications such as Propofol. Our results also demonstrated that 6% (2/33) of the patients achieved 90% or more of the mean calories and protein targets by day 7. The optimal caloric and protein targets, however, have been achieved in 24% and 55% during the second week, respectively. This finding is consistence with other study conducted by Hise, et al. [21] who concluded that minority of medical and surgical ICU patients reached 70% of dietitian recommendation. In contrast Tsuei, et al. [25] reported that 57% (8/14) of open abdomen patients who received EN with duration ranging between 4 to 35 conservative days met at least 80% of estimated or measured energy expenditure. Because open abdomen patients with delayed abdominal closure had multiple injuries that require multiple surgeries, interruption of feeding could be the main reason of inadequate feeding. Other study reported that surgery (27%) is the most common cause of feeding interruption in trauma ICU patients [26].
Failure to meet the prescribed target has been shown to prompt adverse outcomes [27-30]. It is interesting to note that unadjusted survival rate was significantly higher in the group who achieved ≥ 90% of protein target and the rate of TPN use were also higher in the same group. The present study suggests that patients with delayed closure of the abdomen may need TPN and adequate nutritional supply. In patients without open abdomen, similar finding was observed by Woodcock, et al. [31] who found high mortality rate and high incidence of inadequate nutrition in the group received EN. In cancer patients, Pearlstone, et al. [32] documented that level of plasma amino acid repletion was much higher in patients who had received TPN compared to EN or Libitum oral feeding. On the other hand, our data showed that achieving optimal caloric target seems not to affect the survival rate. In agreement with Strack van Schijindel, [28] who found that achieving both energy and protein target in mechanically ventilated critically ill patients had significant better survival rate than those achieve only energy target.
Other clinical outcomes such as sepsis, pneumonia, ICU length of stay, and duration of open abdomen was not significant between optimal and suboptimal protein or caloric intake. Although the differences were not statistically significant, our study demonstrated that there is a reduction in complication namely sepsis in patients who met the caloric and protein target, along with a trend towards reduction in the mortality for those who met the optimal caloric target. Verily, a significant difference may be observed with increasing number of participants in future studies. Our data also reflected that provision optimal amount of energy and early feeding might not be important in determining outcomes. Moreover, early feeding (≤ 4 days) may not in fact be a crucial factor to achieve optimal amount of calorie and protein. Previous studies have reported improved outcomes with early EN in trauma patients with or without open abdomen. [33-35] For instance, in open abdomen study conducted by Dissanaike, et al. [33] showed early enteral feeding has significantly less rate of pneumonia compared with control group (43.8% vs. 72.1%, p = 0.008). However, no significant difference in mortality, length of ventilator days, ICU days or hospital days were observed between groups.
The present study is different from other studies because of many reasons. Firstly, the study investigated the adequacy of both calorie and protein in open abdomen patients, in particularly, with a delayed abdominal closure. The study further investigated the impact of achieving the optimal target on clinical outcomes. Secondly, the delivered energy was collected from various sources, namely, TPN, dextrose infusion and Propofol.
Our study has substantial limitations; this is a retrospective study with a small sample size and with no distinction made between trauma and general surgery patients. Lack of biomedical nutritional markers at the time of admission may also add to its limitations because it is hard to determine whether the patients were underfed or adequately fed at the time of surgery. We believe that these findings should be confirmed by large prospective and multi-center studies.
Conclusions
Based on the Penn State equation estimation, the vast majority of open abdomen patients in this study were insufficiently fed during their two weeks of ICU stay. Patients who achieved their protein target at 14 days were more likely to survive than the group who did not achieve that target. As well, TPN use was higher in the group with a higher survival rate. Achievement of optimum calories, however, did not seem to affect patient clinical outcomes. Due to many challenges associated with open abdomen patients, the careful monitoring of their energy needs may potentially improve their nutritional status and subsequently their clinical outcomes. Further studies are needed to investigate the correlation between outcomes, initiation of feeding, and the route of feeding in this critically ill population.
References
- Sutton E, Bochicchio GV, Bochicchio K, et al. (2006) Long term impact of damage control surgery: a preliminary prospective study. J Trauma 61: 831-834.
- McClave SA, Snider HL (1994) Understanding the metabolic response to critical illness: factors that cause patients to deviate from the expected pattern of hypermetabolism. New Horiz 2: 139-146.
- Walker RN, Heuberger RA (2009) Predictive equations for energy needs for the critically ill. Respir Care 54: 509-521.
- Cartwright MM (2004) The metabolic response to stress: a case of complex nutrition support management. Crit Care Nurs Clin North Am 16: 467-487.
- Plank LD, Hill GL (2000) Sequential metabolic changes following induction of systemic inflammatory response in patients with severe sepsis or major blunt trauma. World journal of surgery 24: 630-638.
- Finnerty CC, Mabvuure NT, Ali A, et al. (2013) The surgically induced stress response. JPEN J Parenter Enteral Nutr 37: 21S-29S.
- Pickkers P, Hoedemaekers A, Netea MG, et al. (2004) Hypothesis: Normalisation of cytokine dysbalance explains the favourable effects of strict glucose regulation in the critically ill. The Netherlands journal of medicine 62: 143-150.
- Cerra FB, Benitez MR, Blackburn GL, et al. (1997) Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest 111: 769-778.
- Opper FH, Burakoff R (1994) Nutritional support of the elderly patient in an intensive care unit. Clin Geriatr Med 10: 31-49.
- Frankenfield D (2011) Validation of an equation for resting metabolic rate in older obese, critically ill patients. JPEN J Parenter Enteral Nutr 35: 264-269.
- Frankenfield DC, Coleman A, Alam S, et al. (2009) Analysis of estimation methods for resting metabolic rate in critically ill adults. JPEN J Parenter Enteral Nutr 33: 27-36.
- Frankenfield D, Smith JS, Cooney RN (2004) Validation of 2 approaches to predicting resting metabolic rate in critically ill patients. JPEN J Parenter Enteral Nutr 28: 259-264.
- Powell NJ, Collier B (2012) Nutrition and the open abdomen. Nutr Clin Pract 27: 499-506.
- Cheatham ML, Safcsak K, Brzezinski SJ, et al. (2007) Nitrogen balance, protein loss, and the open abdomen. Crit Care Med 35: 127-131.
- Fleck A, Raines G, Hawker F, et al. (1885) Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet 1: 781-784.
- Friese RS (2012) The open abdomen: definitions, management principles, and nutrition support considerations. Nutr Clin Pract 27: 492-498.
- Open Abdomen Advisory P, Campbell A, Chang M, et al. (2009) Management of the open abdomen: from initial operation to definitive closure. Am Surg 75: S1-22.
- Hadley JS, Hinds CJ (2002) Anabolic strategies in critical illness. Curr Opin Pharmacol 2: 700-707.
- Wray CJ, Mammen JM, Hasselgren PO (2002) Catabolic response to stress and potential benefits of nutrition support. Nutrition 18: 971-977.
- Mifflin MD, St Jeor ST, Hill LA, et al. (1990) A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241-247.
- Hise ME, Halterman K, Gajewski BJ, et al. (2007) Feeding practices of severely ill intensive care unit patients: an evaluation of energy sources and clinical outcomes. J Am Diet Assoc 107: 458-465.
- Souba WW (1997) Nutritional support. The New England journal of medicine 336: 41-48.
- Heyland DK, Schroter-Noppe D, Drover JW, et al. (2003) Nutrition support in the critical care setting: current practice in canadian ICUs--opportunities for improvement? JPEN J Parenter Enteral Nutr 27: 74-83.
- Heyland DK, Dodek P, Canadian Critical Care Clinical Practice Guidelines C, et al. (2003) Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 27: 355-373.
- Tsuei BJ, Magnuson B, Swintosky M, et al. (2003) Enteral nutrition in patients with an open peritoneal cavity. Nutr Clin Pract 18: 253-258.
- Morgan LM, Dickerson RN, Alexander KH, et al. (2004) Factors causing interrupted delivery of enteral nutrition in trauma intensive care unit patients. Nutr Clin Pract 19: 511-517.
- Petros S, Engelmann L (2006) Enteral nutrition delivery and energy expenditure in medical intensive care patients. Clin Nutr 25: 51-59.
- Strack van Schijndel RJ, Weijs PJ, Koopmans RH, et al. (2009) Optimal nutrition during the period of mechanical ventilation decreases mortality in critically ill, long-term acute female patients: a prospective observational cohort study. Crit Care 13: R132.
- Rubinson L, Diette GB, Song X, et al. (2004) Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med 32: 350-357.
- Villet S, Chiolero RL, Bollmann MD, et al. (2005) Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 24: 502-509.
- Woodcock NP, Zeigler D, Palmer MD, et al. (2001) Enteral versus parenteral nutrition: a pragmatic study. Nutrition 17: 1-12.
- Pearlstone DB, Lee JI, Alexander RH, et al. (1995) Effect of enteral and parenteral nutrition on amino acid levels in cancer patients. JPEN J Parenter Enteral Nutr 19: 204-208.
- Dissanaike S, Pham T, Shalhub S, et al. (2008) Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am Coll Surg 207: 690-697.
- Collier B, Guillamondegui O, Cotton B, et al. ( 2007) Feeding the open abdomen. JPEN J Parenter Enteral Nutr 31: 410-415.
- Moore FA, Feliciano DV, Andrassy RJ, et al. (1992) Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 216: 172-183.
Corresponding Author
Kosar A Khwaja, Laboratoire de Physique Fondamentale et Appliquée, UFR SFA, University Nangui Abrogoua, 02 BP 801, Abidjan 02, Cote D'Ivoire, Tel: +225-02-36-30-08.
Copyright
© 2016 Hassan ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.