Suppression of Free Fatty Acids by Oral Glucose in Patients with Cystic Fibrosis
Abstract
Aim
The oral glucose tolerance test (OGTT) is part of the routine management and readily detects impaired insulin secretion in the majority of adult patients with cystic fibrosis (CF). To assess an activity of endogeneous insulin on adipose tissue in these patients, we analyzed serum levels of insulin and free fatty acids (FFA) in response to oral glucose since glucose-induced insulin decreases FFA and thus, suppression of FFA reflects an insulin effect on adipose tissue.
Methods
Adult patients with end-stage CF lung disease without known dysglycemic disorder and a control group of healthy subjects were included. We assessed levels of two adipokines, adiponectin and leptin, and suppression of FFA during OGTT. Insulin secretion was estimated by the insulinogenic index (IGI), and whole body and adipose tissue insulin sensitivity by the indices ISIcomp and ISIfat, respectively.
Results
OGTTs were performed in 39 patients and 23 controls. Plasma glucose at 2h was higher and IGI was lower in patients with CF than in controls. ISIcomp and ISIfat were similar in patients with CF and in controls. During OGTT, FFA decreased to a comparable extent in both groups. Leptin was lower whereas total adiponectin tended to be higher and its HMW form was significantly higher in patients with CF than in controls.
Conclusion
Residual insulin (in face of apparently normal insulin sensitivity and increased energy expenditure and - possibly adiponectin-enhanced - FFA oxidation) appears to be sufficient for maintaining FFA homeostasis in patients with advanced CF lung disease.
Keywords
Oral glucose tolerance test, Cystic fibrosis related diabetes, Insulin sensitivity, Free fatty acids, Adiponectin, Leptin
Abbreviations
A1c: Glycated Haemoglobin; AUC: Area Under Curve; CF: Cystic Fibrosis; CFRD: Cystic Fibrosis Related Diabetes; CFTR: Cystic Fibrosis Transmembrane Conductance Regulator; FEV1: Forced Expiratory Volume in 1 Second; GH: Growth Hormone; h: Hours; HMW: High Molecular Weight; IGF-1: Insulin-Like Growth Factor-1; IGI: Insulinogenic Index; IGT: Impaired Glucose Tolerance; INDET: Indeterminate Glucose Tolerance; IQR: Interquartile Range; ISIcomp: Whole Body Insulin Sensitivity Index; ISIfat: Adipose Tissue Insulin Sensitivity Index; NGT: Normal Glucose Tolerance; OGT: Oral Glucose Tolerance; OGTT: Oral Glucose Tolerance Test; PG: Plasma Glucose U/V: Unit/Volume
Introduction
Cystic fibrosis (CF) is a multi-organ disease, characterized by chronic systemic inflammation and high-energy expenditure. Exocrine pancreatic insufficiency often develops in the first year of life. Malnutrition and low body weight are common clinical findings in CF despite enzyme replacement therapy. Endocrine pancreas insufficiency appears later in course of disease. However, improved symptomatic treatment regimens and better outcomes following lung transplantation have contributed to an improved overall life expectancy in patients with CF over the last years [1]; Thus, disorders of glucose homeostasis increasingly emerge in clinical practice. Up to half of adult patients with CF have CF related diabetes (CFRD). CFRD is related to increasing age, duration of exocrine pancreatic failure, and the Phe508del homozygous state. In addition, oral glucose tolerance test (OGTT) detects impaired glucose tolerance (IGT; Fasting, ≤ 7.0 mmol/l and 2h plasma glucose (2hPG), ≥ 7.8 mmol/l and ≤ 11.0 mmol/l) or indeterminate glucose tolerance (INDET; fasting, ≤ 7.0 mmol/l and 2hPG, ≤ 7.7 mmol/l, but 1hPG ≥ 11.1 mmol/l) in another substantial proportion of patients [2,3]. At our centre, the majority of patients (75%) are diagnosed with CFRD at the time of evaluation and listing for lung transplantation [4]. Inflammatory pancreatic tissue destruction and subsequent islet cell loss is probably a main cause of CFRD and impaired glucose tolerance [5,6]. While the decreased insulin secretion is well accepted in the pathogenesis of CFRD, the role of insulin resistance is less clear, and data are conflicting [6].
Patients with CFRD rarely present with ketoacidosis, suggesting that a complete lack of insulin action is rather exceptional in patients with CF. Adipose tissue is often reduced in patients with CF, and clearance of energy-providing substrates such as glucose and FFA is high even if ambient insulin concentrations are relatively low, i.e. non-insulin-mediated glucose and FFA disposal may be increased. Patients with CF have increased resting energy expenditure, related to the CF transmembrane conductance regulator (CFTR) gene, pancreatic and lung function, and inflammatory and nutritional status [7,8]. FFA release from stored triglycerides (lipolysis) is very sensitive to suppression by insulin, and in most conditions, insulin appears to control FFA levels by reducing their endogenous appearance rate [9]. Epidemiological studies have linked increased FFA levels to obesity, insulin resistance and type-2 diabetes. The adipose tissue insulin sensitivity index as proposed by Belfiore (ISIfat) is based on suppression of FFA in an OGTT by endogeneous insulin; and the ISIfat was decreased in such patients [10,11]. However, studies applying this method in patients with CF are lacking.
Adiponectin is by far the most abundant adipokine, it has anti-inflammatory and insulin-sensitizing properties. It circulates in several forms; the most abundant are high molecular-weight (HMW) multimers. In patients with obesity, insulin resistance, hyperinsulinemia, and type 2 diabetes, the levels of adiponectin and specifically its HMW form are reduced [12,13]. Adiponectin is elevated in patients with type 1 diabetes and in patients with anorexia nervosa [14-16].
Leptin is predominantly secreted from adipocytes; its serum levels usually correlate positively with fat mass and body weight. Therefore, elevated serum leptin levels are common in obesity and possibly thereby associated with insulin resistance and hyperinsulinemia. In contrast, anorectic and underweight patients show low leptin levels [15].
The main goal of our study was to assess suppression of FFA during OGTT; moreover, we measured adiponectin and leptin in patients with CF evaluated for lung transplantation.
Methods
Subjects
Over a 10-year period, we recruited consecutive patients with CF undergoing evaluation for lung transplantation. Inclusion criteria were age ≥ 18 years and agreement to participate in the study. Exclusion criteria were known previous fasting PG ≥ 7 mmol/l, insulin treatment, corticosteroid therapy and BMI ≥ 30 kg/m2. We used control subjects who had an OGTT for other reasons (i.e., diagnostic evaluation of a potential hypoglycemic disorder, or exclusion of acromegaly) and in whom a dysglycemic disorder was excluded [17]. Study patients and controls were matched for age and gender. The study was approved by the Local Ethics Committee and written informed consent was obtained.
CF status
All patients with CF undergoing evaluation for lung transplantation had forced expiratory volume in 1 second (FEV1) measured by spirometry and their body weight by an electronic scale. All data were collected within 1 week of OGTT. CF genotype status was retrieved from patients' records.
Laboratory values and oral glucose tolerance test
All patients and the control group had laboratory analysis as described below and underwent a standard 75 g OGTT. In the morning after an overnight fast, a peripheral intravenous line was placed into the antecubital vein of all subjects followed by the ingestion a solution with 75 g glucose. Blood samples for glucose, insulin and FFA were obtained at baseline (mean of two samples, taken 15 min and immediately before) as well as 30, 60, 90, and 120 min after glucose load. According to the results of the OGTT, study patients were divided in two groups: one group with CFRD and the other without CFRD. CFRD was diagnosed when glucose readings 120 minutes after glucose load were ≥ 11.1 mmol/l.
Laboratory measurements
Venous PG was measured by the hexokinase method (Beckman Analyzer; Fullerton, CA, USA), serum insulin by solid phase radioimmunoassay (CIS Bio International, Oris Industries, Gif-Sur-Yvette, France) and FFA by an enzymatic endpoint calorimetric assay (Wako Diagnostics, 1600 Bellwood Road, Richond, VA 23237-1326, USA). Glycated Haemoglobin (A1c) was measured by immunoturbidimetric method in EDTA blood samples. Adiponectin was measured by EIA (R&D Systems, Inc., 614 McKinley Place NE, Minneapolis, MN 55413) and its high molecular weight (HMW) multimer form by ELISA (ALPCO, 26-G Keewaydin Drive, Salem, NH 03079). Leptin was measured by Human Leptin ELISA Kit (Cat. EZHL-80SK, EMD Millipore Corporation, St. Charles, Missouri 63304 USA).
β-cell function and insulin sensitivity indices
To estimate β-cell function, the insulinogenic index (IGI) was calculated as proposed by Wareham, i.e., as the ratio of the increment of serum insulin (between 0 and 30 min) to PG at 30 min after the glucose load [18]. Whole body insulin sensitivity index (ISIcomp) was calculated by data obtained during the OGTT according to Matsuda and DeFronzo: ISIcomp = 10,000/square root of [(mean plasma insulin × mean glucose during OGTT) × (fasting PG × fasting plasma insulin)]. Insulin sensitivity of adipose tissue (ISIfat) was estimated as proposed by Belfiore [10]: ISIfat = 2/[(area under curve (AUC)INSp × AUCFFAp) + 1], where AUCINSp and AUCFFAp represent insulinemic and FFA areas under curve (AUC) during OGTT of the person under study divided by the mean inulinemic or FFA AUC of control group. AUC was calculated as unit/volume (U/V) × min-1 = [(U/V0' + U/V30') × 30/2)] + [(U/V30' + U/V60') × 30/2)] + [(U/V60' + U/V90') × 30/2)] + [(U/V90' + U/V120') × 30/2)].
Statistics
Data are presented as median and interquartile range (IQR) since some of the values were not normally distributed. Accordingly, the statistical evaluation for potential differences between the groups of patients and controls was done by unpaired Wilcoxon rank-sum test. p < 0.05 was considered significant.
Results
Characteristics of patients with CF
Data are shown in Table 1. Thirty nine patients with CF (21 women; median age 24 (IQR 21-35) y and 23 controls (12 women; median age 31 (IQR 23-35) y were included in the study. Nineteen patients were homozygous and 14 were heterozygous for Phe508del, respectively; six patients had other CF-defining genotypes. The CF-group was fairly homogenous as all patients were undergoing lung transplantation assessment, implicating that all of them were suffering from severe lung disease, dominating their markedly reduced health status. Their median FEV1 was 28 (IQR 21-38)%. BMI was significantly lower, fasting PG was borderline and A1c on average markedly higher, albumin was lower and CRP higher in patients with CF than in controls.
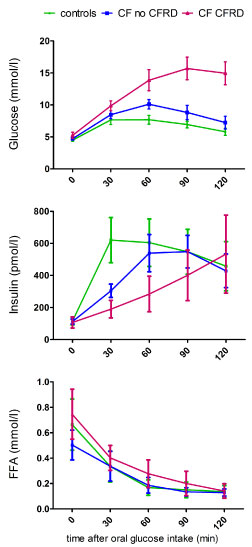
Plasma glucose during OGTT
Data are shown in Table 2 and Figure 1. Fasting PG was similar in patients without CFRD and controls and slighly higher in patients with CFRD. PG 2 hours after glucose intake was much higher in patients with CF than in controls. Compared to control group, the 2hPG was only slightly increased (p < 0.05) in patients without CFRD and much higher (p < 0.0001) in those with CFRD (by definition, ≥ 11.1 mmol/l). A new diagnosis of CFRD was made in 14 (36%) patients. In addition, 10 (26%) patients with CF had IGT, and 4 (10%) INDET (both subgroups included in the 25 CF no CFRD in Table 1 and Figure 1).
Insulin, OGTT-derived indices and FFA suppression
Data are shown in Table 2 and Figure 1. Insulin measured before and 2 hours after glucose intake was similar in patients with CF and controls. However, in patients with CF the insulin peak was delayed, leading to a significantly lower IGI in patients with CF compared to controls. The decrease in IGI was more pronounced in patients with CFRD, and less marked (intermediate) in those with IGT and INDET. Of note, patients without CFRD but especially those with IGT or INDET also showed delayed insulin secretion with a peak after 90 minutes, having a low IGI but normal AUC of insulin. In contrast to the significantly decreased IGI in CF patients, ISIcomp and ISIfat were similar in patients in controls and CF-patients (and independent whether CFRD was diagnosed or not). Despite lower and delayed insulin secretion (resulting in higher glucose values) in patients with CF, FFA decreased to a similar extent as in controls, irrespectively of whether subjects had CFRD or not.
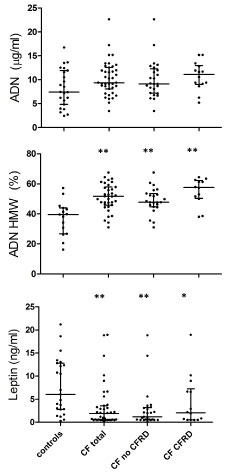
Adiponectin and Leptin
Data are shown in Table 2 and Figure 2. Total adiponectin was similar in patients with CF and controls (with a trend for higher values in the former group), and HMW-adiponectin was significantly higher in patients with CF than in controls. Leptin was significantly lower in patients with CF than in controls.
Discussion
Our results confirm previous reports on high diabetes incidence in patients with CF, and the low IGI is in line with the generally accepted view of impaired insulin secretion as an important pathogenic mechanism in CFRD. Nevertheless, oral glucose suppressed FFA to a similar extent in patients with CF compared to controls.
Patients with type 2 diabetes have impaired insulin-mediated FFA suppression by OGTT, and therefore, a low ISIfat [10]. Carlson, et al. reported on a small subgroup of elderly patients with isolated post challenge hyperglycemia (i.e., normal fasting and diabetic 2hPG values), similar to the majority of our CF patients. This subgroup was characterized by increased FFA levels (basal and during OGTT), despite increased insulin levels at 2h [19]. The authors concluded that elevated FFA not only reflect but also contribute to insulin resistance and elevated 2hPG levels. Although not consistently insulin resistant as most obese patients with type 2 diabetes, fluctuating insulin sensitivity contributes to changes in glucose tolerance of patients with CF and normal glucose tolerance (NGT), INDET or IGT; this may well account for the characteristically high variability in oral glucose tolerance (OGT) in patients with CF [2,20,21]. It is conceivable that in patients with a given reduced insulin secretion and limited capacity to adapt appropriately to an increased insulin resistance, OGT may rapidly change over time, more than in healthy individuals. Insulin resistance is to be expected in the context of inflammation and low muscle mass; it may be found by hyperinsulinemic glucose clamp studies where skeletal muscle is the main target for high rates of glucose uptake. ISIcomp calculated by data obtained during OGTTs addresses more closely the importance of insulin sensitivity at ambient insulin levels, while the hyperinsulinemic clamp method preferentially addresses effects of high dose insulin on skeletal muscle. Normal insulin sensitivity is also in line with clinical observations in patients with CFRD, who usually need less insulin than patients with type 2 diabetes for blood glucose control. However, exogenous insulin requirements can change dramatically within a relatively short period in CFRD patients, an observation that illustrates again the contribution of insulin sensitivity. Using indices (ISIcomp and ISIfat) derived from endogenous insulin values; we found no evidence for insulin resistance in our patients with CF.
Milunsky, et al. [22] analyzed FFA during OGTT in children with CF several decades ago. Their (mostly pre-pubertal) patients had slightly elevated glucose values, marked insulinopenia, and increased growth hormone (GH) levels. Increased GH secretion is presumably a consequence of decreased insulin-like growth factor-1 (IGF-1) as expected in CF patients (and found in our CF patients, not shown) and favours insulin resistance, lipolysis and an increase in FFA. Nevertheless, FFA levels in their patients with CF were similar to those of controls [22]. Consistent with these findings, Hammana, et al. [23] found normal FFA suppression during OGTTs in patients with CF under conditions reflecting insulin action of everyday life conditions. The authors concluded that insulin levels remained sufficiently high to inhibit lipolysis and to stimulate lipogenesis, showing a striking dichotomy between postprandial glucose and FFA/lipid control in patients with CF [23]. The number of patients in the Hammana, et al. [23] study (16 per group) was relatively low, not including subjects more severely affected by their lung disease. In our study, we included many patients with more advanced CF lung disease, very severe insulin deficiency and markedly impaired glucose tolerance, yet, we reach the same conclusions as Hammana, et al. [23], namely that CF patients show abnormal post challenge glucose but not FFA excursions.
To date, adiponectin may also be high in patients with CF and insulinopenia but data have not been consistent. Elevated adiponectin levels have been found in some [24,25], but not all [26] studies, possibly related to inflammation, chronic energy deficit and wasting. According to our results, HMW-adiponectin is often increased in severely ill patients. Hyperadiponectinemia has been shown to be associated with underweight and malnutrition and normalizes after refeeding; particularly HMW-adiponectin is increased in patients with anorexia [15]. It remains unclear whether insulin can downregulate adiponectin in patients with anorexia and in patients with CF. Furthermore, it is unknown whether high adiponectin levels contribute to or merely reflect wasting. According to studies in animals, adiponectin acts in the brain to increase energy expenditure [27], and adiponectin stimulates FFA oxidation [28] and may enhance clearance of FFA. This may contribute to normal FFA levels in patients with CF and insulin deficiency. Although not directly estimated in our study subjects, increased resting energy expenditure in our patients with CF is likely. Given that adiponectin increases insulin sensitivity by stimulating FFA oxidation, the high adiponectin could explain the “unexpectedly” normal insulin sensitivity. At low insulin concentrations, glucose disappearance rates correlate with adiponectin in controls and in patients with type1 diabetes [16], but whether the same is true for glucose and FFA disappearance in patients with CF has not yet been specifically studied.
Distinct patient and control populations most likely explain different findings regarding adiponectin in CF studies, moreover, interfering drug treatment and differences in methodology. All of our patients had end-stage CF lung disease (so that they were probably not ideal to check for a possible relation between disease severity and adiponectin elevation). They were matched with controls for age but not for BMI, also characteristically having lower albumin and higher CRP levels than the controls; they did not receive corticosteroid or insulin treatment.
Similar to our results, Cohen, et al. [29] found decreased levels of leptin in adult patients with advanced CF. Their patients with severe CF were also not weight-matched to controls, and fat mass was closely related to leptin, suggesting that decreased leptin levels were more likely a consequence than a cause of weight loss. Ziai, et al. [30] found comparable leptin levels between CF patients and BMI-matched controls, demonstrating correlations between insulin and leptin levels after adjusting for gender and fat mass. Insulin increases plasma leptin concentrations in normal subjects and patients with type-2 diabetes [31] but corresponding studies have not been performed in patients with CF.
Limitations of our study include the extended observation period and single-center design, our heterogeneous, incompletely matched control group, and the lack of body composition and energy expenditure data. On the other hand, our study cohort was homogenous regarding the stages of their CF lung disease.
The impact of given insulin concentrations on the regulation of PG, or estimated appearance and disappearance rates of glucose from and into target tissues (liver and muscle) define the terms insulin sensitivity and resistance. Yet, insulin has additional important effects such as inhibition of lipolysis and proteolysis. Adipose tissue is not a dominant tissue for overall glucose disposal, nevertheless, is an important target tissue of insulin action. FFA suppression and ISIfat have been used to estimate insulin sensitivity and resistance of adipose tissue to actions of insulin [10], although insulin is not the only determinant for FFA (as for glucose) appearance and disappearance rates. In patients with CF, increased GH could favour lipolysis, and low insulin attenuate its most important brakes.
Most often, however, production and release of energy-providing substrates appear to remain well matched to increasing demands in patients with CF (at the expense of body mass, unless met by appropriate nutrition). Beyond the control of glucose and FFA levels, insulinopenia in these patients is especially deleterious for protein metabolism and skeletal muscle in these patients [4,32]. Food-induced insulin secretion and IGF-1 levels both tend to be low in patients with CF, yet both play a central role in maintaining healthy muscle. Insulin treatment may increase hepatic IGF-1 production and attenuate cytokine-induced protein breakdown, having beneficial effects on maintenance of skeletal muscle mass and nutritional status in these patients.
Normal fasting glucose values (and insulin sensitivity) in many patients with CF may explain the unfortunately often delayed detection of insulinopenia and frequent lack of timely recommendation for insulin replacement. Depot insulin injections may not be the most appropriate first line treatment, rather, we strongly advocate - independently of fasting glucose and A1c values, close clinical observations in individuals with CF as important bases to propose food-related (preprandial injection) insulin therapy.
Conclusions
In summary, we conclude that despite markedly impaired insulin secretion, oral glucose suppressed FFA to a similar extent in patients with CF as in healthy subjects. Residual insulin in the face of increased energy expenditure (and possibly adiponectin-enhanced-FFA oxidation) and apparently normal insulin sensitivity appears to be sufficient for maintaining FFA homeostasis in patients with CF.
Conflicts of Interest Disclosure
Dr. Schnyder reports personal fees from Philhuman Stiftung during the conduct of the study. There are no conflicts of interest.
Acknowledgements
We would like to thank Beate Sick, Institute of Biostatistics University Zurich, for statistical support, and the Philhuman Foundation for financial support.
References
- Elborn JS (2016) Cystic fibrosis. Lancet (London, England) 388: 2519-2531.
- Boudreau V, Coriati A, Hammana I, et al. (2016) Variation of glucose tolerance in adult patients with cystic fibrosis: What is the potential contribution of insulin sensitivity? J Cys Fibros 15: 839-845.
- Coriati A, Ziai S, Azar M, et al. (2016) Characterization of patients with cystic fibrosis presenting an indeterminate glucose tolerance (INDET). J Cys Fibros 15: 127-132.
- Hofer M, Schmid C, Benden C, et al. (2012) Diabetes mellitus and survival in cystic fibrosis patients after lung transplantation. J Cys Fibros 11: 131-136.
- Lohr M, Goertchen P, Nizze H, et al. (1989) Cystic fibrosis associated islet changes may provide a basis for diabetes. An immunocytochemical and morphometrical study. Virchows Archiv A Pathol Anat Histopathol 414: 179-185.
- Lombardo F, De Luca F, Rosano M, et al. (2003) Natural history of glucose tolerance, beta-cell function and peripheral insulin sensitivity in cystic fibrosis patients with fasting euglycemia. Eur J Endocrinol 149: 53-59.
- Fried MD, Durie PR, Tsui LC, et al. (1991) The cystic fibrosis gene and resting energy expenditure. J Pediatr 119: 913-916.
- Bell SC, Saunders MJ, Elborn JS, et al. (1996) Resting energy expenditure and oxygen cost of breathing in patients with cystic fibrosis. Thorax 51: 126-131.
- Carpentier AC, Frisch F, Brassard P, et al. (2007) Mechanism of insulin-stimulated clearance of plasma nonesterified fatty acids in humans. Am J Physiol Endocrinol Metab 292: E693-E701.
- Belfiore F, Iannello S, Volpicelli G (1998) Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose, and FFA levels. Molecular Genetics and Metabolism 63: 134-141.
- Holt HB, Wild SH, Wareham N, et al. (2007) Differential effects of fatness, fitness and physical activity energy expenditure on whole-body, liver and fat insulin sensitivity. Diabetologia 50: 1698-1706.
- Arita Y, Kihara S, Ouchi N, et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79-83.
- Basu R, Pajvani UB, Rizza RA, et al. (2007) Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: Differential regulation from nondiabetic subjects. Diabetes 56: 2174-2177.
- Imagawa A, Funahashi T, Nakamura T, et al. (2002) Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes care 25: 1665-1666.
- Modan-Moses D, Stein D, Pariente C, et al. (2007) Modulation of adiponectin and leptin during refeeding of female anorexia nervosa patients. The Journal of clinical endocrinology and metabolism. 92: 1843-1847.
- Combs TP, Snell-Bergeon JK, Maahs DM, et al. (2015) Adiponectin-SOGA Dissociation in Type 1 Diabetes. The Journal of clinical endocrinology and metabolism 100: E1065-E1073.
- Schnyder MA, Benden C, Faulenbach M, et al. (2016) Insulin secretion abnormalities in patients with cystic fibrosis. J Cys Fibros 15: e52-e53.
- Wareham NJ, Phillips DI, Byrne CD, et al. (1995) The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 12: 931.
- Carlson OD, David JD, Schrieder JM, et al. (2007) Contribution of nonesterified fatty acids to insulin resistance in the elderly with normal fasting but diabetic 2-hour postchallenge plasma glucose levels: The Baltimore Longitudinal Study of Aging. Metabolism 56: 1444-1451.
- Costa M, Potvin S, Hammana I, et al. (2007) Increased glucose excursion in cystic fibrosis and its association with a worse clinical status. J Cys Fibros 6: 376-383.
- Scheuing N, Holl RW, Dockter G, et al. (2014) High variability in oral glucose tolerance among 1,128 patients with cystic fibrosis: A multicenter screening study. PLoS One 9: e112578.
- Milunsky A, Bray GA, Londono J, et al. (1971) Insulin, glucose, growth hormone, and free fatty acids. Determinations in patients with cystic fibrosis. Am J Dis Child 121: 15-19.
- Hammana I, Coderre L, Potvin S, et al. (2009) Dichotomy between postprandial glucose and lipid profiles in adults with cystic fibrosis: A pilot study. J Cyst Fibros 8: 128-134.
- Moriconi N, Kraenzlin M, Muller B, et al. (2006) Body composition and adiponectin serum concentrations in adult patients with cystic fibrosis. J Clin Endocrinol Metab 91: 1586-1590.
- Panagopoulou P, Fotoulaki M, Manolitsas A, et al. (2008) Adiponectin and body composition in cystic fibrosis. J Cyst Fibros 7: 244-251.
- Hammana I, Malet A, Costa M, et al. (2007) Normal adiponectin levels despite abnormal glucose tolerance (or diabetes) and inflammation in adult patients with cystic fibrosis. Diabetes Metab 33: 213-219.
- Qi Y, Takahashi N, Hileman SM, et al. (2004) Adiponectin acts in the brain to decrease body weight. Nature medicine 10: 524-529.
- Yamauchi T, Kamon J, Minokoshi Y, et al. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288-1295.
- Cohen RI, Tsang D, Koenig S, et al. (2008) Plasma ghrelin and leptin in adult cystic fibrosis patients. J Cyst Fibrosis 7: 398-402.
- Ziai S, Belson L, Malet A, et al. (2012) The association between leptin and insulin levels in adults with cystic fibrosis. Diabetes Metab 38: 34-39.
- Malmstrom R, Taskinen MR, Karonen SL, et al. (1996) Insulin increases plasma leptin concentrations in normal subjects and patients with NIDDM. Diabetologia 39: 993-996.
- Rafii M, Chapman K, Stewart C, et al. (2005) Changes in response to insulin and the effects of varying glucose tolerance on whole-body protein metabolism in patients with cystic fibrosis. Am J Clin Nutr 81: 421-426.
Corresponding Author
Marie-Angela Schnyder, Division of Endocrinology, Diabetes and Clinical Nutrition, Inselspital, Freiburgstrasse 15, Bern, Switzerland.
Copyright
© 2019 Schnyder M-A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.